Abstract
In this study, the facile and sustainable synthesis of highly microporous carbons is explored to reduce the extensive use of harsh activating agents and solvents. The role of potassium citrate (PC) as a greener activating agent in addition to the conventional ZnCl2 is investigated in the mechanochemical solvent-free preparation of highly microporous carbon materials from chestnut tannin (CT), a biomass-type carbon precursor. A small amount of potassium citrate as a chemical activator coupled with CO2 activation at 700 °C afforded carbons with higher specific surface area (1256 m2 g−1) and larger micropore volume (0.54 cm3 g−1) as compared to the carbons activated with both PC and ZnCl2. The high microporosity of the PC-activated carbon materials, significantly enlarged after CO2 activation from micropore volume of 0.16 to 0.54 cm3 g−1, makes them favorable for CO2 adsorption, as evidenced by high adsorption capacity of 3.55 mmol g−1 at ambient conditions (25 °C, 1 bar). This study shows that the solvent-free mechanochemical processing of tannin in the presence of PC is a promising method for obtaining highly microporous carbon materials.
1. Introduction
Carbon dioxide (CO2) is one of the most concerning greenhouse gases these days and most CO2 emissions are caused by human activity such as industrialization and fossil fuel combustion in vehicles [1]. A rapid increase in the CO2 emission rate in this century has accelerated global climate change [1]. It has been reported that atmospheric CO2 concentration has tremendously increased, from 280 ppm in the industrial revolution era to 416.5 ppm in 2020 and is predicted to rise to almost 600 ppm by 2100 [1]. Therefore, the development of CO2 removal technology is a crucial and challenging issue that urgently needs to be resolved. One of the most popular and effective CO2 capture strategies is adsorption on solid adsorbents such as metal–organic frameworks (MOFs), zeolite imidazolate frameworks (ZIFs), zeolites, porous alumina, and porous carbon materials, due to their low energy consumption and excellent regeneration ability [1].
Porous carbon materials have been well-known as good adsorbents because of their high CO2 uptake capability, high surface area and great stability [1]. Many strategies for the synthesis of porous carbon materials have been reported, such as hard templating [2,3,4], soft templating [5,6,7] and conventional chemical activation [7,8,9]. However, these solution-based processes require a large amount of solvent and harsh and/or expensive chemicals, which make them time-consuming and unfeasible [10,11]. For these reasons, the solvent-free mechanochemical synthesis of porous carbon materials has a great potential, because it is a green and sustainable process.
Mechanochemical synthesis has been recognized as an effective, ecofriendly alternative to the conventional preparation methods. The chemical reactions during mechanochemical synthesis are initiated by mechanical forces [11,12,13,14,15,16] such as shearing, stretching, compression, grinding, etc. [11] The energy provided by mechanochemistry is sufficient to induce the physical and chemical transformation of the materials without applying any additional external energy [11]. During mechanochemical milling, defects are introduced in the precursors, which act as active sites and can propagate into fractures, increasing specific surface area of the resulting porous carbons [11]. Furthermore, the mechanochemical processes can occur in solvent-free conditions or require a minimal volume of solvent [11,12], which not only reduces the amount of solvent used, but also eliminates the sample-drying step. This advantage makes the mechanochemical synthesis an alternative to the conventional wet chemical synthesis procedures, which are complicated and require large amounts of solvents. Moreover, mechanochemical synthesis is recognized as a low-cost [17,18] and reproducible process [18], which has a high potential to be used for industrial-scale production of materials [18]. There are many reports on the mechanochemical synthesis of various types of carbon materials [11,12,19,20], including the fabrication of N-doped carbons with high surface area [21]. Therefore, mechanochemical synthesis was selected in this study.
Tannin is a naturally occurring polymer containing polyphenols that have been widely used as a substitute for conventional phenolic precursors [20] such as phenol and resorcinol [5,22]. Tannin is considered an environmentally benign chemical [20,23,24], despite a similar chemical reactivity to phenol. In addition, tannin is abundant since it can simply be obtained from biomass extraction [24]. The cost of tannin is listed at a significantly lower price than traditional phenolic resources [25]. There are several studies reporting the use of tannin as a carbon precursor to fabricate carbon materials through various synthesis routes. In 2020, Phuriragpitikhon and coworkers [5] successfully prepared ordered mesoporous carbon (OMC) from commercial mimosa tannin through a self-assembly process and one-step CO2 activation. The as-prepared OMCs, which possessed relatively high specific surface area and well-developed micro-/mesopore texture, were used for CO2 adsorption applications. In the same year, tannin-derived N-doped mesoporous carbon was mechanochemically synthesized by Zhao and coworkers and used for gas capture application with high adsorption selectivity [26]. Later in 2021, Pérez-Rodríguez and coworkers [24] demonstrated the use of pine tannin to prepare a high surface area biochar (2190 m2 g−1) through pyrolysis and a subsequent KOH activation approach. In 2022, sodium-ion battery anode materials were prepared by Tonnoir and coworkers through polycondensation of a commercial mimosa tannin, furfuryl alcohol and formaldehyde [22]. After pyrolysis at high temperature, these materials showed high specific surface area and excellent electrochemical performance [22]. These studies confirm that tannin has a significant potential as a biomass-type carbon precursor.
To achieve high porosity of carbons, various activating agents such as KOH [27,28,29], NaOH [30,31] and ZnCl2 [32,33,34,35] have been used. Among them, ZnCl2 has been recognized as an efficient activator to create micropores due to its comparatively small ionic radius [36]. Recently, Głowniak and coworkers [10] successfully prepared tannin-derived porous carbon through mechanochemical synthesis using urea as a nitrogen source, ZnCl2/NaCl eutectic salt as a template and potassium salts as activating agents. By coupling the salt-templating and mild chemical activation in a single step of mechanochemical processing, the resulting carbons exhibited high specific surface area and well-developed micropores that were responsible for high CO2 uptake [10]. Although many researchers reported on the use of potassium salts as activating agents, to our knowledge, there is no comparative study between the mild potassium salts and conventional activators on the activation effectiveness in the synthesis of porous carbons.
Here, this mechanochemical processing of tannin in the presence of potassium salt as a mild activating agent in combination with conventional ZnCl2 activator is further explored. In contrast to the research employing potassium salts as activators, studies focusing on the use of potassium citrate (representative of mild potassium salts) as a supplement or substitution for harsh chemical activating agents are rather rare. This work shows the role of potassium citrate as a mild activating agent integrated with ZnCl2 to create a greener procedure for the preparation of porous carbon materials while retaining high microporosity and great CO2 adsorption performance.
2. Materials and Methods
2.1. Chemicals and Reagents
Commercial chestnut tannin was obtained from Silva-Chimica, Cuneo, Italy. Zinc chloride anhydrous (ZnCl2, ≥99.995%) was purchased from Sigma-Aldrich, MO, USA. Potassium citrate monohydrate (C6H5K3O7·H2O, >95 wt%) was purchased from Spectrum Chemical Mfg. Corp, NJ, USA. Deionized water (DI water) was used in the material washing step after carbonization.
2.2. Materials Synthesis
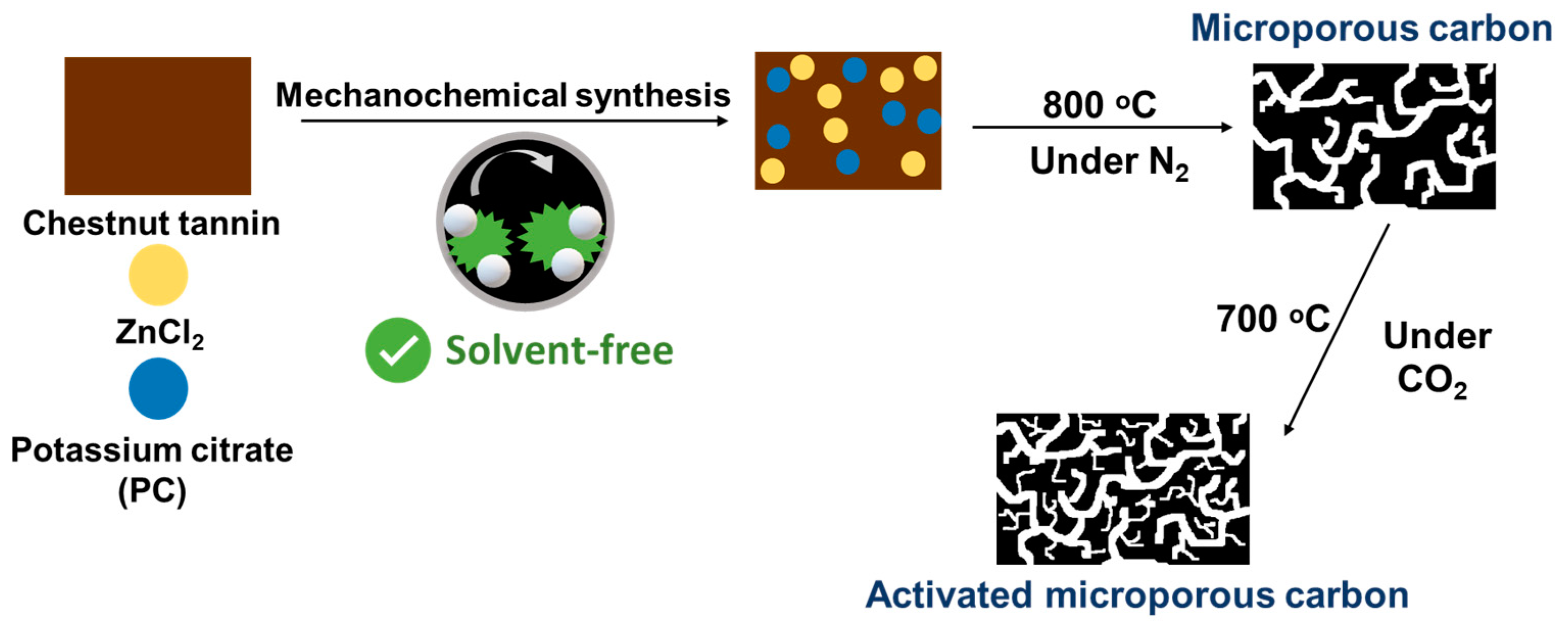
In a typical synthesis, as illustrated in Figure 1, 1.0 g of chestnut tannin (CT), 0.25 g of ZnCl2 (Zn) and 0.2 g of potassium citrate monohydrate (PC) were mechanochemically ground in a PM200 planetary ball mill (Retsch, Germany) at 500 rpm for 3 h. The fine brownish products were then carbonized in a quartz tube under N2 atmosphere at 800 °C for 1.5 h, using a heating rate of 1 °C min−1 and N2 gas flow rate of 75 mL min−1. The carbonized products were subsequently washed with hot DI water several times to remove impurities. The obtained products were labeled as CTZnPC. Finally, CO2 activation was performed on those samples at 700 °C for 3 h, with a ramp rate of 1 °C min−1 and CO2 gas flow rate of 75 mL min−1, to enhance the microporosity of the resulting carbons. The CO2-activated samples were denoted as CTZnPC-A. A control experiment was conducted by directly carbonizing pristine chestnut tannin without any activators at 800 °C and the product was designated as CT-800.
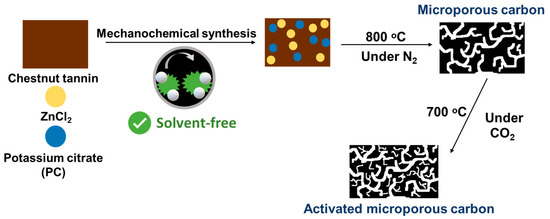
Figure 1.
Schematic illustration of the synthesis of activated microporous carbon via solvent-free mechanochemical processing of chestnut tannin in the presence of ZnCl2 (Zn) and potassium citrate (PC) activators, followed by carbonization at 800 °C under N2 atmosphere and physical CO2 activation at 700 °C.
2.3. Characterization
Surface morphology of pre- and post-activated carbon materials was investigated by scanning electron microscopy (SEM) using an FEI model Quanta 450 FEG at 20 kV. X-ray diffraction spectroscopy (XRD, Rigaku MiniFlex 600) was used to study the turbostratic carbon character of the materials in the 2θ range of 10°–90°. Chemical functionality of the prepared carbon materials was examined by Fourier-transform infrared spectroscopy (FT-IR, vector 33, Bruker) using wavenumbers between 500 and 4000 nm−1. Elemental carbon, hydrogen, and nitrogen composition analysis of the materials (CHN analysis) was performed using Elemental Analyzer TruSpec® Micro Series, Leco.
Surface area and related porosity parameters of the materials were evaluated by using N2 adsorption–desorption isotherms measured at −196 °C using an ASAP 2020 volumetric adsorption analyzer (Micromeritics, GA). CO2 adsorption measurements were performed at 25 °C using a volumetric adsorption analyzer (ASAP 2020, Micromeritics). All samples were degassed at 200 °C for 2 h prior to the N2 and CO2 adsorption, to remove trapped gases and moisture from the carbon materials. Specific surface area of all materials was obtained by using Brunauer–Emmett–Teller (BET) equation for N2 adsorption data at the relative pressure (P/Po) range of 0.05–0.2. Total pore volume (Vtotal) of the materials was evaluated using the quantity adsorbed at a relative pressure of 0.98. Pore size distribution (PSD), volume of fine micropores (pore diameter < 1 nm, Vultra) and micropore volume (pore diameter < 2 nm, Vmicro) of the materials were calculated based on the PSD functions obtained using the manufacturer-provided software, employing 2D non-local density functional theory (2D-NLDFT) calculations. Mesopore volume (Vmeso) of the materials was obtained by subtracting Vmicro from Vtotal.
3. Results and Discussions
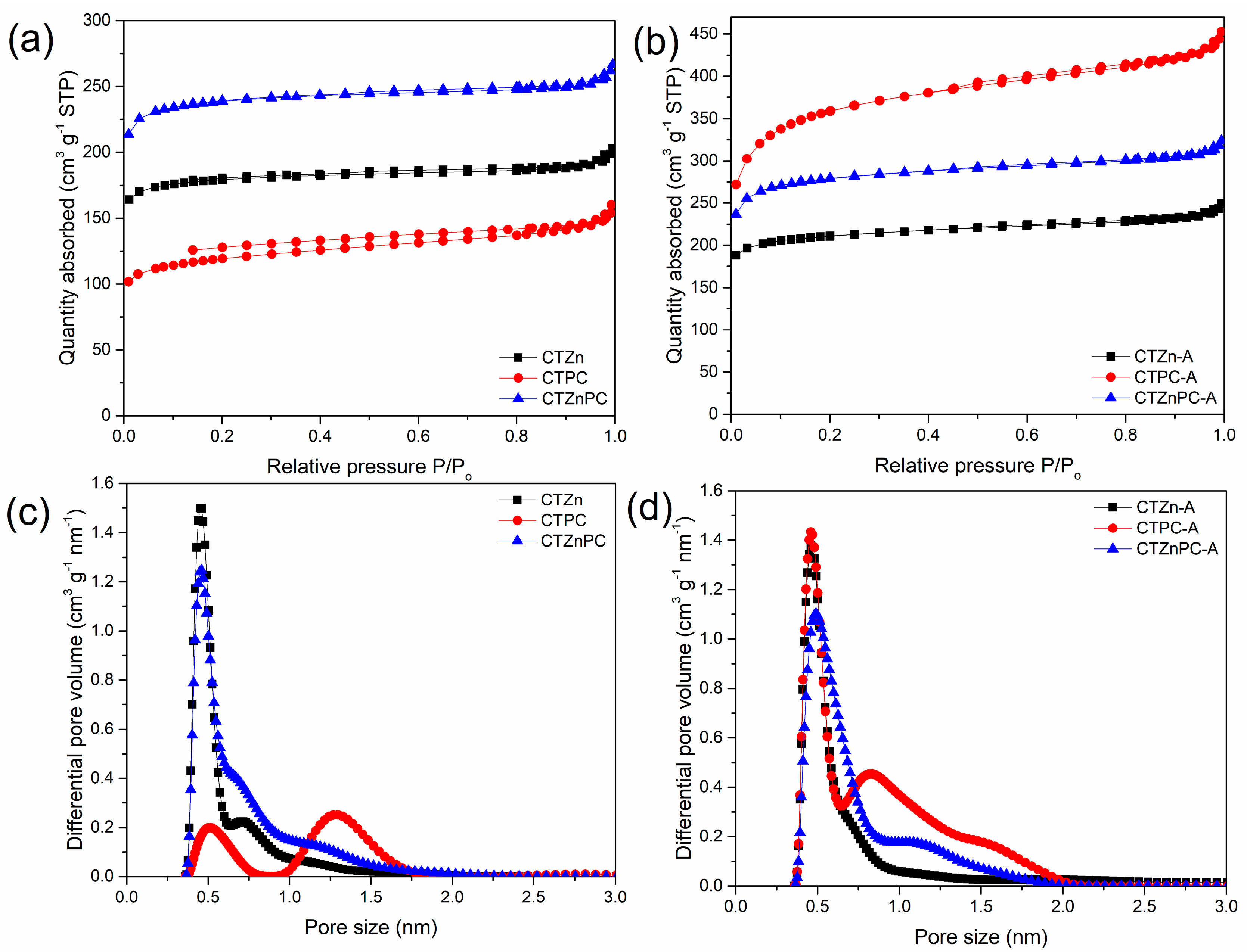
The preparation methodology of carbon materials in this study substitutes the use of hazardous chemicals and extreme conditions with greener chemicals and simplifies the process of making carbon materials, which can be easily scaled up. A series of carbon samples obtained via mechanochemical synthesis by using chestnut tannin as a carbon precursor and potassium citrate monohydrate and zinc chloride as activators are labeled as CTZnPC, where CT refers to the commercial chestnut tannin, PC refers to potassium citrate and Zn refers to zinc chloride. These samples were subjected to CO2 activation (labeled as CTZnPC-A) to increase their microporosity. Nitrogen adsorption–desorption isotherms measured at −196 °C for these samples are presented in Figure 2, with the corresponding pore size distributions obtained by using the 2D-NLDFT calculation method.
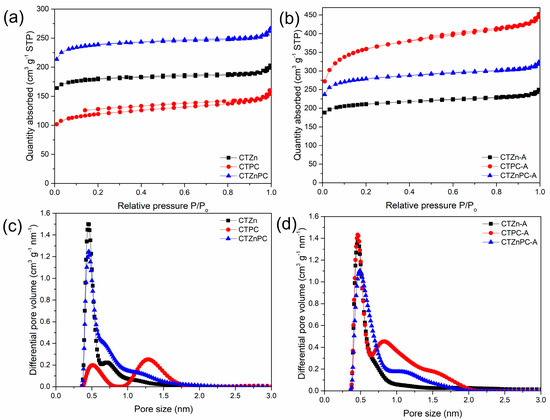
Figure 2.
(a,b) N2 adsorption−desorption isotherms for CTZnPC series of samples: (a) the materials before CO2 activation: CTZn (black line), CTPC (red line) and CTZnPC (blue line), (b) after CO2 activation: CTZn−A (black line), CTPC-A (red line) and CTZnPC-A (blue line). (c,d) Pore size distributions obtained using the manufacturer-provided 2D−NLDFT software for (c) non- CO2 activated carbon samples: CTZn (black line), CTPC (red line) and CTZnPC (blue line) and (d) CO2−activated carbon samples: CTZn−A (black line), CTPC-A (red line) and CTZnPC-A (blue line).
The N2 adsorption–desorption isotherms for the CTZnPC samples before and after CO2 activation, which are presented in Figure 2a,b, exhibit mixed type I and type IV isotherms with small hysteresis loops according to the IUPAC classification. The relatively slow increase in adsorption at higher relative pressures and narrow hysteresis loops that could be observed on the isotherms not only indicate a small amount of mesopores in the samples, but also illustrate the well-developed microporosity. The pore size distribution in the micropore range (pore diameter less than 2 nm), calculated by using 2D-NLDFT software, are shown in Figure 2c,d. Before CO2 activation (Figure 2c), CTZn and CTZnPC show apparent pore size distributions in the fine micropore range, with the highest contribution around 0.5 nm. On the other hand, CTPC, the carbon sample solely activated with PC, shows two distinct pore size ranges at around 0.5 nm and 1.25 nm. After CO2 activation, Figure 2d shows a higher contribution of small micropores. Especially in the case of CTPC-A (red line), there is an evident development of fine microporosity (pore diameter < 1 nm) after CO2 activation, which makes it more attractive for CO2 adsorption [1], while there are only slight changes in the pore size distributions obtained for CTZn and CTZnPC.
The specific surface area, total pore volume, micropore volume and volume of fine micropores for CTZnPC series are presented in Table 1. The control sample without activation, CT-800, was obtained by carbonization of tannin at 800 °C. This sample shows low specific surface area of 49 m2 g−1. Data listed in Table 1 indicate that the use of activating agents in mechanochemical synthesis boosts the surface area of the materials. Surface area improvement occurs even when small amounts of activating agents (0.2–0.25 g per 1 g of tannin) are used, due to the high sensitivity of mechanochemical processing in the presence of additives [11,12,19]. All the samples in CTZnPC series possess considerably high micropore volume (0.16–0.54 cm3 g−1), while the volume of mesopores is noticeably low (0.01–0.08 cm3 g−1). This result is in agreement with the observed shape of nitrogen adsorption isotherms and small hysteresis loops (Figure 2a,b). Moreover, the use of dual activating agents (CTZnPC) afforded materials with high surface area (816 m2 g−1) and substantial total pore volume (Vtotal = 0.37 cm3 g−1) in comparison to the tannin-derived carbon sample obtained without activators. After CO2 activation, the specific surface area, and the total pore volume Vtotal of all materials are significantly increased. In the case of CTZn-A, the surface area slightly increases from 613 m2 g−1 to 723 m2 g−1. While only a small improvement in microporosity is observed, as evidenced by pore size distributions (Figure 2c,d), the mesoporosity is notably enhanced after CO2 activation due to the possible collapse of some original micropores [37,38,39]. In contrast, the microporosity of CTPC-A obtained by using PC as a single in situ activator increases remarkably after post-synthesis CO2 activation, as evidenced by the highest specific surface area and largest micropore volume (0.54 cm3 g−1) in this study. The over three-fold surface area improvement of the CTPC samples from 415 to 1256 m2 g−1 and triple enhancement of the sample micropore volume could be the result of the structural development enabled by the combined in situ activation with PC and post-synthetic activation with CO2. At temperature < 650 °C, PC decomposes into K2CO3 [40,41], as presented in Equation (1). K2CO3 could function as a structural template [41]. At higher temperature, K2CO3 further decomposes into CO2 and K2O [40,41] (Equation (2)). The reactions between these products (CO2 and K2O) and carbon are relevant at high temperature (Equations (3) and (4)) [40,41] for generating micropores that enhance pore volume and surface area.
2K3C6H5O7·H2O → 3K2CO3 + 9C + 7H2O
K2CO3 → K2O + CO2
CO2 + C → 2CO
K2O + C → 2K + CO

Table 1.
Surface area and porosity parameters of the carbon samples calculated from N2 adsorption–desorption isotherms.
Despite adopting two in situ activators and post-synthetic CO2 activation, the surface area of CTZnPC-A only slightly increased from 816 m2 g−1 to 960 m2 g−1 with a small enhancement in microporosity (from 0.29 cm3 g−1 to 0.39 cm3 g−1). Combination of ZnCl2 and PC activators did not improve microporosity. Thus far, this study shows that PC can be used as a single in situ activator, substituting the harsh and corrosive ZnCl2 for the generation of microporosity in carbon materials.
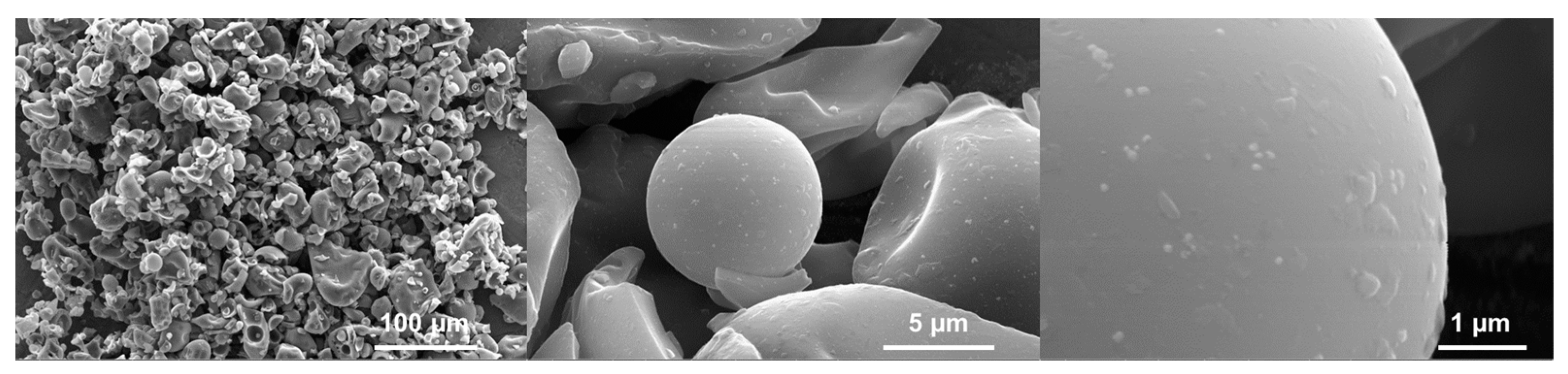
Surface morphology of carbon materials was investigated by scanning electron microscopy (SEM). Without using an activating agent, the carbonized tannin, CT-800, exhibited a smooth surface on the isolated pieces (Figure 3).

Figure 3.
SEM images obtained at 20 kV of CT-800 at different magnifications, presenting the surface morphology of the non-activated tannin-derived carbon sample.
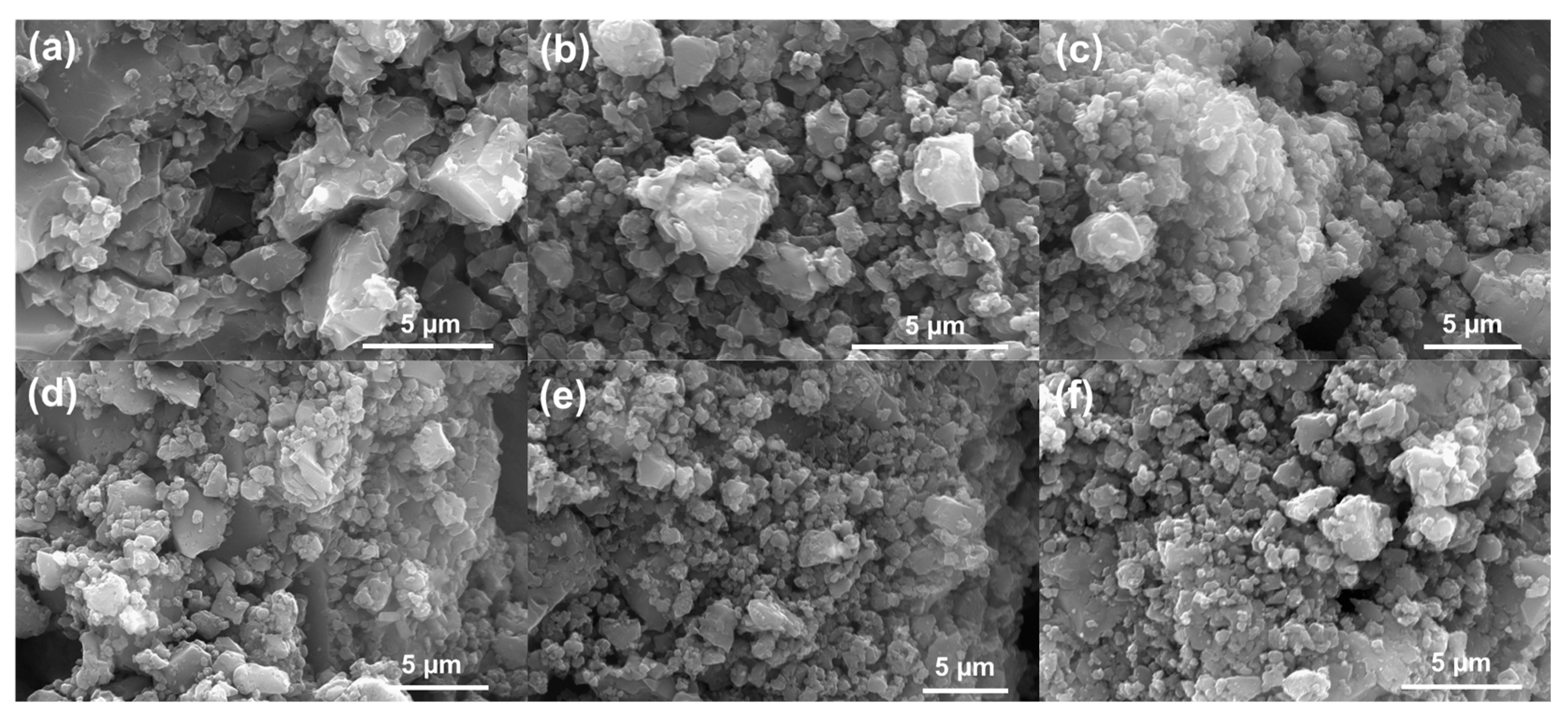
Introduction of activating agents drastically changed the surface morphology to a rough surface with numerous aggregated small particles (Figure 4). Figure 4a–c shows the surface morphology of CTZn, CTPC and CTZnPC, while Figure 4d–f presents images for the CO2-activated carbon samples (CTZn-A, CTPC-A and CTZnPC-A), which suggest that there is no significant change in the morphology before and after physical CO2 activation. This conforms with the BET analysis, showing that the activating agents significantly change structure and provide pathways for CO2 activation to improve microporosity.
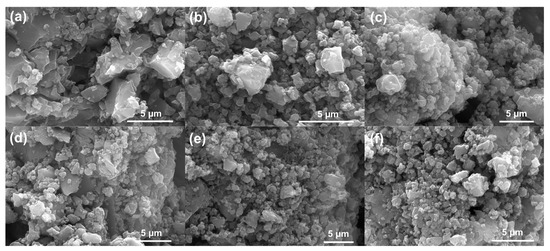
Figure 4.
SEM images obtained at 20 kV represent surface morphology of pre- and post-CO2 activation carbon materials: (a) CTZn, (b) CTPC, (c) CTZnPC, (d) CTZn-A, (e) CTPC-A and (f) CTZnPC-A.
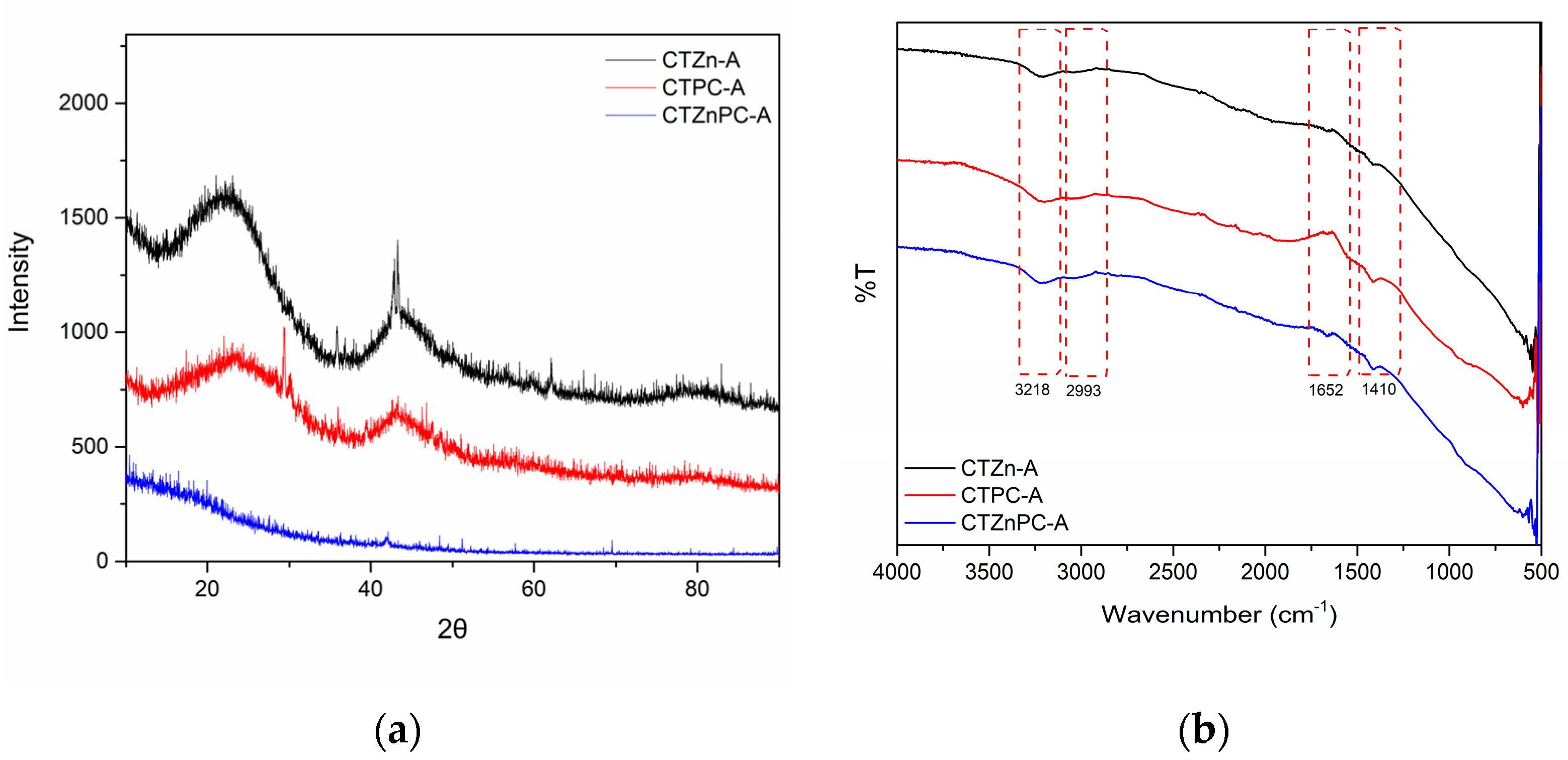
The X-ray diffraction patterns were collected between 2θ of 10°–90°, as presented in Figure 5a. Among CO2-activated samples, CTZn-A and CTPC-A diffraction patterns clearly show broad peaks at 2θ of 22° and 43°, corresponding to the (002) and (100) of graphitic-like carbon [26,42], whereas these peaks are absent in CTZnPC-A diffraction pattern. This indicates that the carbon substructures of CTZnPC-A may be destroyed during heat treatment [43].
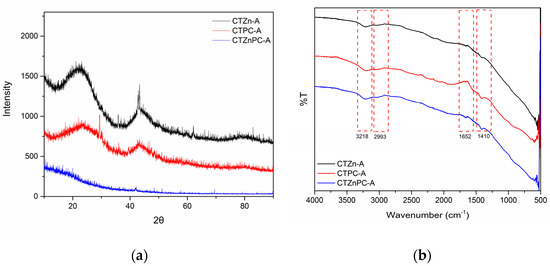
Figure 5.
(a) X-ray diffraction patterns of CO2-activated carbon materials: CTZn−A (black line), CTPC−A (red line) and CTZnPC-A (blue line) from 2θ = 10°–90° (b) FT−IR spectra of CO2−activated carbon materials: CTZn−A (black line), CTPC−A (red line) and CTZnPC−A (blue line) in the wavenumber range of 500−4000 cm−1.
Chemical functionalities of CO2-activated porous carbon materials were studied by Fourier-transform infrared spectrometry (FT-IR) in the wavenumber range of 500–4000 cm−1. The FT-IR spectra of all samples in Figure 5b show similar patterns, which contain broad peaks around 3218 cm−1 corresponding to the O-H stretching vibration [35,44] and 2993 cm−1 referring to the C-H stretching vibration [35], small signals at 1652 cm−1 belonging to the C=C stretching vibration in aromatic rings [35,44] and small peaks at 1410 cm−1 representing the C-C stretching vibration [44]. These chemical functionalities are commonly found in carbon-based materials.
Elemental composition of the materials was determined using an Elemental Analyzer to investigate the percentage of C, H and N in the prepared materials. The results in Table 2 show that many of these materials possess about 1% of nitrogen without using a nitrogen doping source. The presence of nitrogen in tannin-derived samples has been reported elsewhere [45,46,47] because of fixed protein contaminants [48] introduced during the tannin extraction process [45]. Nitrogen originating from tannin facilitates CO2 adsorption via Lewis acid–base interactions [49,50], with the nitrogen species acting as Lewis base sites and CO2 being a Lewis acid.

Table 2.
CHN elemental composition of prepared carbon materials and CO2 adsorption performance of CTZnPC series at 25 °C and 1 bar.
CO2 Capture
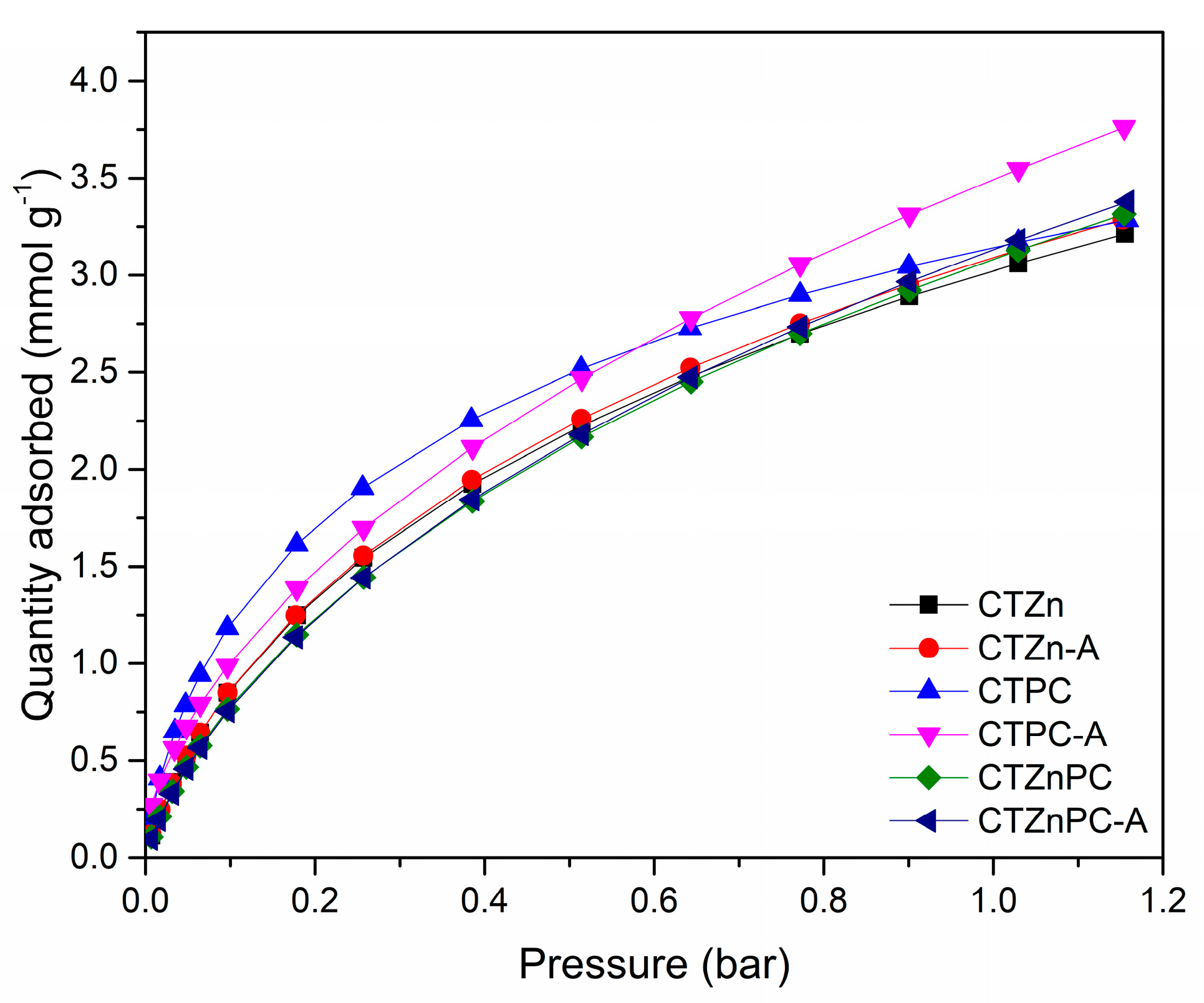
CO2 uptake performance of the materials was studied at 25 °C up to 1.15 bar using a volumetric adsorption analyzer after degassing at 200 °C for 2 h. The CO2 uptake performance at 25 °C is presented in Table 2, using the data at 1 bar to simulate the real ambient conditions.
The results in Figure 6 and Table 2 show that the CO2 capture capacities of all CTZnPC samples are in the range of 3–3.5 mmol g−1, which are comparable to the reported values for microporous carbons. The sample that shows the highest CO2 capacity is CTPC-A (3.55 mmol g−1), which also exhibits the largest micropore volume (0.54 cm3 g−1) and highest specific surface area (1256 m2 g−1). Additionally, CTPC-A shows that the CO2 uptake values are comparable to those observed for traditional KOH-activated carbon materials and the materials prepared via wet chemical synthesis routes, zeolite-based porous materials, or metal organic frameworks (Table 3); for many of these processes, the preparation is a multistep, time-consuming, and more costly process [10]. It is worth mentioning that the high fraction of fine micropores (0.36 cm3 g−1) and large contribution of micropores to the total pore volume (~90%) in CTPC-A are crucial for high CO2 adsorption capacity in comparison to other materials prepared via traditional synthesis routes. Several studies reported that the materials containing pores, which have 2–3 times larger pore diameter than the kinetic diameter of CO2 molecule (0.3 nm) are the most appreciable porous materials for CO2 capture [1]. Even though the nitrogen elemental percentage of CTZn-A (1.24%), CTPC-A (1.20%) and CTZnPC-A (1.16%) are similar, the CO2 uptake capacities of these samples are rather different, as shown in Table 2. This result suggests that the micropore volume and the specific surface area are more beneficial for enhancing CO2 adsorption capacities than the interaction between Lewis base sites. Therefore, the CTPC-A presented in this study is a good candidate for CO2 adsorption in ambient conditions. These results prove that the solvent-free mechanochemical synthesis that has been explored in this study has the potential to be a promising alternative process for the preparation of highly porous carbon materials. In addition, it could be noted that potassium citrate is an excellent green activating agent for the synthesis of highly microporous carbons when coupled with physical CO2 activation, which indicates a promising perspective for the development of carbon materials.
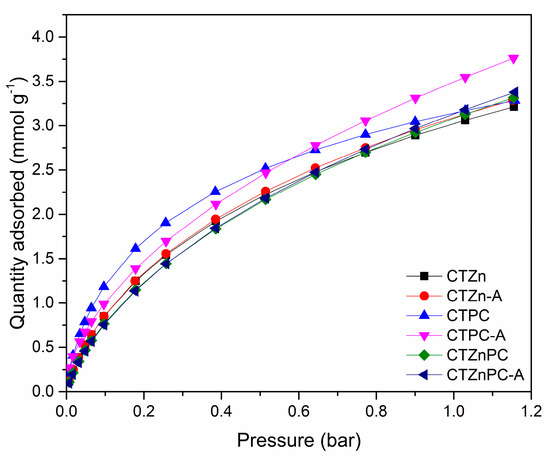
Figure 6.
CO2 adsorption isotherms measured for the CTZnPC sample series, studied at 25 °C in the pressure range of 0.01–1.15 bar.

Table 3.
CO2 adsorption capacities at the ambient conditions of previously reported materials.
In addition to the presented improvements in the synthesis of porous carbon materials, this study could be used as a guideline for the advancement of the recipes for the preparation of microporous carbons from biomass-based precursors. Also, the microporosity of the prepared carbons can be further improved by examining the effect of potassium salts, activators, carbonization temperature and grinding conditions to obtain materials with high microporosity and large specific surface area that are favorable for gas adsorption. Moreover, the combined use of a low-cost process, solvent-free mechanochemical synthesis and a low-cost carbon precursor, tannin, also shows the high potential of scaling up the production of biomass-derived porous carbons for diverse applications.
4. Conclusions
Tannin-derived porous carbon materials were successfully prepared via solvent-free mechanochemical synthesis using ZnCl2 and potassium citrate as activating agents. The SEM images clearly demonstrate the effect of the activating agents towards surface morphology of the materials by turning smooth surface rough. The carbonized materials show high specific surface area in the range 415–816 m2 g−1 after mechanochemical grinding and consequent pyrolysis at 800 °C. These carbon materials were subsequently activated using CO2 at 700 °C to enhance their microporosity and specific surface area, to enhance the CO2 uptake. The specific surface area of activated carbon samples increases up to the range of 723–1256 m2 g−1. The CO2 activation also altered microporosity of the samples. Especially, the micropore volume of the sample activated with potassium citrate shows a significant increase from 0.16 to 0.54 cm3 g−1, which is favorable for CO2 adsorption. The highest CO2 capacity, 3.55 mmol g−1 at 25 °C and 1 bar, is obtained for the mechanochemically prepared carbon from tannin using potassium citrate as the activating agent only, which is caused by the highest specific surface area and largest micropore volume. These results suggest that potassium citrate activates carbon materials better and is less corrosive and toxic than ZnCl2, which means it could be a promising substitute for ZnCl2 in the preparation of microporous carbon materials.
Author Contributions
A.S.: Conceptualization, investigation, methodology, formal analysis, writing—original draft; R.D.: investigation, methodology, formal analysis; L.C.: conceptualization, writing—review & editing; D.D.: conceptualization, writing—review & editing; M.J.: conceptualization, methodology, writing—review & editing, verification of the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the authors.
Acknowledgments
We are grateful to the Department of Chemistry and Biochemistry, College of Art and Science at Kent State University and the Faculty of Science at Prince of Songkla University for facilities and financial support. The first author would like to thank Science Achievement Scholarship of Thailand (SAST) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Z.; Cui, X.; Liu, Z.; Wan, J.; Liu, Y.; Ma, F. Hierarchical Porous Carbon Derived from Coal Tar Pitch by One Step Carbonization and Activation Combined with a CaO Template for Supercapacitors. New J. Chem. 2022, 46, 6078–6090. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Zhang, D.; Lin, Z.; Lin, J.; Li, S.; Guo, S. Construction of 3D Hierarchical Honeycomb Macro/Meso/Micro-Porous Carbon with Soft and Hard Templates for High-Performance Sodium-Ion Batteries. Mater. Lett. 2023, 334, 133737. [Google Scholar] [CrossRef]
- Duraisamy, V.; Venkateshwaran, S.; Thangamuthu, R.; Senthil Kumar, S.M. Hard Template Derived N, S Dual Heteroatom Doped Ordered Mesoporous Carbon as an Efficient Electrocatalyst for Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2022, 47, 40327–40339. [Google Scholar] [CrossRef]
- Phuriragpitikhon, J.; Ghimire, P.; Jaroniec, M. Tannin-Derived Micro-Mesoporous Carbons Prepared by One-Step Activation with Potassium Oxalate and CO2. J. Colloid Interface Sci. 2019, 558, 55–67. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Chen, Y.Q.; Wang, Z.; Wei, J. Synthesis of Mesoporous Carbon Materials from Renewable Plant Polyphenols for Environmental and Energy Applications. Xinxing Tan Cailiao/New Carbon Mater. 2022, 37, 196–222. [Google Scholar] [CrossRef]
- De Souza, L.K.C.; Wickramaratne, N.P.; Ello, A.S.; Costa, M.J.F.; Da Costa, C.E.F.; Jaroniec, M. Enhancement of CO2 Adsorption on Phenolic Resin-Based Mesoporous Carbons by KOH Activation. Carbon 2013, 65, 334–340. [Google Scholar] [CrossRef]
- Yu, Q.; Bai, J.; Huang, J.; Demir, M.; Farghaly, A.A.; Aghamohammadi, P.; Hu, X.; Wang, L. One-Pot Synthesis of Melamine Formaldehyde Resin-Derived N-Doped Porous Carbon for CO2 Capture Application. Molecules 2023, 28, 1772. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of Small Micropores in CO2 Capture by Phenolic Resin-Based Activated Carbon Spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy. Molecules 2021, 26, 1826. [Google Scholar] [CrossRef]
- Szczesniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Dong, X.L.; Lu, A.H. Mechanochemical Synthesis of Porous Carbons and Their Applications in Catalysis. Chempluschem 2020, 85, 866–875. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The Mechanochemical Synthesis of Polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef]
- Schneidermann, C.; Kensy, C.; Otto, P.; Oswald, S.; Giebeler, L.; Leistenschneider, D.; Grätz, S.; Dörfler, S.; Kaskel, S.; Borchardt, L. Nitrogen-Doped Biomass-Derived Carbon Formed by Mechanochemical Synthesis for Lithium–Sulfur Batteries. ChemSusChem 2019, 12, 310–319. [Google Scholar] [CrossRef]
- Dubadi, R.; Weidner, E.; Samojeden, B.; Jesionowski, T.; Ciesielczyk, F.; Huang, S.; Jaroniec, M. Exploring the Multifunctionality of Mechanochemically Synthesized γ-Alumina with Incorporated Selected Metal Oxide Species. Molecules 2023, 28, 2002. [Google Scholar] [CrossRef]
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical Synthesis of Nanoparticles for Potential Antimicrobial Applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef]
- Yuan, R.; Dong, Y.; Hou, R.; Shang, L.; Zhang, J.; Zhang, S.; Chen, X.; Song, H. Structural Transformation of Porous and Disordered Carbon during Ball-Milling. Chem. Eng. J. 2023, 454, 140418. [Google Scholar] [CrossRef]
- Mohamed, M.A.A.; Saadallah, H.A.A.; Gonzalez-Martinez, I.G.; Hantusch, M.; Valldor, M.; Büchner, B.; Hampel, S.; Gräßler, N. Mechanochemical Synthesis of Li-Rich (Li2Fe)SO Cathode for Li-Ion Batteries. Green Chem. 2023. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Zhang, P.; Yang, S.; Zhan, W.; Dai, S. Mechanochemical Synthesis of Ruthenium Cluster@Ordered Mesoporous Carbon Catalysts by Synergetic Dual Templates. Chem. A Eur. J. 2019, 25, 8494–8498. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V. “Green”, Innovative, Versatile and Efficient Carbon Materials from Polyphenolic Plant Extracts. Carbon 2020, 167, 792–815. [Google Scholar] [CrossRef]
- Casco, M.E.; Kirchhoff, S.; Leistenschneider, D.; Rauche, M.; Brunner, E.; Borchardt, L. Mechanochemical Synthesis of N-Doped Porous Carbon at Room Temperature. Nanoscale 2019, 11, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Tonnoir, H.; Huo, D.; Canevesi, R.L.S.; Fierro, V.; Celzard, A.; Janot, R. Tannin-Based Hard Carbons as High-Performance Anode Materials for Sodium-Ion Batteries. Mater. Today Chem. 2022, 23, 100614. [Google Scholar] [CrossRef]
- Phuriragpitikhon, J.; Phinney, E.O.; Jaroniec, M. Potassium Citrate-Assisted Eco-Friendly Synthesis of Tannin-Derived Nitrogen-Doped Micro–Mesoporous Carbon Microspheres. J. Mater. Sci. 2020, 55, 13716–13736. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pinto, O.; Izquierdo, M.T.; Segura, C.; Poon, P.S.; Celzard, A.; Matos, J.; Fierro, V. Upgrading of Pine Tannin Biochars as Electrochemical Capacitor Electrodes. J. Colloid Interface Sci. 2021, 601, 863–876. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V.; Pizzi, A.; Zhao, W. Multifunctional Porous Solids Derived from Tannins. J. Phys. Conf. Ser. 2013, 416, 012023. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, W.; Zhang, P.; Dai, S. Solvent-Free and Mechanochemical Synthesis of N-Doped Mesoporous Carbon from Tannin and Related Gas Sorption Property. Chem. Eng. J. 2020, 381, 122579. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H.; Wu, W.; Lin, D.; Yang, K. Role of Molecular Size of Volatile Organic Compounds on Their Adsorption by KOH-Activated Micro-Mesoporous Carbon. J. Hazard. Mater. 2022, 424, 127355. [Google Scholar] [CrossRef]
- Koyuncu, F.; Güzel, F.; İnal, İ.I.G. High Surface Area and Supermicroporous Activated Carbon from Capsicum (Capsicum annuum L.) Industrial Processing Pulp via Single-Step KOH-Catalyzed Pyrolysis: Production Optimization, Characterization and Its Some Water Pollutants Removal and Supercapacitor Performance. Diam. Relat. Mater. 2022, 124, 108920. [Google Scholar] [CrossRef]
- Wang, S.; Lee, Y.R.; Won, Y.; Kim, H.; Jeong, S.E.; Wook Hwang, B.; Ra Cho, A.; Kim, J.Y.; Cheol Park, Y.; Nam, H.; et al. Development of High-Performance Adsorbent Using KOH-Impregnated Rice Husk-Based Activated Carbon for Indoor CO2 Adsorption. Chem. Eng. J. 2022, 437, 135378. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption Modeling, Thermodynamics, and DFT Simulation of Tetracycline onto Mesoporous and High-Surface-Area NaOH-Activated Macroalgae Carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, Y.; Wang, H.; Song, H. Preparation of Active Carbon through One-Step NaOH Activation of Coconut Shell Biomass for Phenolic Wastewater Treatment. Res. Chem. Intermed. 2022, 48, 1665–1684. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, H.; Jiang, Y.; Li, H.; Tang, P.; Li, D.; Feng, Y. Porous ZnCl2-Activated Carbon from Shaddock Peel: Methylene Blue Adsorption Behavior. Materials 2022, 15, 895. [Google Scholar] [CrossRef]
- Pełech, I.; Staciwa, P.; Sibera, D.; Kusiak-Nejman, E.; Morawski, A.W.; Kapica-Kozar, J.; Narkiewicz, U. The Effect of the Modification of Carbon Spheres with ZnCl2 on the Adsorption Properties towards CO2. Molecules 2022, 27, 1387. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Salamanca, M.; Diaz-Corrales, J.D.; Flórez, E.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the Removal of an Anionic Dye in Textile Wastewaters by Adsorption on ZnCl2activated Carbons from Rice and Coffee Husk Wastes: A Combined Experimental and Theoretical Study. J. Environ. Chem. Eng. 2021, 9, 105685. [Google Scholar] [CrossRef]
- Yağmur, H.K.; Kaya, İ. Synthesis and Characterization of Magnetic ZnCl2-Activated Carbon Produced from Coconut Shell for the Adsorption of Methylene Blue. J. Mol. Struct. 2021, 1232, 130071. [Google Scholar] [CrossRef]
- Li, B.; Hu, J.; Xiong, H.; Xiao, Y. Application and Properties of Microporous Carbons Activated by ZnCl2: Adsorption Behavior and Activation Mechanism. ACS Omega 2020, 5, 9398–9407. [Google Scholar] [CrossRef]
- Lan, X.; Jiang, X.; Song, Y.; Jing, X.; Xing, X. The Effect of Activation Temperature on Structure and Properties of Blue Coke-Based Activated Carbon by CO2 Activation. Green Process. Synth. 2019, 8, 837–845. [Google Scholar] [CrossRef]
- Baek, J.; Lee, H.M.; Roh, J.S.; Lee, H.S.; Kang, H.S.; Kim, B.J. Studies on Preparation and Applications of Polymeric Precursor-Based Activated Hard Carbons: I. Activation Mechanism and Microstructure Analyses. Microporous Mesoporous Mater. 2016, 219, 258–264. [Google Scholar] [CrossRef]
- Guo, S.; Peng, J.; Li, W.; Yang, K.; Zhang, L.; Zhang, S.; Xia, H. Effects of CO 2 Activation on Porous Structures of Coconut Shell-Based Activated Carbons. Appl. Surf. Sci. 2009, 255, 8443–8449. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Zhang, C.; Yang, W.; Han, J.; Jiang, S.; He, S. Potassium Citrate Assisted Synthesis of Hierarchical Porous Carbon Materials for High Performance Supercapacitors. Diam. Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- Yun, S.; Zhang, Z.; Peng, Z.; Yang, C.; Liu, R.; Liao, Y.; Chen, H.-C. Two-Dimensional Hierarchical Porous Carbons with Mesopore-Enriched Architectures for High-Reactivity and Stable Lithium-Ion Full Batteries Energy Materials. J. Mater. Sci. 2022, 57, 5142–5153. [Google Scholar] [CrossRef]
- Lázaro, N.; Castro-Gutiérrez, J.; Ramírez-Vidal, P.; Celzard, A.; Fierro, V.; Algarni, T.S.; Pineda, A.; Luque, R. Mechanochemical Functionalization of Mesoporous Carbons for the Catalytic Transformation of Trans-Ferulic Acid into Vanillin. ACS Sustain. Chem. Eng. 2021, 9, 4704–4710. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Z.; Song, H.; Wang, C.; Subhan, F.; Xing, W.; Yan, Z. The Fabrication of Porous N-Doped Carbon from Widely Available Urea Formaldehyde Resin for Carbon Dioxide Adsorption. J. Colloid Interface Sci. 2014, 416, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Choi, H.; Youk, J.H. Mechanochemical Activation of Zero-Valent Iron on Carbonized Boron-Doped Graphene Dots for Enhanced Sonochemical Dyes Removal. Colloids Interface Sci. Commun. 2021, 45, 100548. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Medjahdi, G.; Ghanbaja, J.; Celzard, A.; Fierro, V. Ordered Mesoporous Carbons Obtained by Soft-Templating of Tannin in Mild Conditions. Microporous Mesoporous Mater. 2018, 270, 127–139. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Fuente, E. Influence of the Pyrolysis Step and the Tanning Process on KOH-Activated Carbons from Biocollagenic Wastes. Prospects as Adsorbent for CO2 Capture. J. Anal. Appl. Pyrolysis 2014, 110, 194–204. [Google Scholar] [CrossRef]
- Blyweert, P.; Nicolas, V.; Macutkevic, J.; Fierro, V.; Celzard, A. Tannin-Based Resins for 3D Printing of Porous Carbon Architectures. ACS Sustain. Chem. Eng. 2022, 10, 7702–7711. [Google Scholar] [CrossRef]
- Cesprini, E.; De Iseppi, A.; Giovando, S.; Tarabra, E.; Zanetti, M.; Šket, P.; Marangon, M.; Tondi, G. Chemical Characterization of Cherry (Prunus Avium) Extract in Comparison with Commercial Mimosa and Chestnut Tannins. Wood Sci. Technol. 2022, 56, 1455–1473. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.; Wulan, B.; Zhang, J. Surface Modification of SnO2 Nanosheets via Ultrathin N-Doped Carbon Layers for Improving CO2 Electrocatalytic Reduction. Chem. Eng. J. 2021, 421, 130003. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Rehman, A.; Munir, M.A.M.; Irshad, S.; Rashid, M.S.; Cheema, A.I. Understanding the Synergy between N-Doped Ultra-Microporous Carbonaceous Adsorbent and Nitrogen Functionalities for High Performance of CO2 sorption. J. Environ. Chem. Eng. 2021, 9, 104646. [Google Scholar] [CrossRef]
- Cai, C.; Fu, N.; Zhou, Z.; Wu, M.; Yang, Z.; Liu, R. Mechanochemical Synthesis of Tannic Acid-Fe Coordination Compound and Its Derived Porous Carbon for CO2 Adsorption. Energy Fuels 2018, 32, 10779–10785. [Google Scholar] [CrossRef]
- Ma, C.; Lu, T.; Shao, J.; Huang, J.; Hu, X.; Wang, L. Biomass Derived Nitrogen and Sulfur Co-Doped Porous Carbons for Efficient CO2 Adsorption. Sep. Purif. Technol. 2022, 281, 119899. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, P.; Barman, S. Synthesis of Sulfur-Doped Porous Carbon for Supercapacitor and Gas Adsorption Applications. Int. J. Energy Res. 2022, 46, 2585–2600. [Google Scholar] [CrossRef]
- Dassanayake, A.C.; Jaroniec, M. Dual Optimization of Microporosity in Carbon Spheres for CO2 Adsorption by Using Pyrrole as the Carbon Precursor and Potassium Salt as the Activator. J. Mater. Chem. A Mater. 2017, 5, 19456–19466. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liu, G.; Yan, Q. In-Situ Pyrolysis of Taihu Blue Algae Biomass as Appealing Porous Carbon Adsorbent for CO2 Capture: Role of the Intrinsic N. Sci. Total Environ. 2021, 771, 145424. [Google Scholar] [CrossRef]
- An, L.; Liu, S.; Wang, L.; Wu, J.; Wu, Z.; Ma, C.; Yu, Q.; Hu, X. Novel Nitrogen-Doped Porous Carbons Derived from Graphene for Effective CO2 Capture. Ind. Eng. Chem. Res. 2019, 58, 3349–3358. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Albazzaz, S.; Ali, U.; Mortari, D.A.; Rashid, T. Development of Biomass Derived Highly Porous Fast Adsorbents for Post-Combustion CO2 Capture. Fuel 2020, 282, 118506. [Google Scholar] [CrossRef]
- Panda, D.; Kumar, E.A.; Singh, S.K. Amine Modification of Binder-Containing Zeolite 4A Bodies for Post-Combustion CO2 Capture. Ind. Eng. Chem. Res. 2019, 58, 5301–5313. [Google Scholar] [CrossRef]
- Sun, L.; Yin, M.; Li, Z.; Tang, S. Facile Microwave-Assisted Solvothermal Synthesis of Rod-like Aluminum Terephthalate [MIL-53(Al)] for CO2 Adsorption. J. Ind. Eng. Chem. 2022, 112, 279–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).