Adsorption of Chromium (VI) from Aqueous Solution Using Palm Leaf-Derived Biochar: Kinetic and Isothermal Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development and Characterization of Biochar
2.3. Batch Adsorption
3. Results and Discussion
3.1. Characterization of Adsorbent
3.1.1. FT-IR Analysis
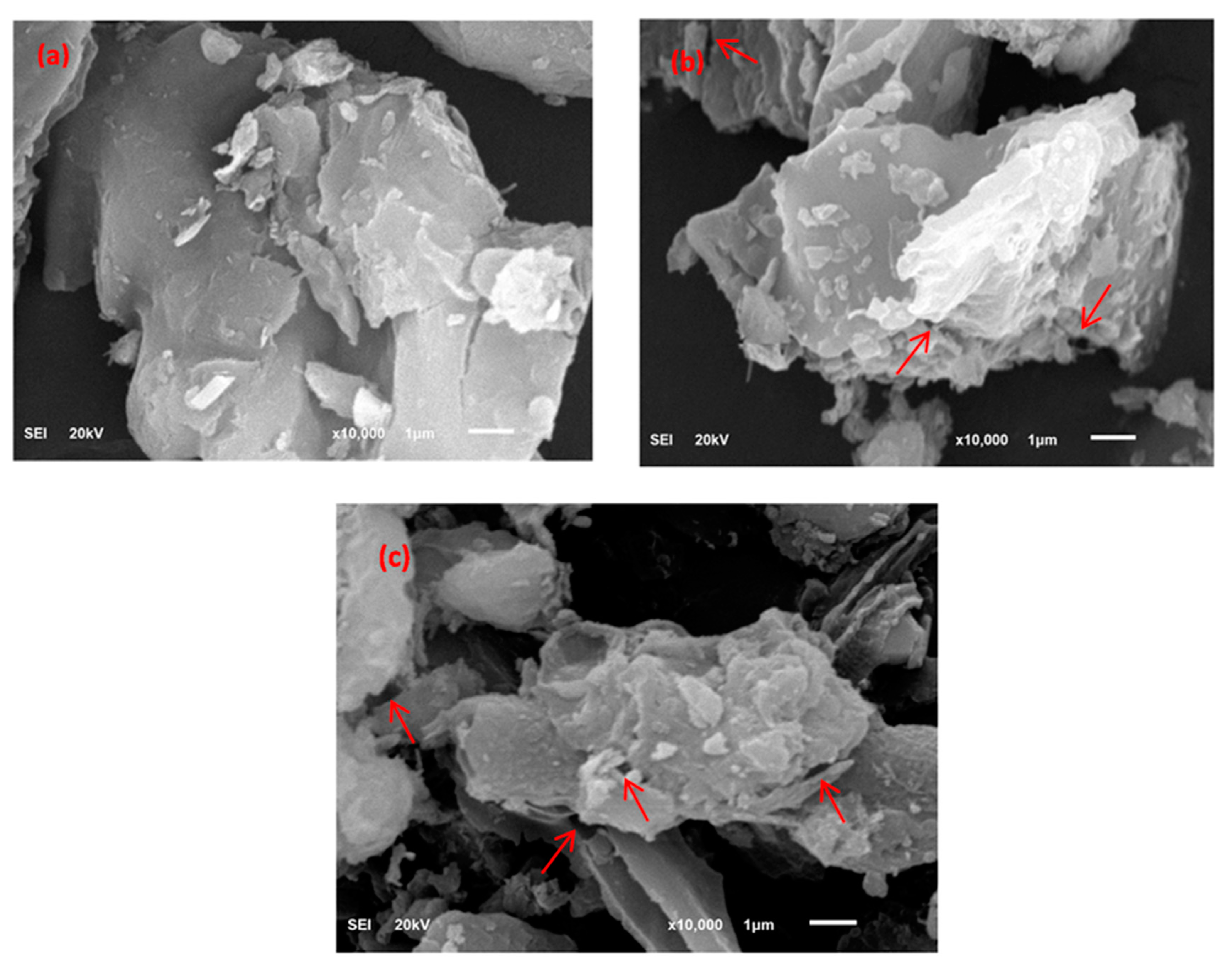
3.1.2. FE-SEM Analysis
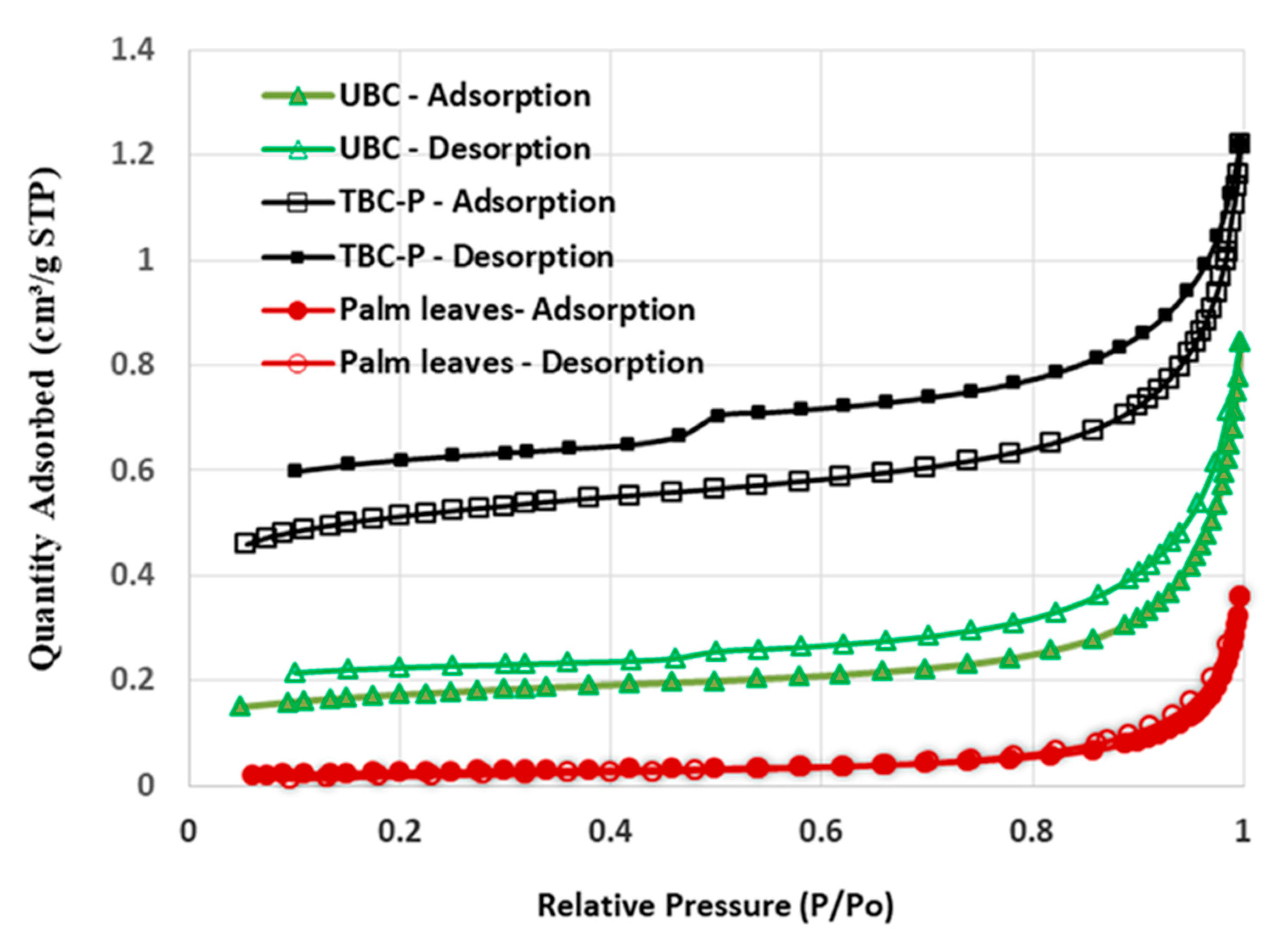
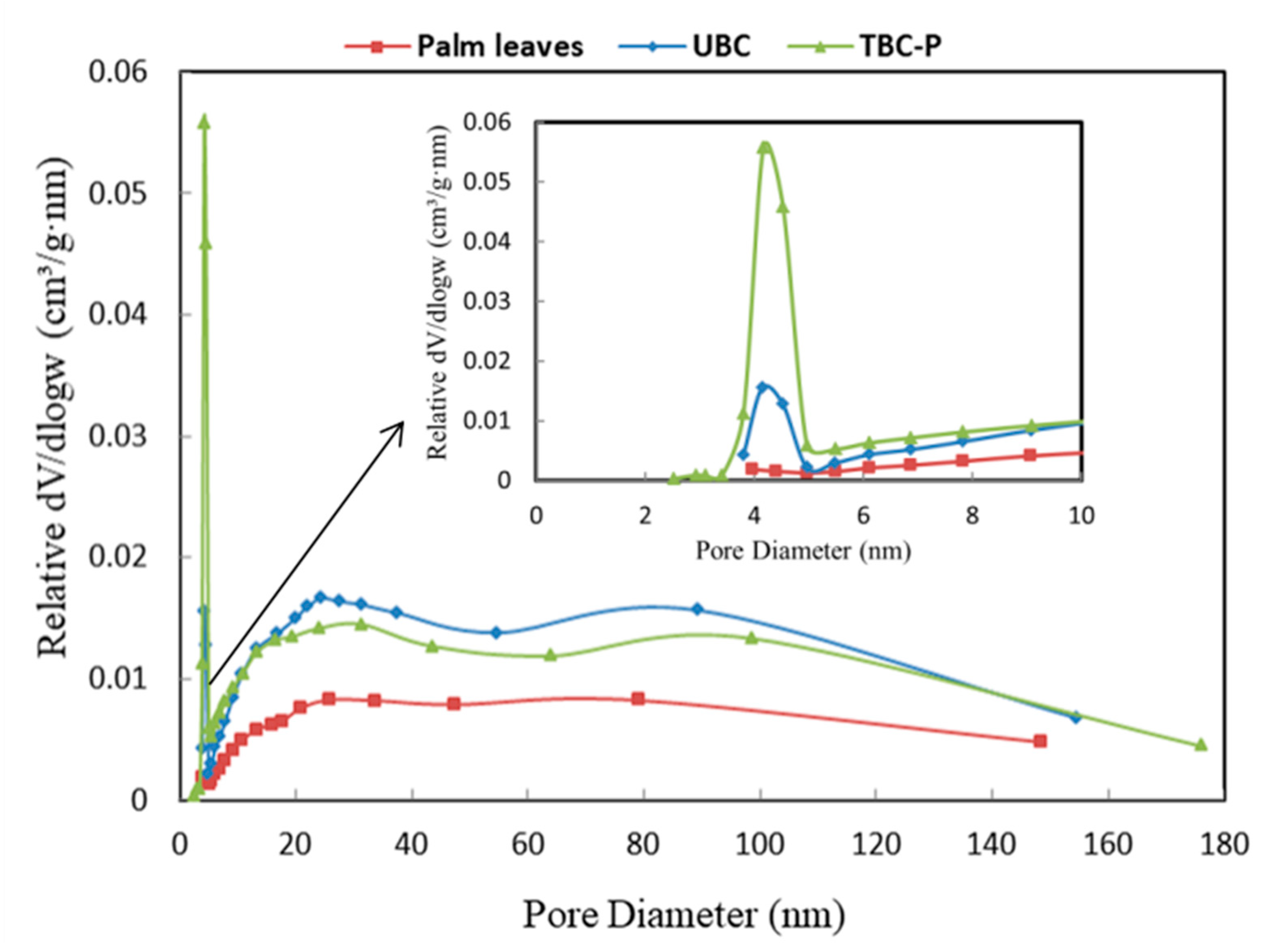
3.1.3. Surface Area Analysis
3.2. Adsorption Studies of Cr (VI)
3.2.1. Effect of Adsorbent Type
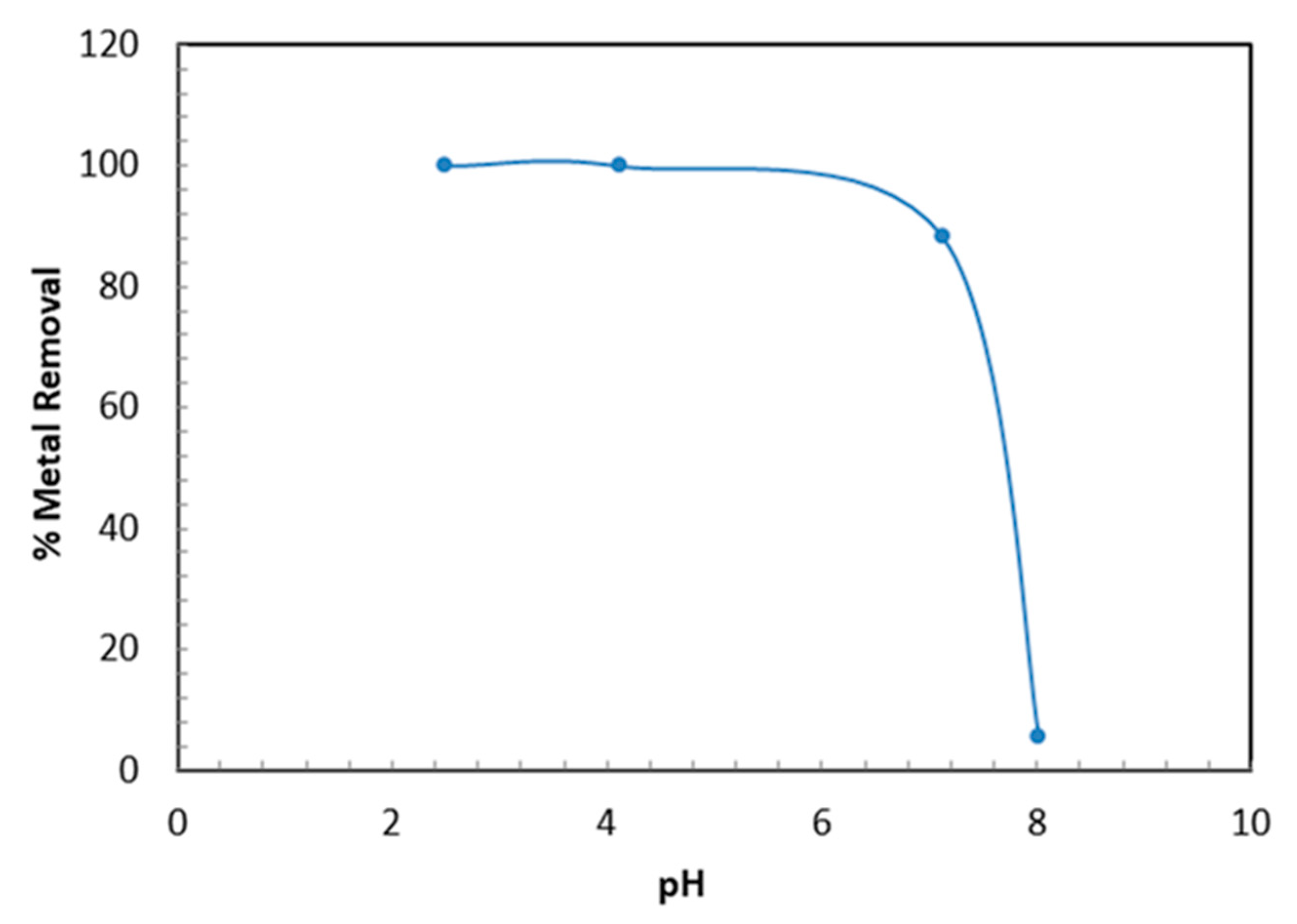
3.2.2. Influence of Solution pH on Metal Uptake
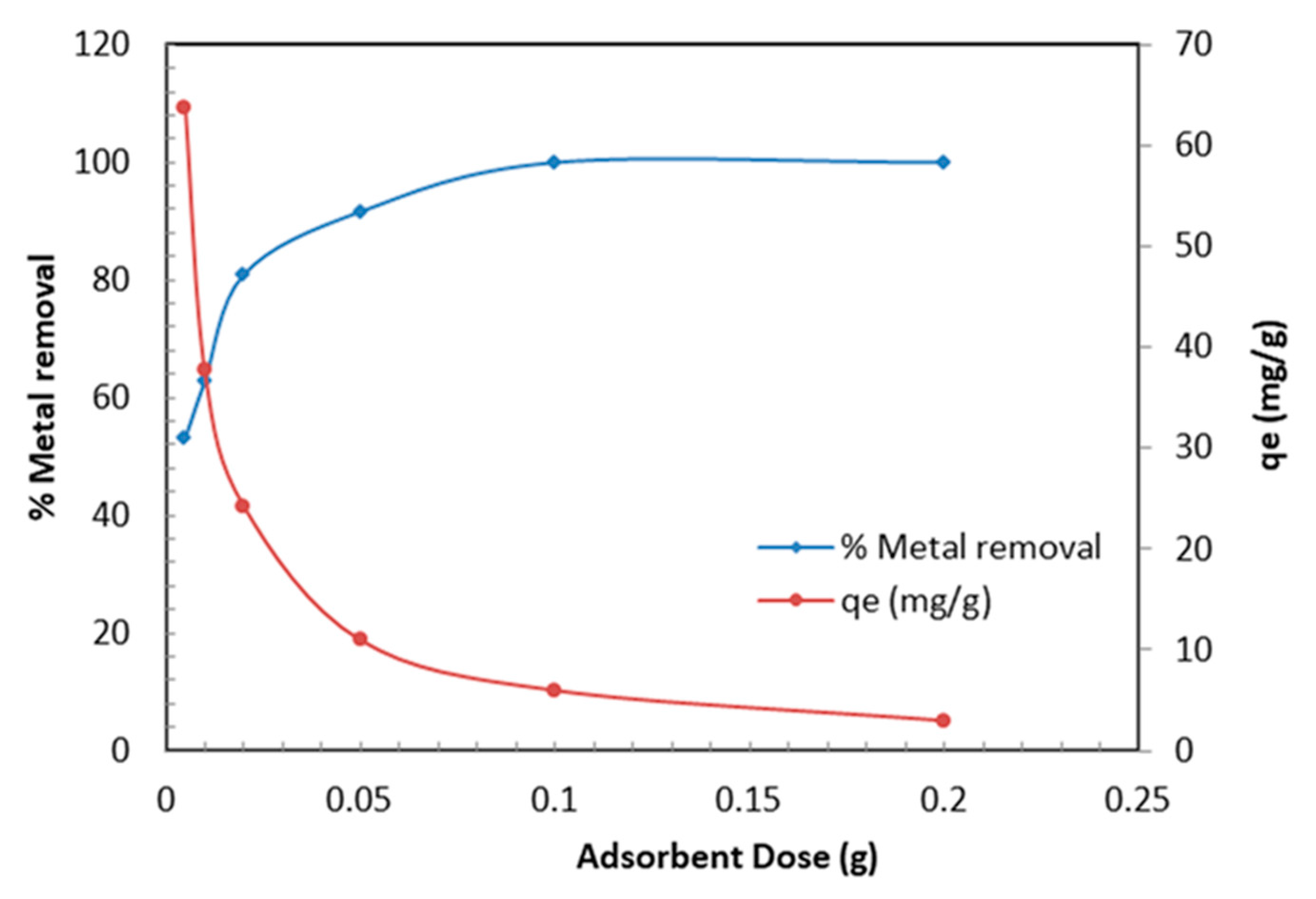
3.2.3. Effect of TBC-P Dose on Cr (VI) Uptake
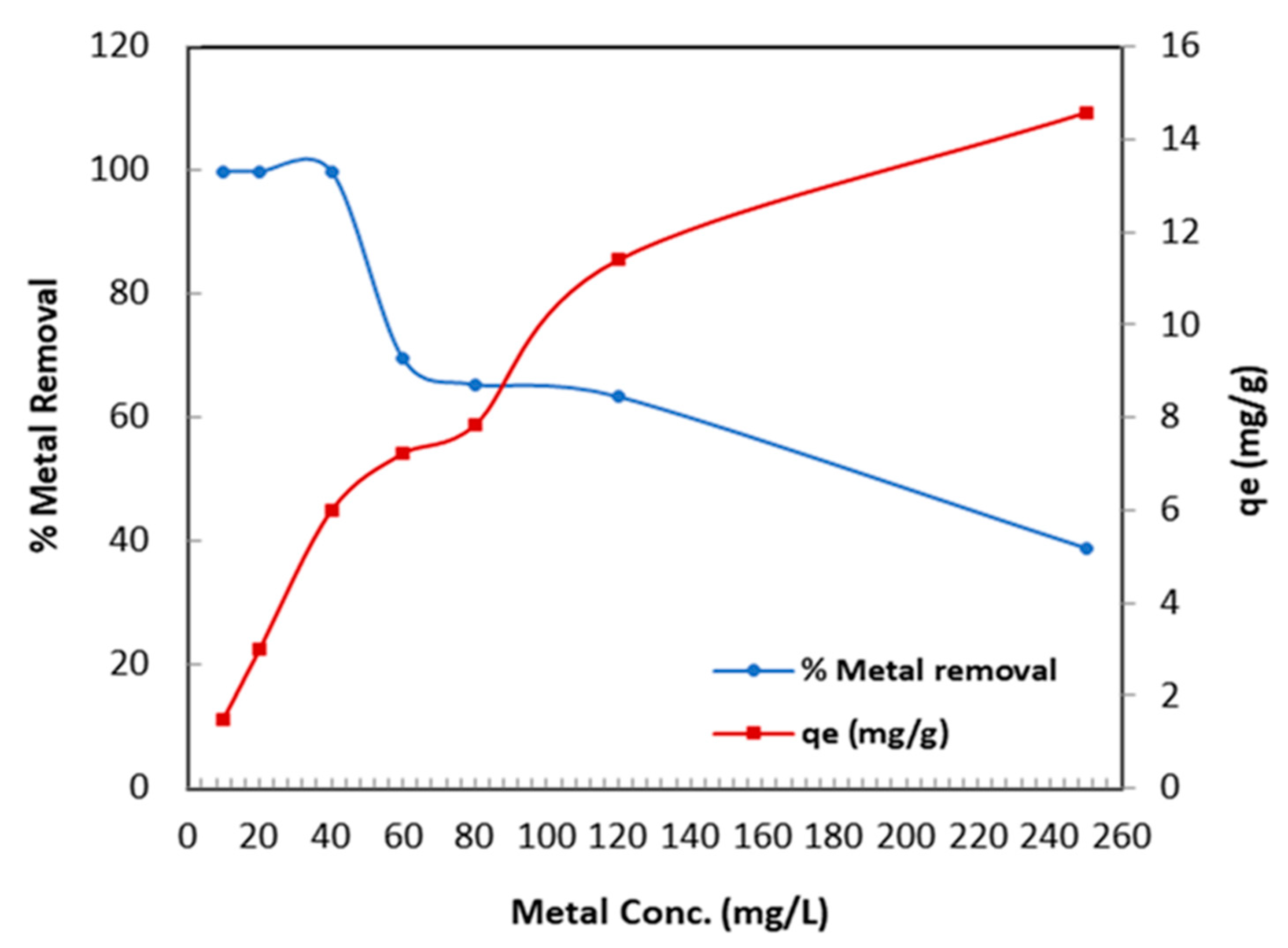
3.2.4. Influence of Metal Initial Concentration
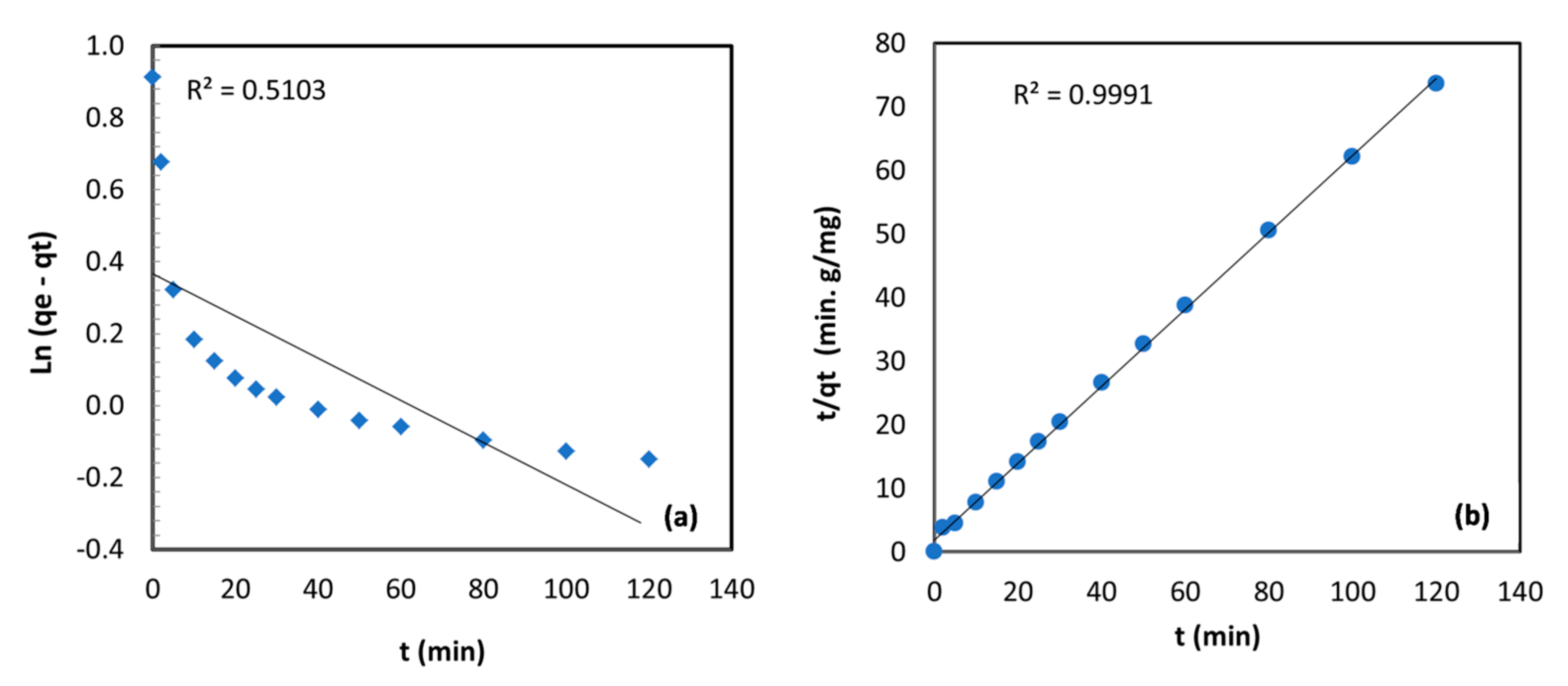
3.2.5. Kinetics Analysis
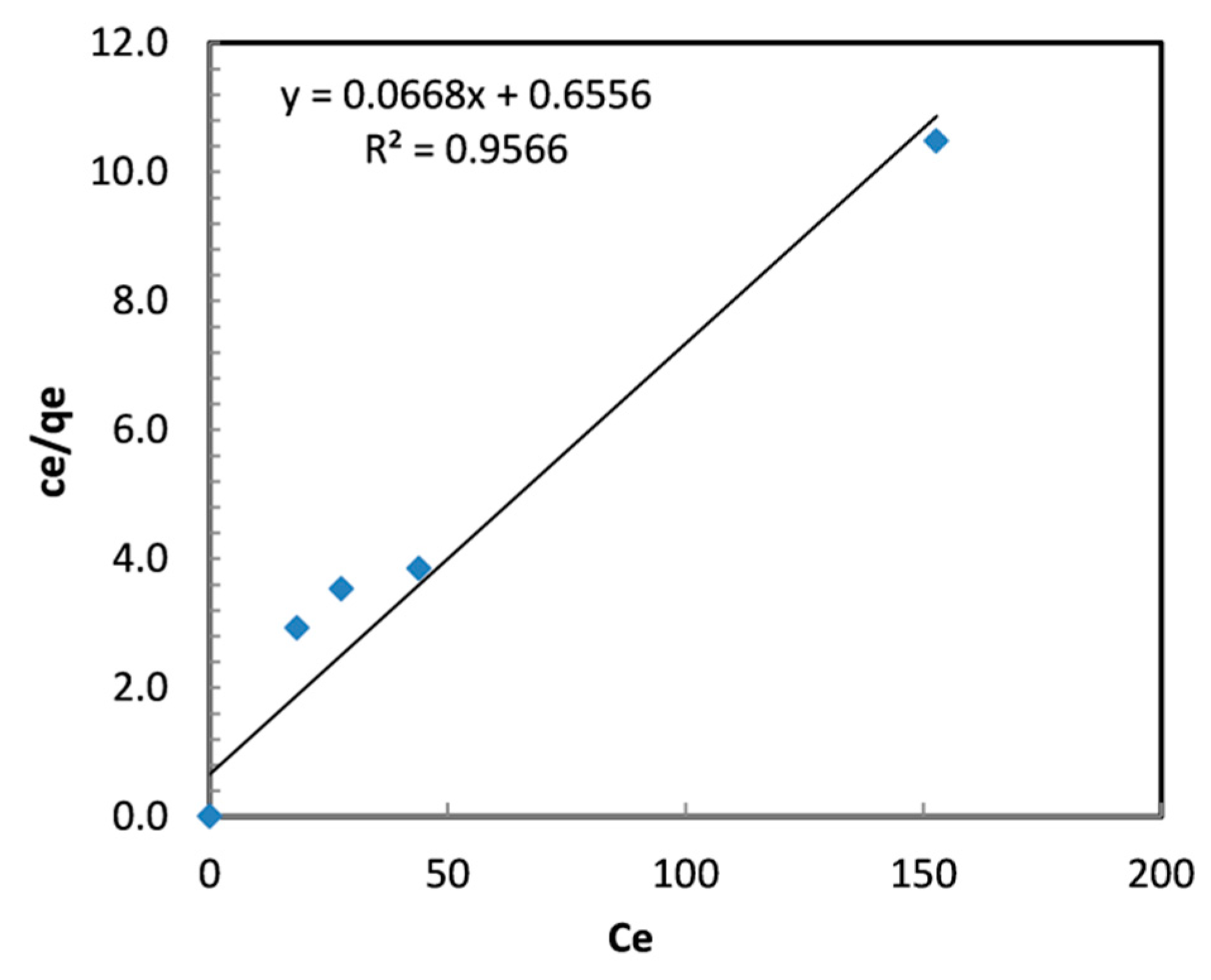
3.2.6. Isotherms Analysis
3.2.7. Comparison of the Cr (VI) Adsorption Capacity with Literature
3.2.8. Regeneration and Reusability of the TBC-P
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Gupta, R.; You, S. Machine learning assisted prediction of biochar yield and composition via pyrolysis of biomass. Bioresour. Technol. 2022, 359, 127511. [Google Scholar] [CrossRef] [PubMed]
- Yrjälä, K.; Ramakrishnan, M.; Salo, E. Agricultural waste streams as resource in circular economy for biochar production towards carbon neutrality. Curr. Opin. Environ. Sci. Health 2022, 26, 100339. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, D.J.; Narula, A.; Chistie, S.M.; Naik, S.U. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Faiad, A.; Alsmari, M.; Ahmed, M.M.; Bouazizi, M.L.; Alzahrani, B.; Alrobei, H. Date palm tree waste recycling: Treatment and processing for potential engineering applications. Sustainability 2022, 14, 1134. [Google Scholar] [CrossRef]
- Song, J.; He, Q.; Hu, X.; Zhang, W.; Wang, C.; Chen, R.; Wang, H.; Mosa, A. Highly efficient removal of Cr (VI) and Cu (II) by biochar derived from Artemisia argyi stem. Environ. Sci. Pollut. Res. 2019, 26, 13221–13234. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Chattree, A.; Dan, S.; Jeyasundari, J.; Rathish, R.J.; Nguyen, T.A.; Rajendran, S. Detection and evaluation of trace metals in soil using nanosensors. In Nanosensors for Smart Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 217–235. [Google Scholar]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Lee, D.J.; Cheng, Y.L.; Wong, R.J.; Wang, X.D. Adsorption removal of natural organic matters in waters using biochar. Bioresour. Technol. 2018, 260, 413–416. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Liu, Y.G.; Wang, H.; Zeng, G.M.; Hu, X.; Guo, Y.M.; Li, T.T.; Chen, A.W.; Jiang, L.H.; Guo, F.Y. Adsorption of copper by magnetic graphene oxide-supported β-cyclodextrin: Effects of pH, ionic strength, background electrolytes, and citric acid. Chem. Eng. Res. Des. 2015, 93, 675–683. [Google Scholar] [CrossRef]
- Puglla, E.P.; Guaya, D.; Tituana, C.; Osorio, F.; García-Ruiz, M.J. Biochar from agricultural by-products for the removal of lead and cadmium from drinking water. Water 2020, 12, 2933. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Szlachta, M. Biochar derived from fruit by-products using pyrolysis process for the elimination of Pb (II) ion: An updated review. Chemosphere 2022, 287, 132250. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, S.; Upadhyay, U.; Sreedhar, I. Comparative studies of heavy metal removal from aqueous solution using novel biomass and biochar-based adsorbents: Characterization, process optimization, and regeneration. Biomass Convers. Biorefinery 2022, 1–3. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Lal Srivastav, A. Biochar as sustainable adsorbents for chromium ion removal from aqueous environment: A review. Biomass Convers. Biorefinery 2022, 1–4. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Zeng, H.; Zeng, H.; Zhang, H.; Shahab, A.; Zhang, K.; Lu, Y.; Nabi, I.; Naseem, F.; Ullah, H. Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 2021, 286, 124964. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Mohapatra, S.; Panda, C.R.; Singh, A. Banana peduncle biochar: Characteristics and adsorption of hexavalent chromium from aqueous solution. Methodology 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Yin, W.; Guo, Z.; Zhao, C.; Xu, J. Removal of Cr (VI) from aqueous media by biochar derived from mixture biomass precursors of Acorus calamus Linn. and feather waste. J. Anal. Appl. Pyrolysis 2019, 140, 86–92. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, C.; Guan, Q.; Ning, P.; Gu, J.; Chen, Q.; Zhang, J.; Miao, R. Enhanced removal of Cr (VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 2018, 195, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Shi, Y.; Gu, J.; Bi, J.; Yuan, H.; Luo, B.; Chen, Y. Aqueous Cr (VI) removal by biochar derived from waste mangosteen shells: Role of pyrolysis and modification on its absorption process. J. Environ. Chem. Eng. 2020, 8, 103885. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr (VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mathabatha, T.I.; Matheri, A.N.; Belaid, M. Peanut Shell-Derived Biochar as a Low-Cost Adsorbent to Extract Cadmium, Chromium, Lead, Copper, and Zinc (Heavy Metals) from Wastewater: Circular Economy Approach. Circ. Econ. Sustain. 2022, 1–20. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, C.; Lv, Y.; Zhang, W.; Du, Y.; Hao, Z.; Zhang, J. Adsorption and coadsorption mechanisms of Cr (VI) and organic contaminants on H3PO4 treated biochar. Chemosphere 2017, 186, 422–429. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Júnior, O.O.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. (Eds.) Biochar: A Guide to Analytical Methods; Csiro Publishing: Clayton, Australia, 2017. [Google Scholar]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Sizirici, B.; Fseha, Y.H.; Yildiz, I.; Delclos, T.; Khaleel, A. The effect of pyrolysis temperature and feedstock on date palm waste derived biochar to remove single and multi-metals in aqueous solutions. Sustain. Environ. Res. 2021, 31, 9. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 18851. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Babu, B.V.; Gupta, S. Adsorption of Cr (VI) using activated neem leaves: Kinetic studies. Adsorption 2008, 14, 85–92. [Google Scholar] [CrossRef]
- Yao, Y.; Bing, H.; Feifei, X.; Xiaofeng, C. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous effluents by rapeseed biomass. New Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Marcu, C.V.; Varodi, C.; Balla, A. Adsorption kinetics of chromium (VI) from aqueous solution using an anion exchange resin. Anal. Lett. 2021, 54, 140–149. [Google Scholar] [CrossRef]
- Sağ, Y.; Aktay, Y. Kinetic studies on sorption of Cr (VI) and Cu (II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem. Eng. J. 2002, 12, 143–153. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Sanaei, D.; Ali, I.; Bhatnagar, A. Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq. 2016, 215, 671–679. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Wang, C.C.; Cheng, J.H.; Zhang, C.L.; Yao, J.J. Removal of Cr (VI) by biochar derived from six kinds of garden wastes: Isotherms and kinetics. Materials 2021, 14, 3243. [Google Scholar] [CrossRef]
- Kar, S.; Equeenuddin, S.M. Adsorption of hexavalent chromium using natural goethite: Isotherm, thermodynamic and kinetic study. J. Geol. Soc. India 2019, 93, 285–292. [Google Scholar] [CrossRef]
- Chandana, L.; Krushnamurty, K.; Suryakala, D.; Subrahmanyam, C. Low-cost adsorbent derived from the coconut shell for the removal of hexavalent chromium from aqueous medium. Mater. Today Proc. 2020, 26, 44–51. [Google Scholar] [CrossRef]
- Niam, A.C.; Fenelon, E.; Ningsih, E.; Mirzayanti, Y.W.; Kristanti, E. High-efficiency adsorption of hexavalent chromium from aqueous solution by Samanea saman activated carbon. Adsorpt. Sci. Technol. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Mechanism of Cr (VI) uptake onto sagwan sawdust derived biochar and statistical optimization via response surface methodology. Biomass Convers. Biorefinery 2023, 13, 709–725. [Google Scholar] [CrossRef]
- Qhubu, M.C.; Mgidlana, L.G.; Madikizela, L.M.; Pakade, V.E. Preparation, characterization and application of activated clay biochar composite for removal of Cr (VI) in water: Isotherms, kinetics and thermodynamics. Mater. Chem. Phys. 2021, 260, 124165. [Google Scholar] [CrossRef]
- Espinoza-Sanchez, M.A.; Arevalo-Nino, K.; Quintero-Zapata, I.; Castro-Gonzalez, I.; Almaguer-Cantu, V. Cr (VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. J. Environ. Manag. 2019, 251, 109595. [Google Scholar] [CrossRef]
- Tripathy, S.; Sahu, S.; Patel, R.K.; Panda, R.B.; Kar, P.K. Efficient removal of Cr (VI) by polyaniline modified biochar from date (Phoenix dactylifera) seed. Groundw. Sustain. Dev. 2021, 15, 100653. [Google Scholar] [CrossRef]
- Liang, M.; Ding, Y.; Zhang, Q.; Wang, D.; Li, H.; Lu, L. Removal of aqueous Cr (VI) by magnetic biochar derived from bagasse. Sci. Rep. 2020, 10, 21473. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr (VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Nawaz, A.; Singh, B.; Kumar, P. H3PO4-modified Lagerstroemia speciosa seed hull biochar for toxic Cr (VI) removal: Isotherm, kinetics, and thermodynamic study. Biomass Convers. Biorefinery 2021, 1–5. [Google Scholar] [CrossRef]

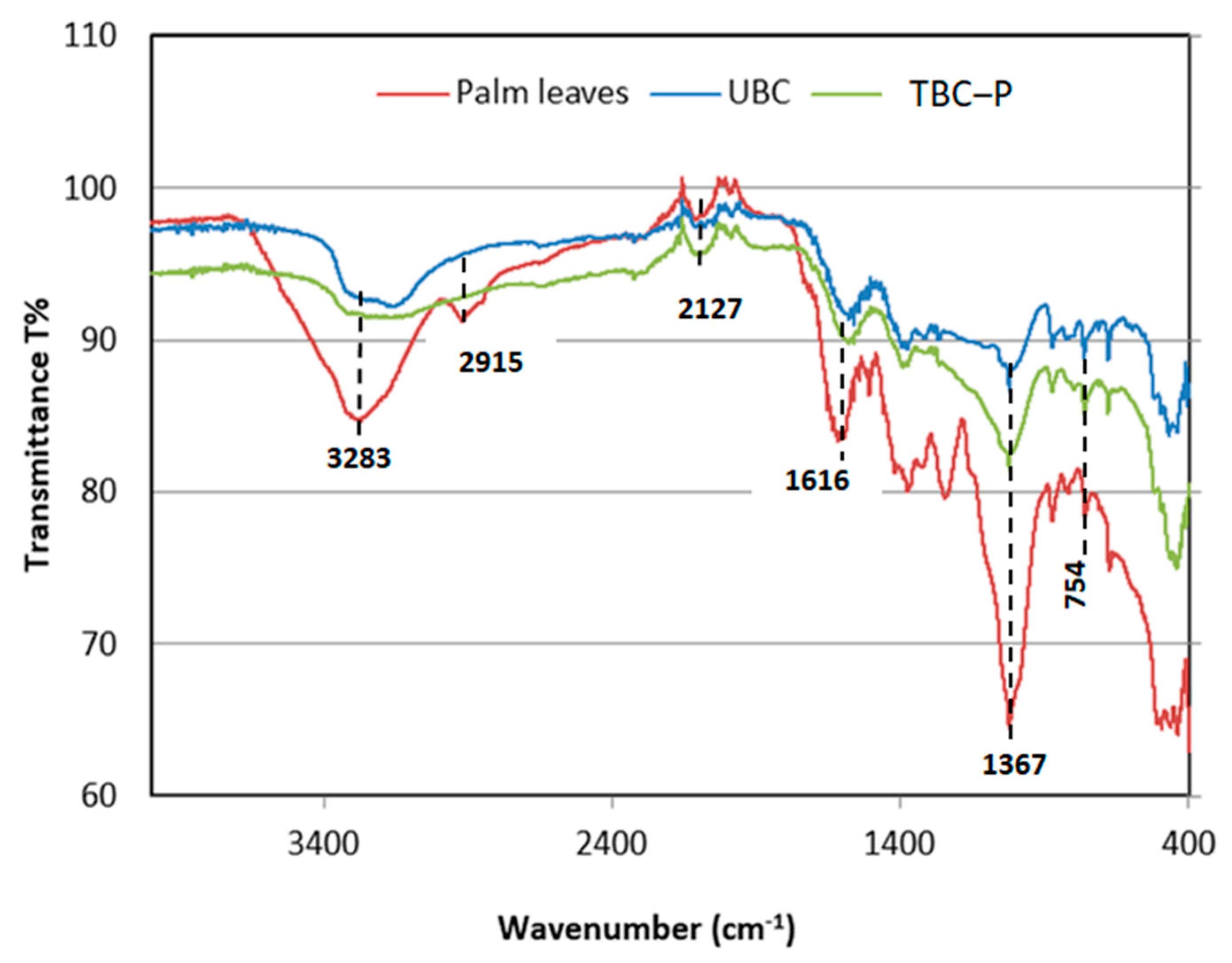
| Wavenumber (cm−1) | Functional Groups | |||
|---|---|---|---|---|
| Reference | Palm Leaves | UBC | TBC-P | |
| 3600–3400 | 3283 | 3376 | 3340 | O-H Stretching of alcohol, carboxylic |
| 3100–3000 | 2915 | - | - | C-H Stretching of alkyne, benzene |
| 2260–2100 | 2127 | 2149 | 2100 | C-C Stretching of alkyne |
| 1650–1600 | 1616 | 1620 | 1577 | C=C Stretching of alkene |
| 1300–1000 | 1367, 1240, 1019 | 1349, 1028 | 1373, 1020 | C-O Stretching of ether, ester |
| 1000–675 | 875, 754, 673 | 680, 463 | 678, 440 | C=C, C-H Stretching of bending |
| Adsorbent | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Palm leaves | 2.030 | 0.0106 | 25.91 |
| UBC | 12.71 | 0.0229 | 16.44 |
| TBC-P | 37.01 | 0.0279 | 9.16 |
| Model | Parameters | |
|---|---|---|
| qe,exp (mg/g) | 2.49 | |
| Pseudo‒first-order | qe,cal (mg/g) | 1.44 |
| k1 (min−1) | 0.0059 | |
| 0.5103 | ||
| Pseudo‒second-order | qe,cal (mg/g) | 1.65 |
| k2 (g/mg·min) | 0.193 | |
| 0.9991 | ||
| T (°C) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| 25 | b (L/mg) | Qmax (mg/g) | R2 | 1/n | KF (mg/g (L/mg) 1/n) | R2 |
| 0.102 | 14.97 | 0.957 | 0.1684 | 5.296 | 0.779 | |
| Adsorbents | Temperature (°C) | pH | C0 (mg/g) | Qmax (mg/g) | References |
|---|---|---|---|---|---|
| Sophora japonica Linn biochar | 25 | 2 | 5–400 | 9.58 | [41] |
| Natural goethite | 25 | 2 | 2–25 | 0.727 | [42] |
| AC derived from the coconut shell | 25 | 2 | 10–30 | 14.62 | [43] |
| Samanea saman activated carbon | 25 | 5 | 5–30 | 0.2893 | [44] |
| Sagwan sawdust-derived biochar | 25 | 2 | 30–100 | 9.62 | [45] |
| Activated clay mineral biochar composite | 25 | 3 | 1–11 | 6.84 | [46] |
| Fungal biomass of Rhizopus sp. | 25 | 2 | 12.5–300 | 8.06 | [47] |
| TBC-P | 25 | 2 | 10–250 | 14.97 | This study |
| Polyaniline-coated date seed-derived biochar | 50 | 5 | 2–100 | 27.3 | [48] |
| Magnetic biochar derived from bagasse | 25 | 2 | 5–300 | 29.08 | [49] |
| Pineapple peel-derived biochar | 25 | 2 | 10–500 | 23.81 | [50] |
| H3PO4- modified Lagerstroemia speciosa hull biochar | 30 | 2 | 25–100 | 41.92 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daffalla, S. Adsorption of Chromium (VI) from Aqueous Solution Using Palm Leaf-Derived Biochar: Kinetic and Isothermal Studies. Separations 2023, 10, 260. https://doi.org/10.3390/separations10040260
Daffalla S. Adsorption of Chromium (VI) from Aqueous Solution Using Palm Leaf-Derived Biochar: Kinetic and Isothermal Studies. Separations. 2023; 10(4):260. https://doi.org/10.3390/separations10040260
Chicago/Turabian StyleDaffalla, Samah. 2023. "Adsorption of Chromium (VI) from Aqueous Solution Using Palm Leaf-Derived Biochar: Kinetic and Isothermal Studies" Separations 10, no. 4: 260. https://doi.org/10.3390/separations10040260
APA StyleDaffalla, S. (2023). Adsorption of Chromium (VI) from Aqueous Solution Using Palm Leaf-Derived Biochar: Kinetic and Isothermal Studies. Separations, 10(4), 260. https://doi.org/10.3390/separations10040260






