Abstract
Since active pharmaceutical ingredients (APIs) are directly related to human health, monitoring and quantifying them in the environment is a crucial and challenging issue. Using capillary-zone electrophoresis (CZE), four frequently used fluoroquinolones (FQs), ciprofloxacin, sparfloxacin, moxifloxacin, and gatifloxacin, were efficiently isolated and measured in pharmaceutical industrial wastewater. Solid-phase extraction (SPE) was developed and used as an efficient sample pretreatment procedure. The capillary electrophoretic procedure’s various parameters were tuned to produce the optimal separation pattern for the drugs under consideration. All of the drugs under study were quantified in a concentration range of 0.5 to 50 µg/mL. After full assay validation in compliance with ICH-Q2B criteria, real wastewater samples were subjected to effective SPE, and the proposed assay was successfully used to determine the examined FQs in real wastewater samples.
1. Introduction
Fluoroquinolones (FQs) are substances used in both human and veterinary medicine to treat a wide range of bacterial illnesses. They are efficient against anaerobic, Gram-positive, Gram-negative, and mycobacterium bacteria. Two bacterial enzymes, DNA gyrase and topoisomerase IV, are inhibited by FQs, which results in their bactericidal activity. The World Health Organization (WHO) has declared that this class of antibiotics is the first-line treatment for complicated urinary tract infections and bacterial diarrhea and the second-line therapeutic intervention for tuberculosis in patients who have developed resistance to the first-line anti-tuberculosis medication. They can be applied for the effective handling of osteomyelitis, several types of wound infections, and respiratory infections. They are also used to treat many sexually transmitted diseases [1,2].
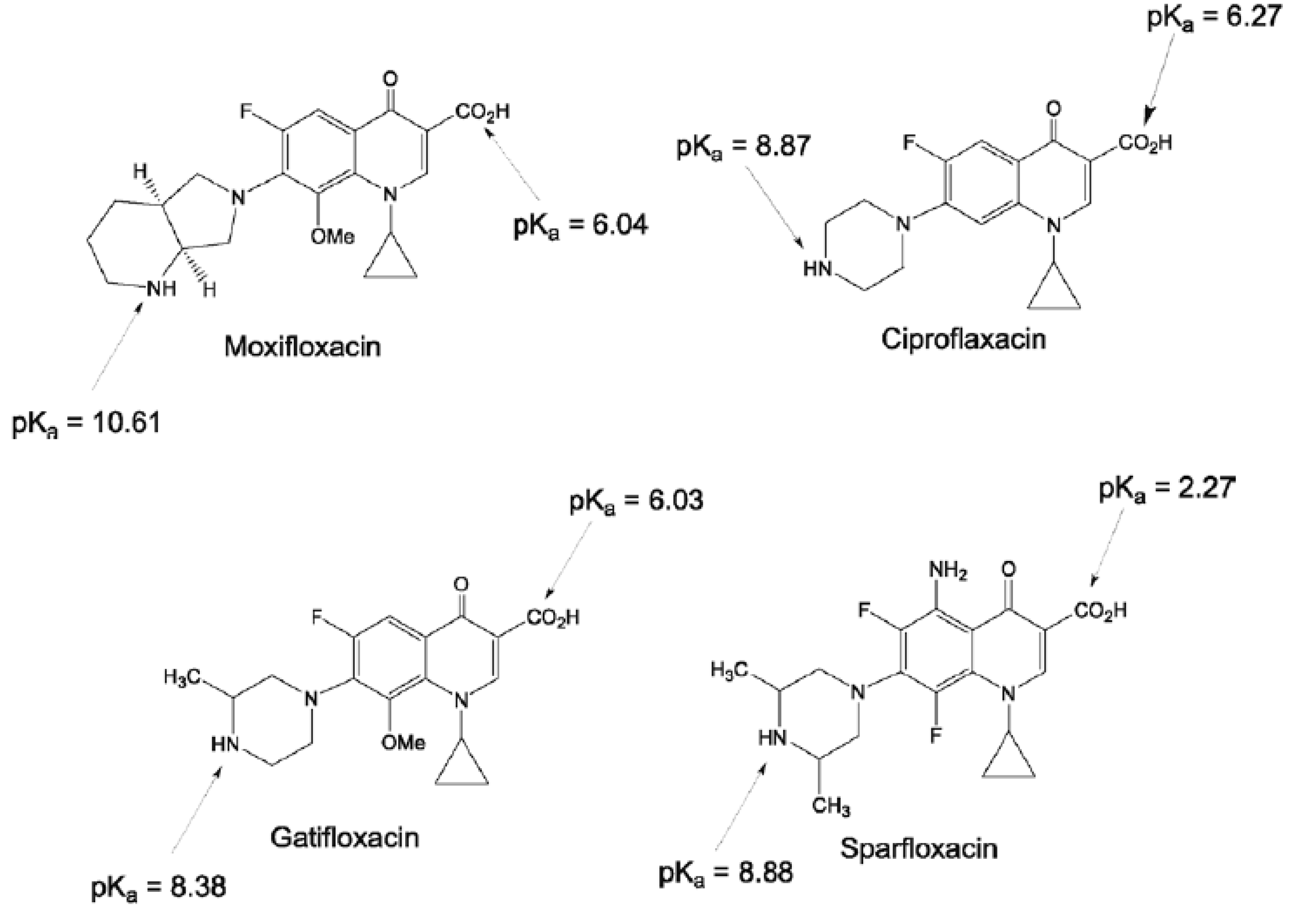
Ciprofloxacin (CPR), sparfloxacin (SPR), moxifloxacin (MOX), and gatifloxacin (GTF) have been chosen as FQs’ representatives. Figure 1 displays the chemical formulae and pKa values of the selected members [3].
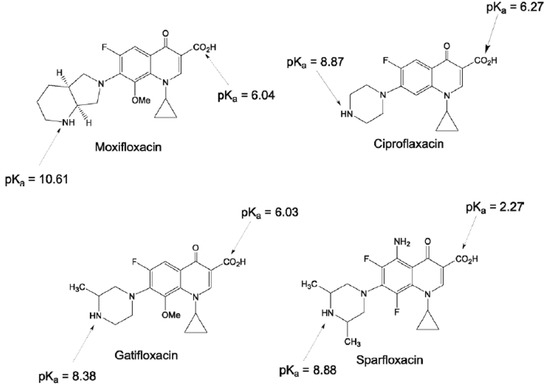
Figure 1.
Chemical structures and pKa values of the selected fluoroquinolones [3].
The extensive usage of FQs in recent years has raised concerns for people since they have a detrimental impact on them. Antibiotic resistance, which is brought on by extended exposure to antibiotic residues, is one of the most significant global public health challenges. Resistant and multi-resistant bacterial strains quickly proliferated after antibiotics started to be widely used. This is a very unfavorable event since diseases brought on by resistant bacteria are very difficult, if not impossible, to treat [4,5]. From this point of view, it is essential to find and keep an eye on the tested drugs in the environment, especially in wastewater effluents.
The quantification of the investigated pharmaceuticals in various sample forms was carried out using a variety of analytical techniques, such as spectrofluorimetry [6,7], high-performance liquid chromatography (HPLC) [8,9,10,11], and liquid chromatography coupled to a mass spectrometric detector (LC-MS/MS) [12,13].
Numerous methods for determining FQs in aquatic habitats have been developed, including HPLC [14,15,16], LC-MS/MS [17,18], and electrochemistry [19]. On the other hand, capillary electrophoresis (CE) has been applied to quantify FQs in different sample forms, including, bovine milk [20], human urine [21], animal tissues [22], pharmaceutical dosage forms [23], and water environments [24,25,26,27,28].
CE can be considered an important analytical tool for the analysis of FQs in the last few years due to its high separation efficiency, low sample and reagent consumption, rapid speed of analysis, and application to a wider selection of samples [29]. CE is preferred to HPLC in FQ analysis due to the presence of a permanent charge on FQs at all pH values, which constitutes a difficulty during their HPLC separation. Unlike in HPLC, the ionized state is an advantageous property in capillary-zone electrophoresis (CZE), in which separation is based on the differences between the electrophoretic mobilities of the analytes [30].
Despite having many benefits, it has drawbacks just like other methods. The relatively high limit of quantification (LOQ) is a well-known drawback. It occurs as a result of a very small sample volume being fed into the capillary and an optical pathway that is only slightly longer than the capillary’s inner diameter [31].
There is no doubt that the exposure of the local environment to industrial effluents containing FQs constitutes a serious danger to the survival of all living things. This claim suggests that it is vital to keep an eye on and measure the researched FQs in pharmaceutical industrial effluents. The objective of this work was to adopt a trustworthy analytical technique that can accurately, sensitively, and selectively detect the examined pharmaceuticals in industrial wastewater.
2. Materials and Methods
2.1. Chemicals, Reagents, and Standard Solutions
All of the chemicals were analytical grade, and the solvents were HPLC quality. The following chemicals were provided by Merck (Darmstadt, Germany): acetonitrile (ACN), methanol (MeOH), sodium hydroxide (NaOH), and boric acid (H3BO3). A 40 mM solution of boric acid adjusted to pH 8.5 using NaOH was used as a background electrolyte (BGE). All of the work was performed with ultrapure water (Milli-Q Plus system, Millipore Bedford, MA, USA).
The examined FQs were provided by Bio Vision (Boston, MA, USA). A certificate stated that the purity of CPR, SPR, MOX, and GTF, respectively, was 99.98%, 99.45%, 99.21%, and 99.84%. The relevant quantity of each drug was dissolved in an ACN:H2O (1:1, v/v) mixture to create a stock standard solution (100 μg/mL) of each FQ, which was then kept in the dark at 4 °C. By diluting the stock solutions with ACN:H2O (1:1, v/v), working standard solutions were freshly prepared.
The samples were processed using Micrón’s Strata-X polymeric reversed-phase extraction cartridges (200 mg, 6 mL; Phenomenex) (Madrid, Spain). The final extract and background electrolyte (BGE) were filtered using Acrodisc nylon membrane syringe filters (0.2 µm, 13 mm; Pall Corp., Washington, NY, USA). The wastewater samples were filtered using a Millipore solvent filter system and nylon membranes (0.2 µm, 47 mm; Supelco, Bellefonte, PA, USA).
2.2. Instrumentation
The experiments were conducted using an Agilent 7100 CE apparatus from Waldbronn, Germany, which included an ultraviolet–visible (UV-Vis) detector and an automatic injector. For separation, a fused-silica capillary with a 75 µm inner diameter and a 64.5 cm length was used (Polymicro Technologies, Phoenix, AZ, USA). Peak areas, migration periods, and other information were measured with the use of the Agilent Chem-Station software. The pH of the liquids was adjusted using a pH-meter from Mettler Toledo (Greifensee, Switzerland).
2.3. Real Samples’ Collection
Real wastewater samples were obtained from a pharmaceutical plant that produces the investigated FQs in tablet form. To avoid any sample deterioration, the samples were stored in opaque glass vials in a cold environment.
2.4. Capillary Preconditioning
In the case of new capillaries, the preconditioning approach involved flushing for 20 min with a 1 M NaOH solution, for 20 min with a 0.1 M NaOH solution, for 2 min with deionized water, and then for 30 min with a BGE solution. The next day, the capillary was flushed with a 0.1 M NaOH solution for 20 min, a 1 M NaOH solution for 5 min, water for 2 min, and BGE for 30 min. The capillary ends were left in the water overnight after being cleaned in water for 20 min each day.
2.5. Electrophoretic Conditions
In order to perform optimal separation, an applied voltage of 16 kV at room temperature was used to separate the studied drugs. The sample solution was hydrodynamically injected for 10 s at 60 mbar. The UV-Vis detection of the studied drugs was performed at 288 nm.
2.6. Method Validation
ICH-Q2B guidelines were applied for assay validation [32].
2.6.1. Linearity
Accurately and independently, various aliquots of the investigated FQs (5–500 µg) were transferred to a series of volumetric flasks (10 mL capacity). To achieve a concentration of 0.5–50 µg/mL for each investigated medication, the volume of each flask was subsequently completed with ACN:BGE (1:3). An elution liquid composed of ACN:H2O:MeOH (2:1:1 by volume) was used for the electrophoretic analysis of the samples.
2.6.2. Accuracy
The percentage of an analyte recovered from a specific quantity can be used to define accuracy [32]. The process under the condition of linearity was used to examine the results from nine samples with concentrations of 5, 10, and 30 µg/mL for each drug under study.
2.6.3. Precision
Precision is illustrated as inter- and intra-day precision, represented as % relative standard deviation for a number of statistically relevant experiments. Each drug’s three concentrations (5, 10, and 30 µg/mL) were tested three times either on the same day (intra-day) or on three successive days (inter-day).
2.6.4. LOD and LOQ
LOD and LOQ are the minimum analyte concentrations having a signal-to-noise (S/N) ratio of 3 and 10, respectively [32].
2.6.5. Robustness
The impact of small alterations on the suggested technique can be used to gauge robustness. This was accomplished by changing the amount of acetonitrile (±1%) in the elution liquid. Additionally, a ±1 kV change in the applied voltage was made.
2.7. Applications
2.7.1. Real Wastewater Sample Pretreatment
The pH of 250 mL samples was adjusted to 6. After the samples had been shaken for 1 min and had been in the dark for at least 30 min, they were filtered to remove suspended particles. After conditioning with 2 mL of MeOH and 2 mL of water at a flow rate of 0.5 mL/min, the samples were run through a Strata-X cartridge (33 m polymeric reversed phase, 200 mg, 6 mL), which was subsequently dried. The analytes were then eluted with ACN:H2O:MeOH (2:1:1 by volume). The extract was dried at 35 °C under a gentle stream of N2. The extract was vortexed for about a minute before being reconstituted in 0.5 mL of ACN:BGE (1:3) and filtered.
2.7.2. Determination of FQs in Spiked Water Samples
In order to obtain the recovered concentrations of 5, 10, and 30 µg/mL of the investigated FQs, samples of distilled and tap water were spiked with the mentioned concentrations of the researched medications. The extracted samples from the spiked ones were then analyzed using the optimized electrophoretic method.
2.7.3. Determination of FQs in Real Wastewater Samples
After going through the optimized sample preparation process, five real wastewater samples were tested under the optimal CE conditions. To determine the unknown concentrations, calibration graphs were utilized. The acquired results were compared with those obtained using reference methods [8,9,10,11] for the determination of CPR, SPR, MOX, and GTF following sample pretreatment using the same extraction procedure.
3. Results
3.1. Optimization of the SPE Procedure
An SPE procedure was used for sample pretreatment to reach appropriate quantification levels for the determination of the studied FQs in the industrial wastewater samples. The cartridge was preconditioned with 4 mL of MeOH:H2O (1:1, v/v) at a flow rate of 0.5 mL/min.
In order to elute the studied drugs from the sorbent, four different eluting mixtures were tested, namely, (a) 4 mL of MeOH, (b) 4 mL of MeOH:H2O (3:1, v/v), (c) 4 mL of ACN:H2O:MeOH (2:1:1 by volume), and (d) 4 mL of H2O:MeOH (3:1, v/v). The (C) elution mixture gave the highest recoveries (Table 1).

Table 1.
Recoveries of the studied FQs from the used sorbent using different elution mixtures.
Given that the chosen sorbents are reversed-phase materials and that the chosen drugs are amphoteric chemicals that can take on cationic, anionic, or zwitterionic forms, the sample’s pH range was tested between 4 and 7, with the pH range between 6 and 7 producing the highest recoveries. Since pH 6 was closest to the native samples’ pH, it was chosen as the pH value (Table 2).

Table 2.
The effect of sample pH on the recovery of the studied FQs during the SPE process.
The resultant eluate was dried under a moderate N2 stream after the best SPE method had been used. In order to obtain the best reconstitution liquid, different solvents, including ACN, BGE, ACN:H2O (1:1, v/v), and ACN:BGE (1:3, v/v), were examined. In each experiment, 0.5 mL of the reconstitution liquid was utilized, and it was vortexed for 1 min. The combinations ACN:BGE (1:3, v/v) and ACN:H2O (1:1, v/v) produced the highest recoveries; however, because of the ACN:H2O (1:1, v/v) mixture’s lower conductivity, which eventually led to a current decrease, ACN:BGE (1:3, v/v) was chosen as the ideal reconstitution solvent (Table 3).

Table 3.
Recoveries of the studied FQs using different reconstitution solvents.
3.2. Optimization of CZE
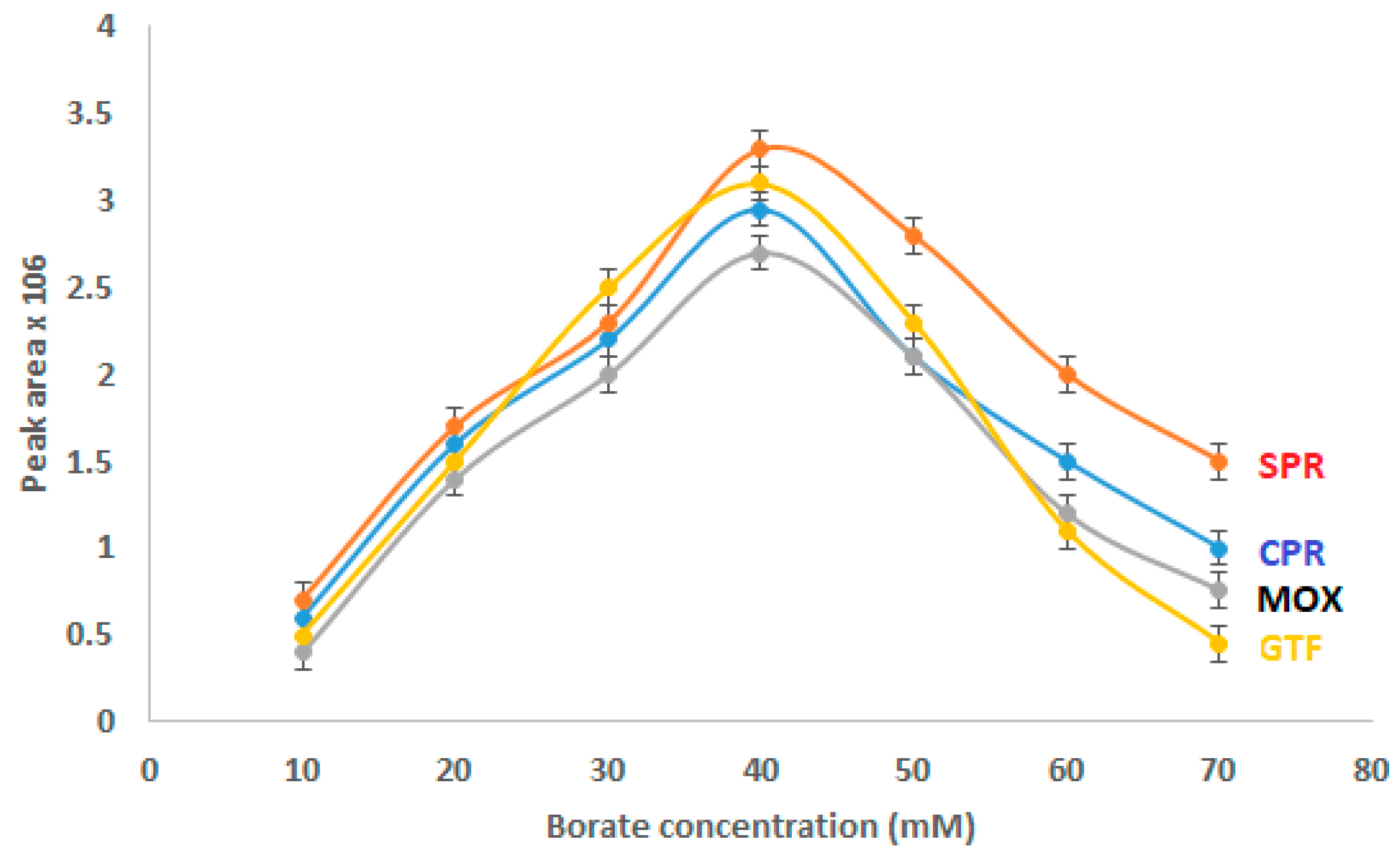
Due to its potential to affect electro-osmotic flow (EOF), Joule heating, ionic strength, and the current generated in the capillary, the electrolyte concentration has a considerable impact on the separation quality. Therefore, the peak area and migration time will be influenced by the electrolyte concentration. The effect of varying borate concentrations (10 mM to 70 mM) in the BGE was investigated (Figure 2). The examined FQs’ concentration was set at 15 µg/mL, and the pH was adjusted to 8.5. With the increase in borate concentration from 10 mM to 40 mM, the peak area of the analytes gradually increased. When the borate concentration was greater than 40 mM, the migration time was prolonged, the peak area was reduced, and the analysis time rose noticeably. So, 40 mM borate was the ideal concentration.
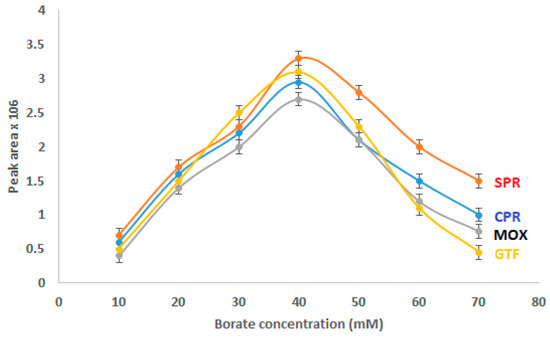
Figure 2.
Effect of borate concentration on CZE performance.
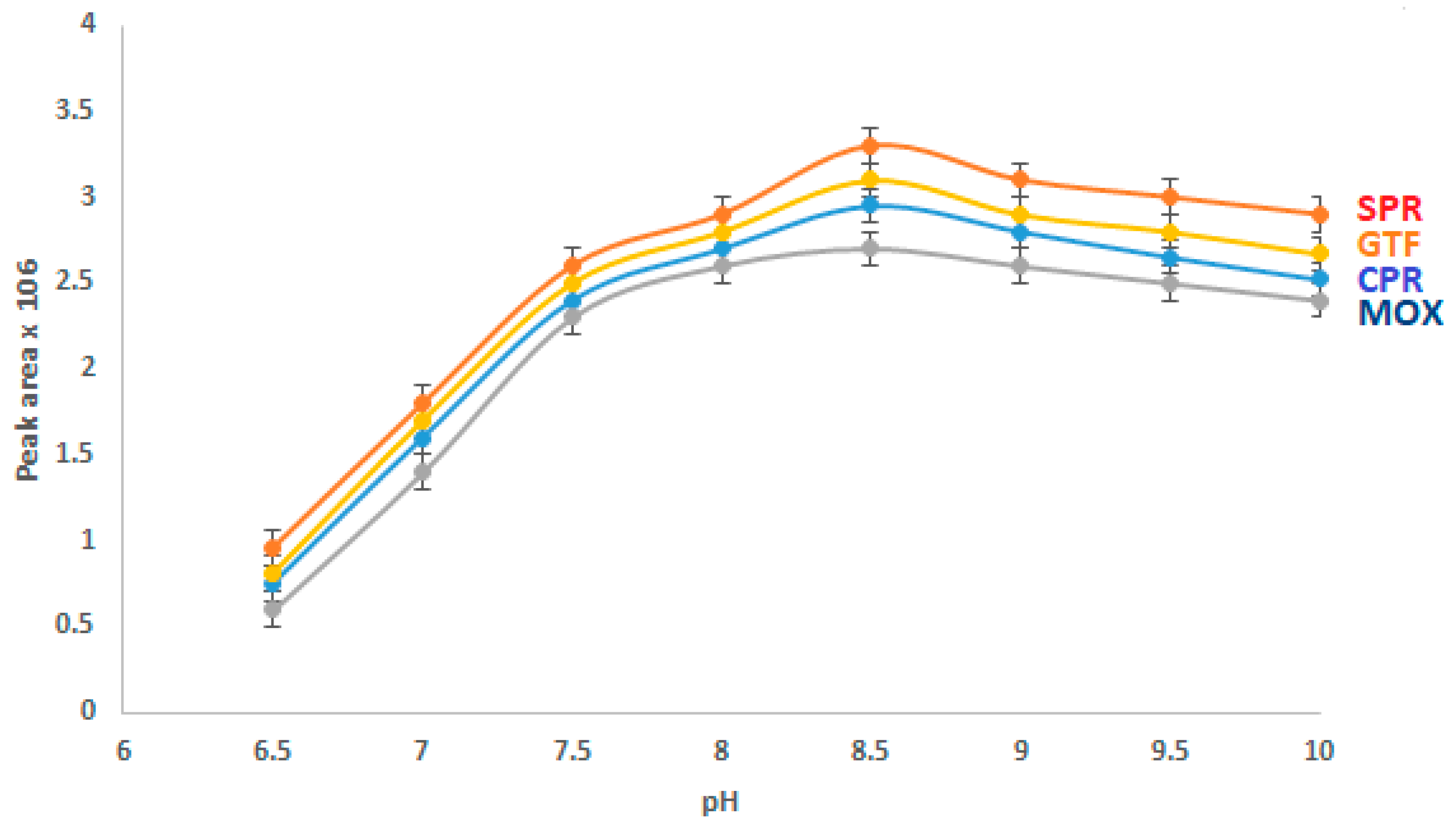
The buffer pH strongly affects the CE analysis of the studied drugs, as it influences the electro-osmotic flow (EOF). The effect of buffer pH on the separation of the analytes was investigated (Figure 3). Borate was present in a concentration of 40 mM, and the analytes were fixed at a concentration of 15 µg/mL. The outcome showed that as the pH of the BGE rose from 6.5 to 8.5, the peak area increased. When the pH was more than 8.5, the migration time was increased and the peak area remained essentially constant. Thus, a BGE pH of 8.5 was selected for subsequent studies.
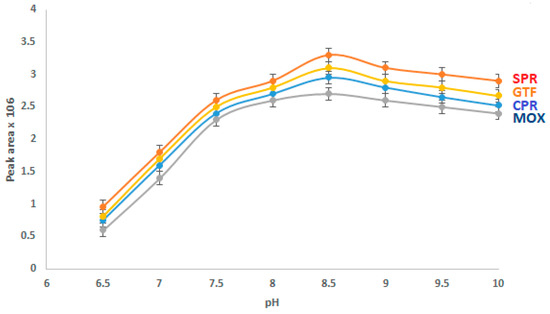
Figure 3.
Effect of the pH value on the CZE performance.
The applied voltage plays an important role in achieving the optimal separation of the investigated drugs. In order to achieve the best balance between the resolution, peak area, and migration time, a voltage of 16 kV was used during the experiment.
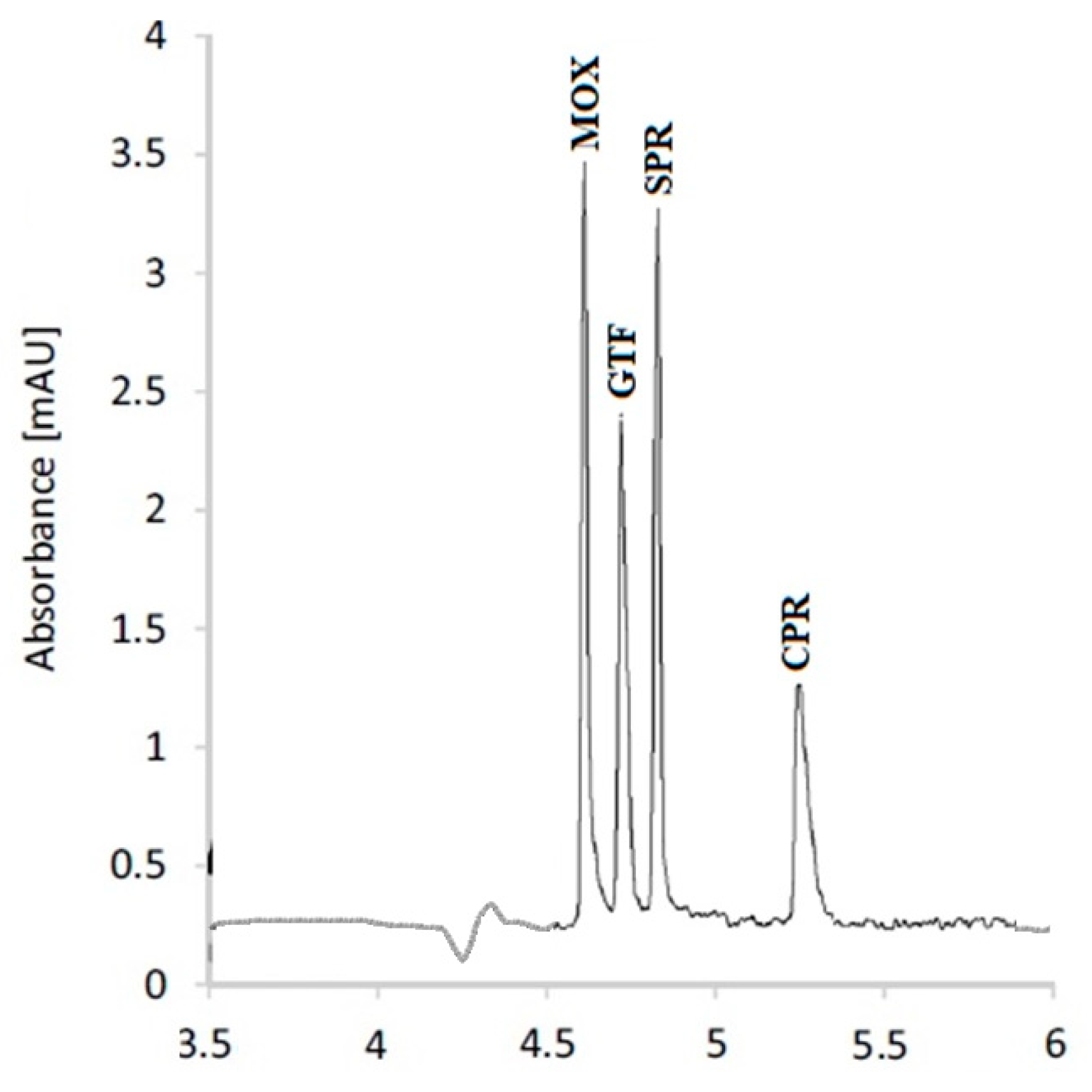
3.3. Separation of the Studied FQs
After the optimization of the experimental parameters, a mixture of the studied FQs containing 10 µg/mL of each was analyzed. The separation pattern is illustrated in Figure 4. The resolution of the separated peaks is calculated with respect to the adjacent peak, according to Equation (1).
where RA,B is the resolution of a pair of adjacent peaks, tA and tB are the migration times of a pair of adjacent peaks, and WA and WB are the peak widths. The Rs values indicate acceptable resolution (Table 4).
RA,B =2(tB − tA)/WA + WB
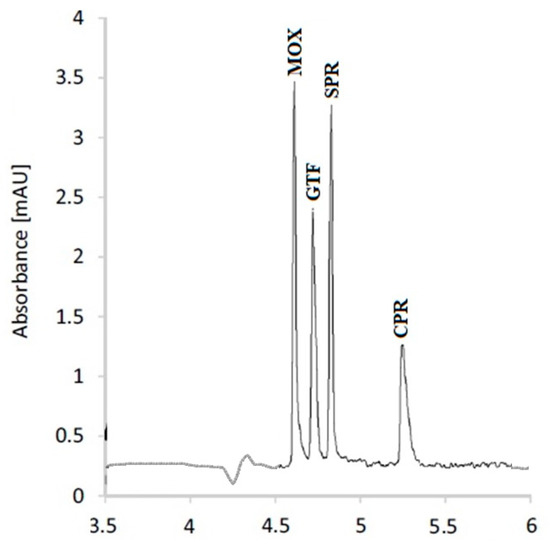
Figure 4.
Capillary electrophoretic separation pattern of the studied FQs.

Table 4.
Validation results of the proposed capillary electrophoretic method.
3.4. Method Validation
Validation was carried out in accordance with ICH-Q2B guidelines [31]. The peak area and drug concentration in the concentration range of 0.5 to 50 µg/mL were shown to be linearly related. It was calculated that the subsequent regression equations would be:
where PA is the peak area, C is the concentration (µg/mL), and r is the correlation coefficient.
PA (MOX) = 402.62 C − 123.06 r = 0.9998
PA (GTF) = 432.37 C + 26.31 r = 0.9989
PA (SPR) = 472.37 C + 26.31 r = 0.9991
PA (CPR) = 605.35 C − 119.67 r = 0.9996
As shown in Table 4, the validation document revealed satisfactory repeatability, accuracy, and intermediate precision. The method’s robustness was further evaluated by making a slight change to the applied voltage and the elution liquid’s composition. The robustness of the recommended method was confirmed by all results, which showed that making these small changes had no appreciable impact on the proposed method. The estimated LOD and LOQ values demonstrated the method’s acceptable sensitivity.
3.5. Method Application
By spiking samples of distilled and tap water with the proposed medicines and then using the optimized procedures for sample preparation and quantification, the effectiveness of the proposed methodologies was evaluated. Table 5 displays the obtained results.

Table 5.
Determination of the studied FQs in the spiked water samples using the proposed method.
Moreover, the optimized sample preparation technique was used to thoroughly treat real wastewater samples, which were subsequently evaluated using the optimized CE procedure. The outcomes were compared with those of reference methods used to quantify CPR, SPR, MOX, and GTF [8,9,10,11] after applying the same sample pretreatment approach (Table 6).

Table 6.
Determination of the studied FQs in actual wastewater samples from industrial pharmaceutical plants.
4. Discussion
Maintaining human health requires careful attention to environmental pollution. Pharmaceutical medications can pose a serious threat to human health if they end up in domestic water or crops since they slowly enter the body and may have serious effects.
The current work showed that it is possible to measure various FQ residues in wastewater effluents produced by the pharmaceutical industry using a sensitive, rapid, and precise capillary electrophoretic method in order to keep an eye on the environmental concentrations of the drugs under study and prevent their adverse effects on humans.
Sample pretreatment via SPE was adjusted, including the choice of the eluting liquid, sample pH, and reconstitution liquid, to obtain the highest sample recovery. Moreover, the capillary electrophoretic setup was optimized. The BGE’s borate content was tuned to 40 mM, resulting in the highest peak area and the shortest analysis time. The pH of the BGE was thoroughly examined. The peak area rose together with the pH until a pH of 8.5 was obtained. The peak area remained nearly constant at this pH level, but the migration time rose as a consequence of the FQs existing in the anionic form above that pH level. In order to achieve the best balance between the resolution, peak area, and migration time, the applied voltage was adjusted t” 16 ’V.
After method optimization, the studied FQs were well separated, as indicated in Figure 4. The resolution factors of the studied FQs exceed unity, confirming acceptable resolution. A detailed validation sheet included all the parameters that were determined for the method’s validation (Table 4). The results pointed to the method’s excellent precision and accuracy. Furthermore, the validation sheet indicated exceptional method robustness, which was confirmed by minor variations in the BGE composition and applied voltage having no appreciable effects on the performance of the described technique. The linearity range, detection, and quantification limits demonstrated excellent method sensitivity. The method was excellent at determining the specified FQs at concentrations that were expected to be present in actual wastewater specimens obtained from pharmaceutical manufacturing plants.
The suggested sample preparation technique and quantification procedure were successfully used to measure the studied FQs in spiked distilled and tap water samples, yielding results that were acceptable and revealing the effectiveness of the sample preparation procedure and the accuracy of the suggested method (Table 5). The recommended approach was also used to quantify the examined compounds in actual wastewater samples. The measured concentrations in the actual wastewater samples were compared to those obtained by using reference methods [8,9,10,11] for the determination of the examined FQs in the same samples. It was concluded that there was good agreement between the results, confirming the validity of the proposed analytical method (Table 6). The developed approach for analyzing the pharmaceuticals under study was found to be simple, sensitive, and appropriate for environmental analysis.
5. Conclusions
To achieve the most successful sample extraction, the SPE technique was adjusted according to the eluent composition, the pH of the sample, and the nature of the reconstitution solvent. Additionally, the CE technique was optimized according to many factors, including the pH of the BGE and the electrolyte concentration. The studied drugs were separated with acceptable resolution, which was demonstrated by calculating the resolution factors (Rs) of the separated peaks and showing values exceeding unity, indicating good resolution.
The optimized procedures were applied to quantify the studied FQs in distilled and tap water samples spiked with the FQs at known concentrations, showing excellent recoveries and confirming the optimal sample extraction procedures. The studied medications were analyzed in actual wastewater effluents using well-optimized procedures, and the results were compared to those obtained by applying reference methods, which showed excellent agreement.
The present work can be considered an environment-friendly procedure, as it uses lower volumes of organic solvents when compared to the HPLC technique.
Our study is unique in that it is the first to quantify the researched FQs in actual wastewater effluents using capillary electrophoresis. The aforementioned analytical method can be successfully used to monitor the examined FQs in wastewater effluents.
Author Contributions
Conceptualization, S.A.A.-G. and A.A.; Data Curation, S.A.A.-G. and A.A.; Formal Analysis, S.A.A.-G.; Investigation, S.A.A.-G. and A.A.; Methodology, S.A.A.-G.; Resources, S.A.A.-G. and A.A.; Software, S.A.A.-G. and A.A.; Supervision, S.A.A.-G.; Validation, S.A.A.-G.; Visualization, S.A.A.-G.; Writing—Original draft, S.A.A.-G.; Writing—Review and Editing, S.A.A.-G. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported via funding from Prince Sattam Bin Abdulaziz University, project number PSAU/2023/R/1444.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This study is supported via funding from Prince Sattam Bin Abdulaziz University, project number PSAU/2023/R/1444.
Conflicts of Interest
The authors declare that they have no competing interests in this manuscript.
Sample Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Sweetman, S.C. Martindale: The Complete Drug Reference, 36th ed.; The Pharmaceutical Press: London, UK, 2009; pp. 302–340. [Google Scholar]
- Tang, K.; Zhao, H. Quinolone Antibiotics: Resistance and Therapy. Infect. Drug Resist. 2023, 10, 811–820. [Google Scholar] [CrossRef]
- O’Neil, M.J. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck: Rahway, NJ, USA, 2006. [Google Scholar]
- Wang, D.; Ning, Q.; Dong, J.; Brooks, B.W.; You, J. Predicting mixture toxicity and antibiotic resistance of fluoroquinolones and their photodegradation products in Escherichia coli. Environ. Pollut. 2020, 262, 114275. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.; Gransden, W. Increasing antibiotic resistance among urinary tract isolates. Arch. Dis. Child. 2003, 88, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Egunova, O.R.; Reshetnikova, I.S.; Kazimirova, K.O.; Shtykov, S.N. Magnetic solid-phase extraction and fluorimetric determination of some fluoroquinolones. J. Anal. Chem. 2020, 75, 24–33. [Google Scholar] [CrossRef]
- Ouyang, Y.Z.; Wu, H.L.; Fang, H.; Wang, T.; Sun, X.D.; Chang, W.W.; Ding, Y.J.; Yu, R.Q. Rapid and simultaneous determination of three fluoroquinolones in animal-derived foods using excitation emission matrix fluorescence coupled with second-order calibration method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117458. [Google Scholar] [CrossRef]
- Vella, J.; Busuttil, F.; Bartolo, N.S.; Sammut, C.; Ferrito, V.; Serracino-Inglott, A.; Azzopardi, L.M.; LaFerla, G. A simple HPLC–UV method for the determination of ciprofloxacin in human plasma. J. Chromatogr. B 2015, 989, 80–85. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Díez, R.; Rodríguez, J.M.; Sierra, M.; García, J.J.; Fernández, N.; Diez, M.J.; Sahagún, A.M. Development and Validation of a Sensitive HPLC Assay for Determination of Sparfloxacin According to the European Medicines Agency Guideline. Separations 2022, 9, 223. [Google Scholar] [CrossRef]
- El-Yazbi, A.F.; Aboukhalil, F.M.; Khamis, E.F.; Elkhatib, M.A.W.; El-Sayed, M.A.; Youssef, R.M. Simple simultaneous determination of moxifloxacin and metronidazole in complex biological matrices. RSC Adv. 2022, 23, 15694–15704. [Google Scholar] [CrossRef]
- Saad, M.N.; Essam, H.M.; Elzanfaly, E.S.; Amer, S.M. Economic chromatographic methods for simultaneous quantitation of some fluoroquinolones and corticosteroids present in different binary ophthalmic formulations. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 271–281. [Google Scholar] [CrossRef]
- Li, J.; Ren, X.; Diao, Y.; Chen, Y.; Wang, Q.; Jin, W.; Zhou, P.; Fan, Q.; Zhang, Y.; Liu, H. Multiclass analysis of 25 veterinary drugs in milk by ultra-high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2018, 257, 259–264. [Google Scholar] [CrossRef]
- Ziarrusta, H.; Val, N.; Dominguez, H.; Mijangos, L.; Prieto, A.; Usobiaga, A.; Etxebarria, N.; Zuloaga, O.; Olivares, M. Determination of fluoroquinolones in fish tissues, biological fluids, and environmental waters by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 6359–6370. [Google Scholar] [CrossRef] [PubMed]
- Seifrtová, M.; Aufartová, J.; Vytlačilová, J.; Pena, A.; Solich, P.; Nováková, L. Determination of fluoroquinolone antibiotics in wastewater using ultra high-performance liquid chromatography with mass spectrometry and fluorescence detection. J. Sep. Sci. 2010, 33, 2094–2108. [Google Scholar] [CrossRef] [PubMed]
- Selahle, S.K.; Nomngongo, P.N. Determination of fluoroquinolones in the environmental samples using vortex assisted dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Int. J. Environ. Anal. Chem. 2020, 100, 282–294. [Google Scholar] [CrossRef]
- Ramos-Payán, M.; Villar-Navarro, M.; Fernández-Torres, R.; Callejon-Mochon, M.; Bello-Lopez, M.A. Electromembrane extraction (EME)—An easy, novel and rapid extraction procedure for the HPLC determination of fluoroquinolones in wastewater samples. Anal. Bioanal. Chem. 2013, 405, 2575–2584. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.W.; Tian, H.; Wei, Q.H.; Liu, B.S.; Bao, G.M.; Liao, M.L.; Peng, J.L.; Huang, X.Q.; Wang, L.Q. High Through-put determination of 28 veterinary antibiotic residues in swine wastewater by one-step dispersive solid phase extraction sample cleanup coupled with ultra-performance liquid chromatography–tandem mass spectrometry. Chemosphere 2019, 230, 337–346. [Google Scholar] [CrossRef]
- Maia, A.S.; Paiga, P.; Delerue-Matos, C.; Castro, P.M.L.; Tiritan, M.E. Quantification of fluoroquinolones in wastewaters by liquid chromatography–tandem mass spectrometry. Environ. Pollut. 2020, 259, 113927. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, S.A.; Arab, H.H.; Albassam, A.A. Potentiometric Determination of Moxifloxacin by Solid-Contact ISEs in Wastewater Effluents. Chemosensors 2022, 10, 146. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wei, S.; Yao, S.; Zhang, J.; Huang, H. Selective extraction and determination of fluoroquinolones in bovine milk samples with montmorillonite magnetic molecularly imprinted polymers and capillary electrophoresis. Anal. Bioanal. Chem. 2016, 408, 589–598. [Google Scholar] [CrossRef]
- Kośka, I.; Purgat, K.; Kubalczyk, P. Simple, fast and reliable CE method for simultaneous determination of ciprofloxacin and ofloxacin in human urine. Sci. Rep. 2022, 12, 7729. [Google Scholar] [CrossRef]
- Kośka, I.; Purgat, K.; Głowacki, R.; Kubalczyk, P. Simultaneous Determination of Ciprofloxacin and Ofloxacin in Animal Tissues with the Use of Capillary Electrophoresis with Transient Pseudo-Isotachophoresis. Molecules 2021, 26, 6931. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Z. Simultaneous separation of 12 different classes of antibiotics under the condition of complete protonation by capillary electrophoresis-coupled contactless conductivity detection. Anal. Methods 2022, 14, 174–179. [Google Scholar] [CrossRef]
- Lombardo, M.; Gámiz-Gracia, L.; García-Campaña, A.M.; Cruces-Blanco, C. Sensitive determination of fluoroquinolone residues in waters by capillary electrophoresis with laser-induced fluorescence detection. Anal. Bioanal. Chem. 2010, 396, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Deng, Y.; Zhao, M.Z.; Zhou, Y.L.; Zhang, X.X. Highly-sensitive detection of eight typical fluoroquinolone antibiotics by capillary electrophoresis-mass spectroscopy coupled with immunoaffinity extraction. RSC Adv. 2018, 8, 4063–4071. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Hernandez-Borges, J.; Borges- Miquel, T.M.; Rodrıguez-Delgado, M.A. Dispersive liquid– liquid microextraction combined with nonaqueous capillary electrophoresis for the determination of fluoroquinolone antibiotics in waters. Electrophoresis 2010, 31, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Herrera, A.V.; Ravelo-Perez, L.M.; Hernandez-Borges, J.; Afonso, M.M.; Palenzuela, J.A.; Rodriguez-Delgado, M.A. Oxidized multi-walled carbon nanotubes for the dispersive solid-phase extraction of quinolone antibiotics from water samples using capillary electrophoresis and large volume sample stacking with polarity switching. J. Chromatogr. A 2011, 1218, 5352–5361. [Google Scholar] [CrossRef]
- Springer, V.H.; Lista, A.G. In-line coupled single drop liquid-liquid-liquid microextraction with capillary electrophoresis for determining fluoroquinolones in water samples. Electrophoresis 2015, 36, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Baciu, T.; Borrull, F.; Neusus, C.; Aguilar, C.; Calull, M. Capillary electrophoresis combined in-line with solid-phase extraction using magnetic particles as new adsorbents for the determination of drugs of abuse in human urine. Electrophoresis 2016, 37, 1232–1244. [Google Scholar] [CrossRef]
- Rusu, A.; Hancu, G.; Völgyi, G.; Tóth, G.; Noszál, B.; Gyéresi, A. Separation and Determination of Quinolone Antibacterials by Capillary Electrophoresis. J. Chromatogr. Sci. 2014, 52, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Purgat, K.; Olejarz, P.; Kośka, I.; Głowacki, R.; Kubalczyk, P. Determination of homocysteine thiolactone in human urine by capillary zone electrophoresis and single drop microextraction. Anal. Biochem. 2020, 596, 113640. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. In ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2 (B); PharmaLogica, Inc.: Charlotte, NC, USA, 2005.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).