SFC and CE—A Comparison of Two Orthogonal Methods for the Analysis of Dihydrochalcones in Apple Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials and Sample Preparation
2.3. Analytical Instrumentation
2.4. Method Validation
3. Results and Discussion
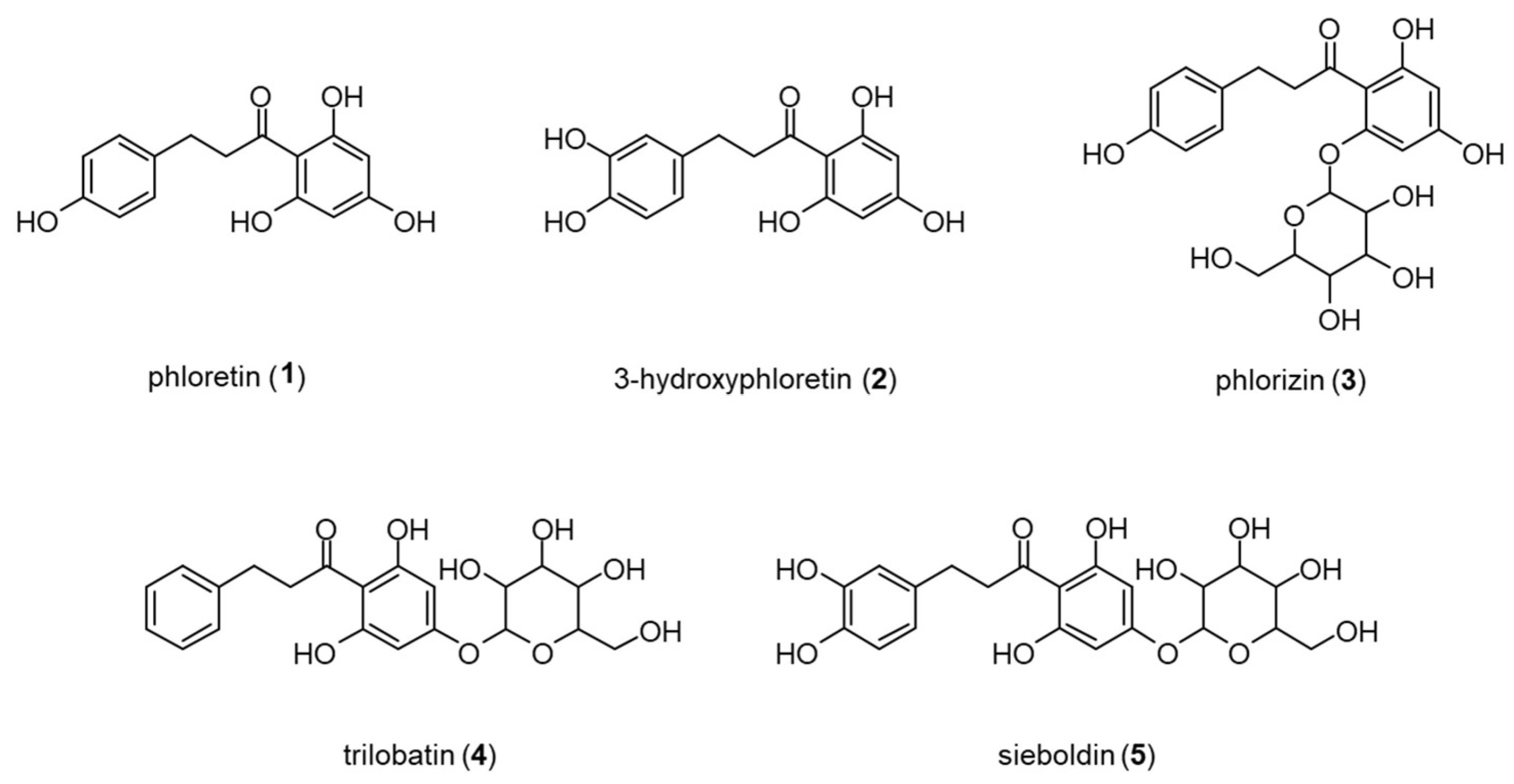
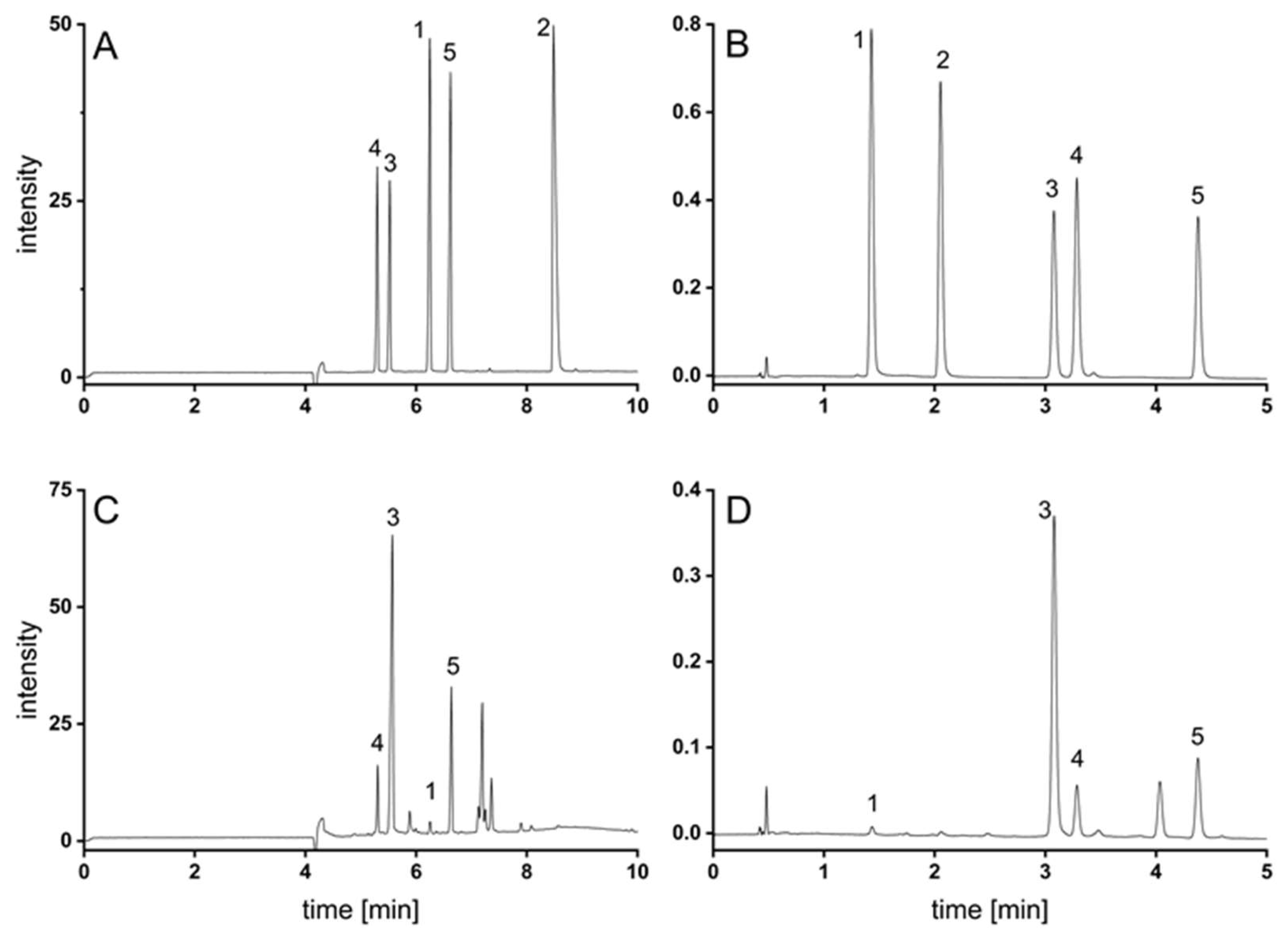
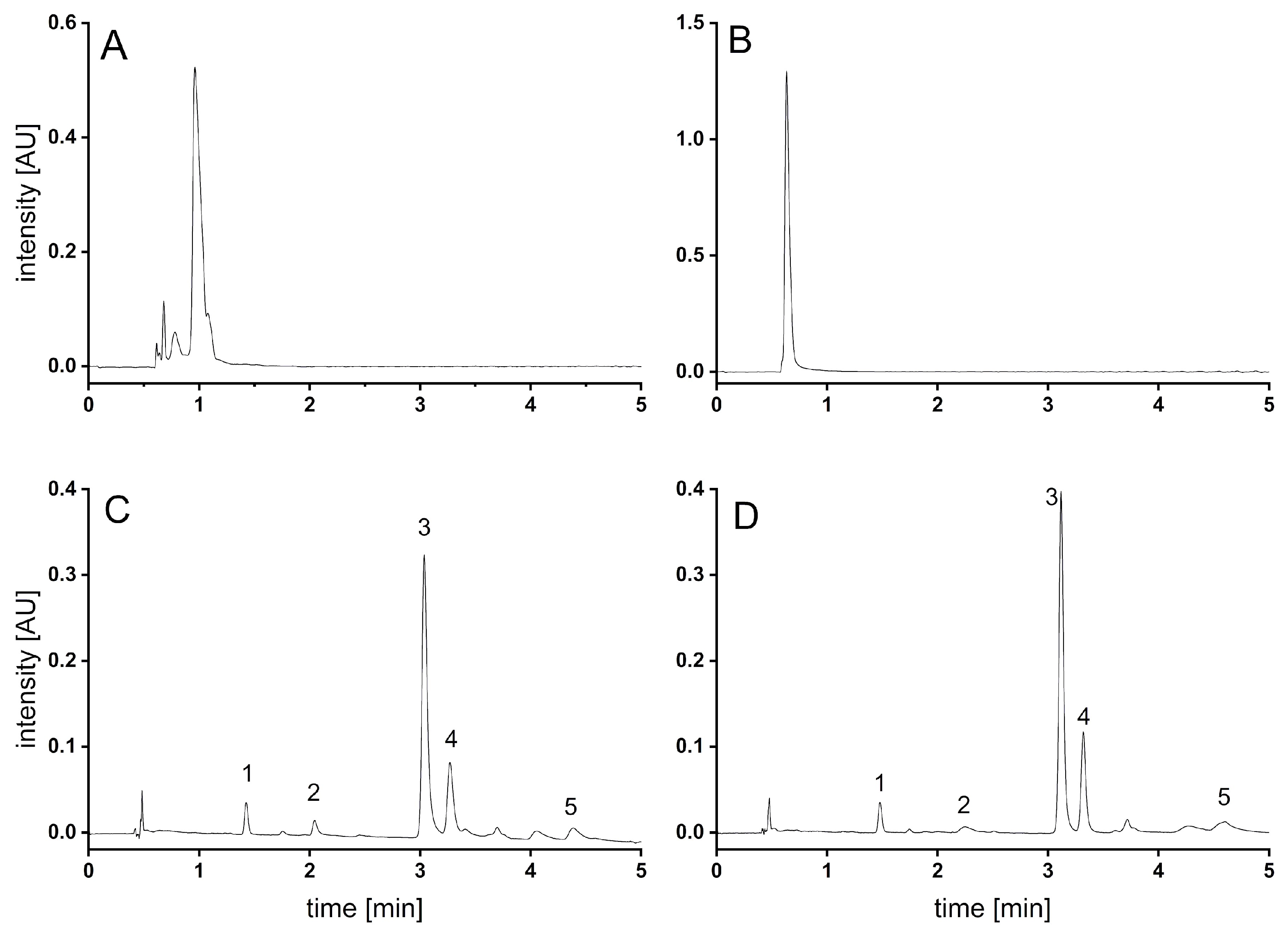
3.1. Method Development
3.1.1. Supercritical Fluid Chromatography
3.1.2. Capillary Electrophoresis
3.2. Method Validation
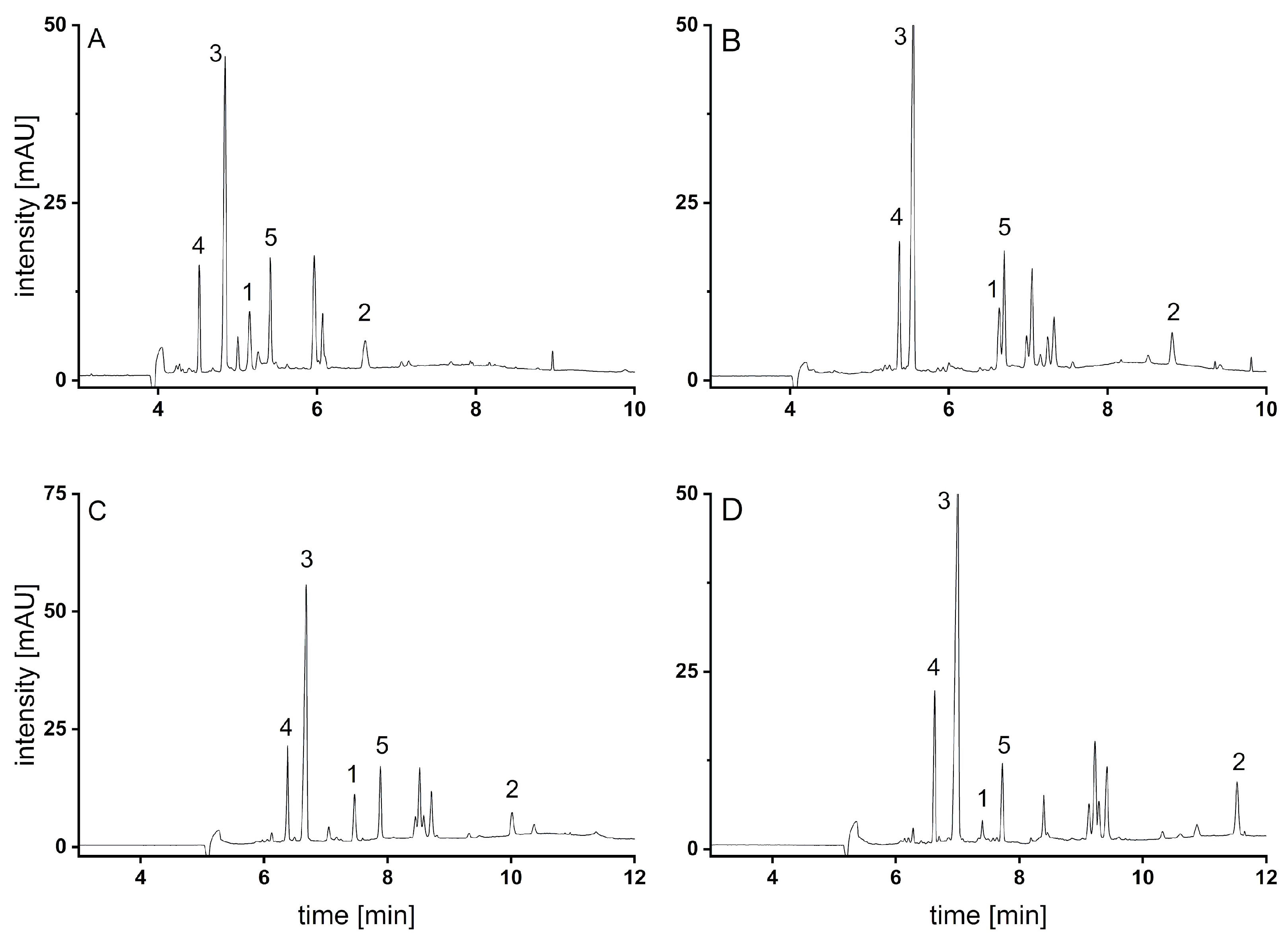
3.3. Analysis of Malus sp. Samples
3.4. Comparison of Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Strack, D. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Gufler, V.; Ngoc, H.N.; Stuppner, H.; Ganzera, M. Capillary electrophoresis as a fast and efficient alternative for the analysis of Urceola rosea leaf extracts. Fitoterapia 2018, 125, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scheuba, J.; Wronski, V.-K.; Rollinger, J.M.; Grienke, U. Fast and Green—CO2 Based Extraction, Isolation, and Quantification of Phenolic Styrax Constituents. Planta Med. 2017, 83, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- West, C. Current trends in supercritical fluid chromatography. Anal. Bioanal. Chem. 2018, 410, 6441–6457. [Google Scholar] [CrossRef]
- Riu, J.; Barceló, D. Chapter 17 Application of capillary electrophoresis in environmental analysis. Tech. Instrum. Anal. Chem. 2000, 21, 739–787. [Google Scholar] [CrossRef]
- Zwerger, M.; Ganzera, M. Analysis of boswellic acids in dietary supplements containing Indian frankincense (Boswellia serrata) by Supercritical Fluid Chromatography. J. Pharm. Biomed. Anal. 2021, 201, 114106. [Google Scholar] [CrossRef]
- Hartmann, A.; Ganzera, M. Supercritical Fluid Chromatography—Theoretical Background and Applications on Natural Products. Planta Med. 2015, 81, 1570–1581. [Google Scholar] [CrossRef]
- Eisath, N.G.; Sturm, S.; Stuppner, H. Supercritical Fluid Chromatography in Natural Product Analysis—An Update. Planta Med. 2017, 84, 361–371. [Google Scholar] [CrossRef]
- Rabanes, H.R.; Guidote, A.M., Jr.; Quirino, J.P. Capillary electrophoresis of natural products: Highlights of the last five years (2006–2010). Electrophoresis 2011, 33, 180–195. [Google Scholar] [CrossRef]
- Ganzera, M.; Nischang, I. The Use of Capillary Electrochromatography for Natural Product Analysis—Theoretical Background and Recent Applications. Curr. Org. Chem. 2010, 14, 1769–1780. [Google Scholar] [CrossRef]
- Li, S.F.Y. Capillary Electrophoresis: Principles, Practice and Applications; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands, 1992; Volume 52. [Google Scholar]
- Unger, M. Capillary Electrophoresis of Natural Products: Current Applications and Recent Advances. Planta Med. 2009, 75, 735–745. [Google Scholar] [CrossRef]
- Ganzera, M.; Krüger, A. Analysis of Natural Products by Capillary Electrophoresis and Related Techniques. Encycl. Anal. Chem. 2014, 1–30. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review. Molecules 2019, 24, 4468. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, X.; Lai, F.; Liang, T.; Wen, J.; Lin, W.; Qiu, J.; Liu, S.; Li, L. Trilobatin as an HIV-1 entry inhibitor targeting the HIV-1 Gp41 envelope. FEBS Lett. 2018, 592, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- de Bernonville, T.D.; Guyot, S.; Paulin, J.-P.; Gaucher, M.; Loufrani, L.; Henrion, D.; Derbré, S.; Guilet, D.; Richomme, P.; Dat, J.F.; et al. Dihydrochalcones: Implication in resistance to oxidative stress and bioactivities against advanced glycation end-products and vasoconstriction. Phytochemistry 2010, 71, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Hsu, F.-L.; Chen, C.-S.; Chern, J.-W.; Lee, M.-H. Constituents from the Formosan apple reduce tyrosinase activity in human epidermal melanocytes. Phytochemistry 2007, 68, 1189–1199. [Google Scholar] [CrossRef]
- Gaucher, M.; de Bernonville, T.D.; Lohou, D.; Guyot, S.; Guillemette, T.; Brisset, M.-N.; Dat, J.F. Histolocalization and physico-chemical characterization of dihydrochalcones: Insight into the role of apple major flavonoids. Phytochemistry 2013, 90, 78–89. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, C.; Zhang, C.; Cai, L.; Chen, H. The protective effect of phloretin in osteoarthritis: An in vitro and in vivo study. Food Funct. 2018, 9, 263–278. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical Basis of Anti-Cancer-Effects of Phloretin—A Natural Dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Hsieh, M.-J.; Yang, S.-F.; Chen, S.-P.; Tsai, W.-C.; Chen, P.-N. Phloretin suppresses metastasis by targeting protease and inhibits cancer stemness and angiogenesis in human cervical cancer cells. Phytomedicine 2019, 62, 152964. [Google Scholar] [CrossRef]
- Kim, U.; Kim, C.-Y.; Lee, J.M.; Oh, H.; Ryu, B.; Kim, J.; Park, J.-H. Phloretin Inhibits the Human Prostate Cancer Cells Through the Generation of Reactive Oxygen Species. Pathol. Oncol. Res. 2019, 26, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhou, N.; Mi, L.; Hu, Z.; Wang, L.; Liu, X.; Zhang, S. Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des. Dev. Ther. 2017, 11, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2011, 39, 5299–5306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, Z.; Wang, A.; Jia, L.; Zhang, X.; Fang, M.; Yi, K.; Li, Q.; Hu, H. Comparative oral and intravenous pharmacokinetics of phlorizin in rats having type 2 diabetes and in normal rats based on phase II metabolism. Food Funct. 2019, 10, 1582–1594. [Google Scholar] [CrossRef]
- Gutierrez, B.L.; Arro, J.; Zhong, G.-Y.; Brown, S.K. Linkage and association analysis of dihydrochalcones phloridzin, sieboldin, and trilobatin in Malus. Tree Genet. Genomes 2018, 14. [Google Scholar] [CrossRef]
- Bai, L.; Guo, S.; Liu, Q.; Cui, X.; Zhang, X.; Zhang, L.; Yang, X.; Hou, M.; Ho, C.-T.; Bai, N. Characterization of nine polyphenols in fruits of Malus pumila Mill by high-performance liquid chromatography. J. Food Drug Anal. 2016, 24, 293–298. [Google Scholar] [CrossRef]
- Petkovska, A.; Gjamovski, V.; Stanoeva, J.P.; Stefova, M. Characterization of the polyphenolic profiles of peel, flesh and leaves of Malus domestica cultivars using UHPLC-DAD-HESI-MSn. Nat. Prod. Commun. 2017, 12, 1934578X1701200111. [Google Scholar] [CrossRef]
- Walia, M.; Kumar, S.; Agnihotri, V.K. UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity. J. Sci. Food Agric. 2015, 96, 1440–1450. [Google Scholar] [CrossRef]
- Mayr, F.; Möller, G.; Garscha, U.; Fischer, J.; Castaño, P.R.; Inderbinen, S.G.; Temml, V.; Waltenberger, B.; Schwaiger, S.; Hartmann, R.W.; et al. Finding New Molecular Targets of Familiar Natural Products Using In Silico Target Prediction. Int. J. Mol. Sci. 2020, 21, 7102. [Google Scholar] [CrossRef] [PubMed]
- ICH Guide Q2 (R1). Validation of Analytical Procedures-Text and Methodology; International Conference on Harmonization: Geneva, Switzerland. 2005. Available online: https://www.ich.org/page/quality-guidelines (accessed on 12 December 2022).
- West, C.; Lemasson, E.; Bertin, S.; Hennig, P.; Lesellier, E. An improved classification of stationary phases for ultra-high performance supercritical fluid chromatography. J. Chromatogr. A 2016, 1440, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Y.; Zhang, F.; Liu, J.; Ren, Z.; Xu, Y.; Liu, T.; Zhou, W.; Li, H.; Zhang, C. An analytical strategy for accurate, rapid and sensitive quantitative analysis of isoflavones in traditional Chinese medicines using ultra-high performance supercritical fluid chromatography: Take Radix Puerariae as an example. J. Chromatogr. A 2019, 1606, 460385. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, J.; Zhao, Y.; Zheng, W.; Pang, X.; Wang, B.; Wang, J.; Li, Q.; Chen, X.; Zhang, J.; et al. Comprehensive analysis and quality assessment of Herba Epimedii from multiple botanical origins based on ultra-high performance supercritical fluid chromatography coupled with quadrupole time-of-flight mass spectrometry and photodiode array detector. J. Supercrit. Fluids 2019, 149, 1–9. [Google Scholar] [CrossRef]
- Zehrmann, N.; Zidorn, C.; Ganzera, M. Analysis of rare flavonoid C-glycosides in Celtis australis L. by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2010, 51, 1165–1168. [Google Scholar] [CrossRef]
- De Paepe, D.; Servaes, K.; Noten, B.; Diels, L.; De Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. An improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 2013, 136, 368–375. [Google Scholar] [CrossRef]
- Hasemann, P. Leistungsfähigkeit von MEKC-Methoden und Chiralen Trennungen Mittels Kapillarelektrophorese: Entwicklung, Validierung und Anwendung Kapillarelektrophoretischer Methoden. Ph.D. Thesis, TU Braunschweig, Braunschweig, Germany, 2011. [Google Scholar]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Waters, R.; Nazemipour, M.; Bland, M.; Altman, D.G. Bland-Altman methods for comparing methods of measurement and response to criticisms. Glob. Epidemiol. 2020, 3, 100045. [Google Scholar] [CrossRef]
- Xu, M.; Fralick, D.; Zheng, J.Z.; Wang, B.; Tu, X.M.; Feng, C. The Differences and Similarities Between Two-Sample T-Test and Paired T-Test. Shanghai Arch. Psychiatry 2017, 29, 184–188. [Google Scholar] [CrossRef]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Regression equation | y = 4517.7x + 923.76 y = 0.409x − 1.072 | y = 4034.1x − 12932 y = 0.708x + 2.433 | y = 2402.6x + 4736.6 y = 0.230x − 0.173 | y = 3057.4x − 9252.6 y = 0.226x − 0.103 | y = 2662.3x − 10703 y = 0.387x − 0.556 |
| R2 | 0.9998 0.9999 | 0.9999 0.9993 | 0.9994 0.9999 | 0.9999 0.9999 | 0.9999 0.9999 |
| LOD a | 0.49 0.98 | 0.49 0.98 | 0.98 0.98 | 0.98 0.98 | 0.98 0.98 |
| LOQ a | 1.47 2.94 | 1.47 2.94 | 2.94 2.94 | 2.94 2.94 | 2.94 2.94 |
| Precision | |||||
| intra-day b | 1.40 5.73 | - - | 1.65 3.61 | 2.02 5.15 | 2.59 5.12 |
| inter-day c | 1.65 3.78 | - - | 1.09 2.54 | 0.98 5.15 | 0.70 2.89 |
| Accuracy d | |||||
| high spike | 98.2 (0.50) 95.3 (0.84) | 97.7 (0.56) 100.6 (0.84) | 101.2 (0.97) 96.2 (1.37) | 101.5 (1.19) 95.4 (1.26) | 101.4 (0.89) 98.7 (1.38) |
| medium spike | 98.7 (0.66) 98.3(0.91) | 99.4 (1.15) 95.8 (1.12) | 100.6 (0.35) 97.3 (0.77) | 102.0 (0.32) 100.5 (1.36) | 102.3 (1.09) 96.7 (1.14) |
| low spike | 99.9 (0.50) 95.2 (0.87) | 97.0 (0.56) 98.6 (1.13) | 102.5 (0.40) 96.4 (0.96) | 101.4 (1.18) 96.9 (1.68) | 102.4 (0.89) 97.9 (0.84) |
| Sample | Malus-1 | Malus-2 | Malus-3 | Malus-4 | Malus-5 | Malus-6 | Malus-7 | |
|---|---|---|---|---|---|---|---|---|
| Compound | ||||||||
| 1 | - - | 0.10 (0.78) 0.14 (1.99) | - - | - - | 0.20 (0.30) 0.24 (2.98) | 0.08 (0.30) 0.13 (1.55) | - - | |
| 2 | 0.41 (0.72) 0.37 (1.42) | - - | - - | - - | - - | - - | - - | |
| 3 | - - | 9.53 (0.73) 9.50 (0.98) | 15.57 (0.89) 15.45 (2.78) | - - | 3.64 (0.89) 3.55 (2.54) | 6.44 (1.36) 6.29 (2.43) | 5.68 (1.50) 5.52 (1.51) | |
| 4 | 2.54 (0.79) 2.62 (1.22) | 1.24 (1.21) 1.43 (1.22) | - - | 2.66 (1.39) 2.77 (0.93) | 1.59 (0.99) 1.72 (2.93) | 0.64 (1.18) 0.59 (2.81) | 1.21 (0.85) 1.27 (1.57) | |
| 5 | 14.15 (0.21) 14.36 (0.50) | 2.41 (0.71) 2.58 (1.70) | - - | 7.30 (1.67) 7.44 (2.96) | Det. Det. | 0.40 (1.49) 0.38 (2.64) | 3.35 (0.61) 3.37 (1.35) | |
| Σ of DHCs | 17.09 17.39 | 13.28 13.65 | 15.57 15.45 | 9.96 10.21 | 5.43 5.51 | 7.56 7.39 | 10.24 10.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwerger, M.; Contratti, S.; Mariano, V.; Ganzera, M. SFC and CE—A Comparison of Two Orthogonal Methods for the Analysis of Dihydrochalcones in Apple Leaves. Separations 2023, 10, 239. https://doi.org/10.3390/separations10040239
Zwerger M, Contratti S, Mariano V, Ganzera M. SFC and CE—A Comparison of Two Orthogonal Methods for the Analysis of Dihydrochalcones in Apple Leaves. Separations. 2023; 10(4):239. https://doi.org/10.3390/separations10040239
Chicago/Turabian StyleZwerger, Michael, Sarah Contratti, Valentina Mariano, and Markus Ganzera. 2023. "SFC and CE—A Comparison of Two Orthogonal Methods for the Analysis of Dihydrochalcones in Apple Leaves" Separations 10, no. 4: 239. https://doi.org/10.3390/separations10040239
APA StyleZwerger, M., Contratti, S., Mariano, V., & Ganzera, M. (2023). SFC and CE—A Comparison of Two Orthogonal Methods for the Analysis of Dihydrochalcones in Apple Leaves. Separations, 10(4), 239. https://doi.org/10.3390/separations10040239






