Abstract
Moringa oleifera L. tree (Mo) has emerged as a rich alternative source of bioactive compounds to design cosmetic formulations. Supercritical carbon dioxide fluid extraction (SFE-CO2) was successfully applied on the screening of Mo seed, leaf, and root extracts. The extraction yield was evaluated by response surface methodology (RSM), for pressure and temperature ranges of 117–273 bar and 41–60 °C, respectively, using a design of experiments (DOE). The pressure significantly affected the results (), with the highest extraction efficiency obtained at conditions above 195 bar. The extracts’ composition, evaluated by gas chromatography-mass spectrometer (GC-MS), revealed an increasing correlation between the pressure, total extract solubility, and mass of extract at a constant temperature, due to the higher extraction yield. Seed extracts presented more than 80% of oleic acid in relative composition (8.04 mgcompound∙gplantpart−1). Leaf extracts performed well for the obtainment of linolenic acid (>20%; 3.10 mg∙g−1), nonacosane (>22%; 0.46 mg∙g−1), and α-tocopherol (>20%; 0.21 mg∙g−1). Mo root resulted in higher relative composition for sterol molecules, despite its very low affinity with CO2. The most promising bioactive compounds, oleic acid and α-tocopherol, were more abundant when operating at 250 bar at 45 °C and 195 bar at 55 °C, for Mo seed and leaf SFE-CO2 extracts, respectively.
1. Introduction
The properties of Moringa oleifera L. tree (Mo) as a traditional medicine treatment option for skin injuries, malnutrition, and other diseases have been well-known for centuries [1]. However, the scientific community only recently recognised Mo’s rich phytochemical composition and bioactive potential [1,2,3], resulting in increased use of its oils and extracts in commercial goods, such as cosmetic, pharmaceutical, therapeutical, and nutraceutical formulations [4,5,6].
Previous studies have shown that the different plant parts—leaf, root, and seed—present interesting but distinctive compositions and properties. For example, Mo leaves have revealed significant amounts of quercetin, kaempferol, myricetin, rutin, and lutein compounds, responsible for anti-inflammatory and antioxidant activities [3,7]. These flavonoid compounds extracted from Mo leaves have been shown to enhance the proliferation and viability of the cellular response to tissue injuries [4]. Additionally, the presence of isothiocyanate molecules indicates a pharmacological potential related to chronic inflammations [8], with diverse functional properties [9]. In vivo tests have shown the anti-inflammatory and antioxidant activities in cosmetic products using Mo leaf extracts as an ingredient. Male volunteers had skin hydration improved by up to 40%, avoiding more than 50% of the trans-epidermal water loss and preventing UV radiation effects after topical application of Mo active cream for a 12-week testing period [10]. Mo-based formulations also presented inflammatory response by reducing atopic dermatitis, triggered by proinflammatory cytokine-related mRNA cells (TNF-α, CCL17, IL-1β, IL-6) and mitogen-activated protein kinases (TNF-α/IFN-γ) in rat ears [11]. Due to the presence of condensed tannins and flavonoids, Mo root extracts presented a significant antioxidant ability, up to 70 times higher than the reference ascorbic acid, and thus provided potential for skin and anti-ageing products [12]. Other studies showed that Mo root extracts have an analgesic effect and a confirmed action on rheumatoid arthritis due to the presence of 1,3-dibenzyl urea and aurantiamide acetate compounds [13]. Moreover, a higher antifungal activity toward C. albicans was found in Mo root extracts compared to the commercial Nystatin ointment [14], as well as activity against S. aureus, B. subtilis and E. coli [12].
Fatty acids and sterols are generally found in the Mo seeds composition, predominantly oleic, palmitic, and behenic acids, but also brassicasterol, campesterol, and stigmasterol phytochemicals [15]. The lipidic fraction plays an important role as an active ingredient in topical products, providing high skin hydration and anti-ageing potential [15,16]. Sterols and fatty acids are functional compounds widely used to increase the antioxidant potential of skin care formulations, helping the cream spreadability and creating a barrier against environmental stressors such as UVB radiation [15,17]. Furthermore, Mo seed extracts have been shown to present antibiofilm, antioxidant, and anti-inflammatory effects due the presence of tocopherols and phenolic acid compounds [3,15,18].
The Mo benefits can be achieved by using the fresh plant or by applying extraction and concentration techniques. Conventional extraction methodologies, such as hydrodistillation [3], maceration [4], and Soxhlet [19], have been used to obtain Mo extracts; however, numerous drawbacks have been reported (e.g., thermal decomposition of target compounds, long extraction time, and residual toxic solvents in the extracts) [20,21]. A new generation of consumers and, thus, industry, have increasingly been urging the use of safe and environmentally friendly alternatives. The scientific community has pointed out green technologies as a solution to overcome both quality and environmental issues. The ‘green technology’ term can be ascribed to other extraction methods, such as pressurised hot-water extraction [22], microwave-assisted extraction [23], and ultrasound extraction [24]. These methods usually result in high total extraction yields.
Supercritical extraction using carbon dioxide (SFE-CO2) has been extensively used as an alternative green technology, resulting in extracts with higher compound selectivity, as the extraction yield of a specific compound or group of compounds can be enhanced by tuning the pressure and temperature. Supercritical CO2 presents diffusion coefficients similar to gases and low viscosities similar to liquids, resulting in a decrease in surface tension and an increase in the solvent penetration into the plant matrix [17]. The extraction ability of CO2, a non-polar solvent, can also be enhanced using a co-solvent such as ethanol. The mixture modifies the solvent characteristics and improves the polar molecules’ affinity [25].
Parameters such as plant material granulometry, extraction time, CO2 flow rate, and use of co-solvent are frequently considered in optimisation processes [26,27]. Recognised as a GRAS (Generally Recognised as Safe) process by the United States FDA (Food and Drug Administration) and European Union statements, SFE-CO2 has been previously applied to obtain Mo seed [26,27,28,29,30,31,32,33,34] and leaf extracts [25,35,36]. Moreover, statistical methodologies, namely the response surface methodology (RSM), were used to improve the extraction of the target compounds. RSM is a reliable mathematical tool to describe correlated and independent factors upon a response of interest [27]. However, no work has reported a full screening of the main Mo plant parts, which is helpful to identify promising compounds or a blend of compounds to incorporate in cosmetic products as an ingredient.
This study aims to assess SFE-CO2 extracts obtained from Mo leaves, seeds, and roots, using the RSM to define the best extraction conditions in terms of the extraction yield and phytochemical composition, in view of their potential use as skincare ingredients.
2. Materials and Methods
2.1. Chemicals
Carbon dioxide (CAS 124-38-9, food grade, 99%) was purchased from Air Liquid (Paris, France); n-hexane (CAS 110-54-3, 99%) and ethanol absolute (CAS 64-578-6, ≥99%) were obtained from Supelco (Madrid, Spain). The analytical standards for oleic acid (CAS 112-80-1, 99%), linolenic acid (CAS 463-40-1, ≥99%), nonacosane (CAS 630-03-5, 99%), and α-tocopherol (CAS 10191-41-0, 99%) were acquired from Sigma Aldrich (Madrid, Spain).
2.2. Plant Material
Mo plant material was supplied by Moringa del Sur (Malaga, Spain, moringadelsur.com, accessed on 12 January 2023). Leaves and roots were harvested in May and September of 2021, respectively, and seeds were collected during the harvesting season of 2020. Both aerial materials were received having been previously dried at room temperature, while the roots were submitted to freeze-drying (55C, CoolSafe, Beverwijk, The Netherlands) at −55 °C for 72 h. All samples were ground for 20 s (Hr7762/90 Mini Chopper, Philips Walita, Amsterdam, The Netherlands) and carefully sieved (D-42781, Retsch, Haan, Germany). The grinding of leaves and seeds resulted in small particles with granulometry between 0.50 and 0.70 mm. Root grinding resulted in a powder with a particle size below 0.50 mm.
2.3. Supercritical Fluid Extraction Using Carbon Dioxide
The extraction procedures were conducted on bench-scale SFE-CO2 equipment [37]. The pressurisation system included a CO2 cylinder up to 50 bar and an isocratic pump (JASCO PU-2080 Plus) with a cooled head, with a constant flow rate below 5 mL∙min−1. The pressured CO2 flowed, in continuous mode, to an extraction cell made of stainless steel with a capacity of 200 mL and designed to reach a pressure of 300 bar, with a temperature ranges from 40 to 80 °C. The system was also equipped with a pump (JASCO, PU-4180), optionally used to add ethanol as the co-solvent. The extractions were monitored using a pressure transducer and controlled by a back pressure regulator (BPR), working as a programmed set-point. The BPR and additional depressurisation valves were maintained at temperatures up to 90 °C by a resistance strip and controlled by a thermostat to avoid dry ice clogging caused by the free expansion of CO2, also known as the Joule-Thomson effect. Finally, the extracted compounds were collected in a single separator, kept at 5 °C and below 40 bar—it could work up to 100 bar—while the CO2 reduced to atmospheric pressure by two needle valves to be quantified using a mass flow meter.
In this work, the extraction conditions were set according to the limits of the equipment and the target molecules to be obtained: pressure and temperature range of 117 to 273 bar and 41 to 69 °C, respectively. The extractions were performed in continuous mode using a CO2 flow rate of 4 mL∙min−1 for 120 min, and 10 g of sample in each experiment. At the end of each extraction, the apparatus was cleaned with 200 mL of ethanol absolute, and CO2 was pumped for 15 min to recover the entire extracted material. Finally, the solvent was removed in a rotary vacuum evaporator (RE100B, Bibby Scientific Ltd., Stone, UK) at 40 °C, and the extracts were subjected to an air-drying process at the same temperature (Venticell, MMM Medcenter, Planegg, Germany) until reaching a constant weight. For storage and further analysis, the leaf and root extracts were then dissolved in 25 mL of ethanol, the seed products solubilised in 25 mL of n-hexane, and all extracts were stored at −20 °C.
The extraction yield, , (in dry weight) and the solubility of the extracts were, respectively, obtained by:
Additional isothermal properties of CO2 (see Table 1) were obtained in the online database NIST (National Institute of Standards and Technology) [38].

Table 1.
DOE procedure (22 + 2 central points, twoblocks) for Mo seed, leaf, and root SFE-CO2 extracts, and their thermodynamic properties.
2.4. Design of Experiments and Response Surface Analysis
A design of experiments (DOE) with 22 + 2 central points and twoblocks was applied to the extraction of each plant part, as shown in Table 1. The central point (0,0) was chosen as the mid-range pressure and temperature, while the two blocks were used to reduce the variations in the results. A second-order polynomial equation (quadratic model) was used to fit the experimental data obtained from DOE, expressed as:
where is the dependent/response variable (extraction yield), and and are the coefficient constants for the intercept, linear, quadratic, and interaction terms, respectively. and correspond to the independent variables, in this case, pressure and temperature, and ε is the experiment residual.
The independent variables are represented by the surface and contour plots, which reveal the optimum extraction condition.
The significant differences were assessed by ANOVA (analysis of variance) and Tukey tests, with (significance level; 95% confidence), and the RSM was evaluated using Statistica StatSoft (version 14, USA).
2.5. Chemical Composition Assays by GC-MS
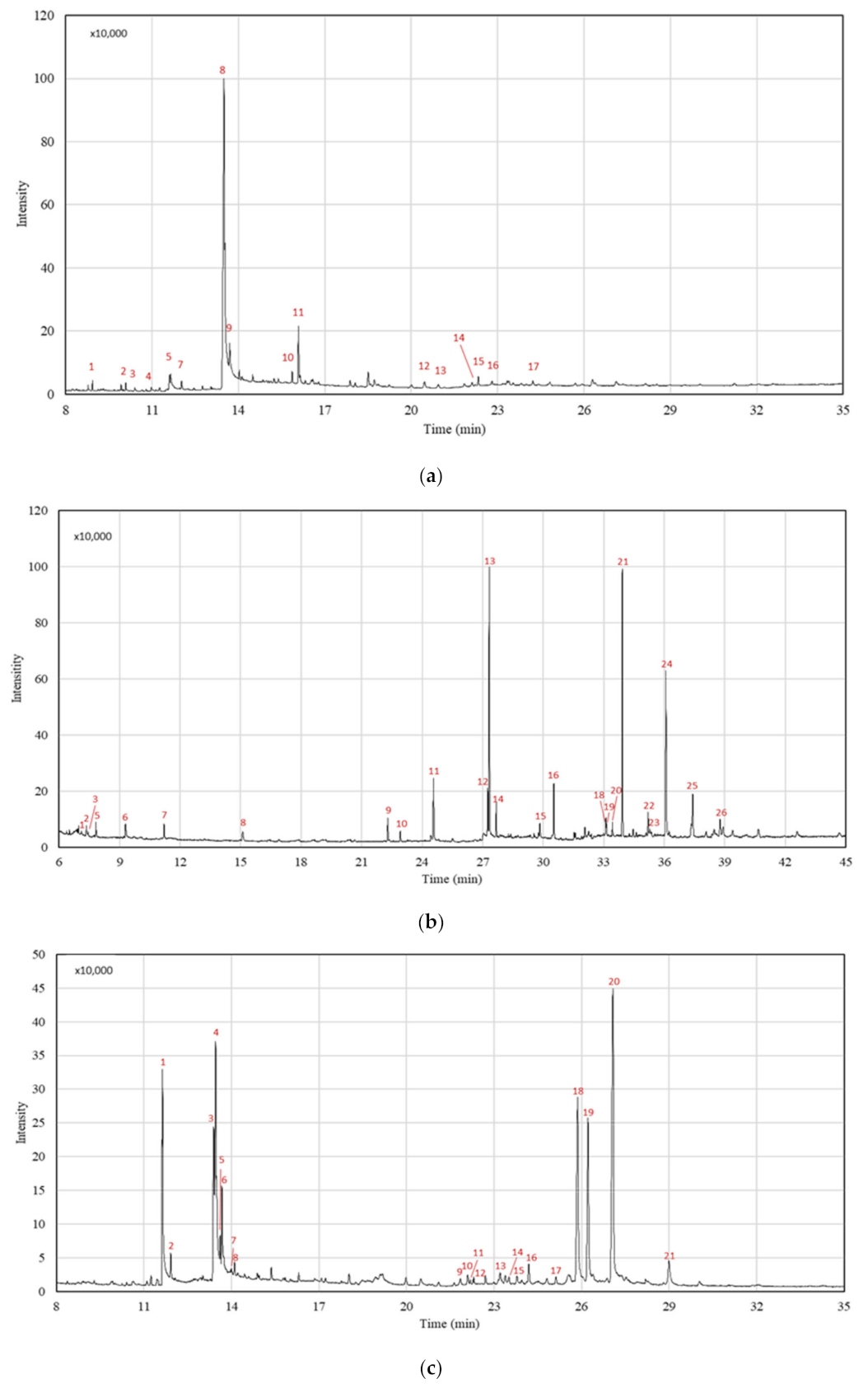
The volatile compounds of the two experiments achieving the highest extraction yield for each Mo plant part were assessed using gas chromatography (GC) (TQ8040 NX Triple Quadrupole, Shimadzu, Kyoto, Japan) (Table 1). Both central points were also analysed to validate the DOE procedure.
This equipment worked with an ion-trap mass spectrometer (MS), a splitless injector, an automatic sampler (AOC-20s+i), and a cross-bonded fused column (30 m × 0.25 mm, 0.25 μm film thickness) for low-polarity phases (Rxi-5Sil MS, Restek, Bellefonte, PA, USA). All samples were analysed in triplicate, and the volume of 1 μL was injected at 280 °C, carried out at 1 mL∙min−1 by ultrapure helium operating in linear velocity flow control mode. The mass scanning was set at m/z 40–500, and the ion and interface temperatures were kept at 250 °C and 260 °C, respectively.
The oven was isothermally optimised for each Mo plant part to guarantee ideal compound separation. The temperature programming for seed extracts comprised 40 °C for 1 min, then increased from 40 to 200 °C for 2 min at 20 °C∙min−1, 200 to 250 °C for 5 min at 10 °C∙min−1, and finally, 250 to 280 °C for 1 min at 20 °C∙min−1. Leaf and root extracts were analysed at 40 °C for 1 min, raised to 200 °C for 2 min at a rate of 7 °C∙min−1, followed by an increase to 250 °C for 2 min at 15 °C∙min−1, and then established at 280 °C for 1 min at 20 °C∙min−1.
Each molecule was identified as previously described by Kessler et al. [39]. Briefly, the mass spectra of each molecule were compared to those obtained in the database software from the National Institute of Standards and Technology (NIST 21, 27, 107, 147), and their respective linear retention indices (LRI) were calculated based on the Kovats retention index equation, by analysing a homologous series of alkanes (C8-C20) under the same chromatographic conditions. The selected target compounds were quantified through calibration curves of their respective analytical standards.
3. Results and Discussions
3.1. Extraction Yield of Mo SFE-CO2 Extracts
Table 1 shows the results of the ten experiments performed following the DOE. The extraction yields, , resulted in values between 0.10% and 3.38%, 0.22% and 1.83%, and 0.02% and 0.18% for seed, leaf, and root plant materials, respectively.
The data show the pressure and temperature influence on the extraction results. Higher pressures at constant temperature enhanced the CO2 density and increased the extraction yield. On the other hand, by keeping the pressure constant and increasing the temperature, there was a tendency for higher mass transfer resistance between the sample surface and the supercritical phase, reflected in a decrease in the extraction yield [26,35]. This behaviour was mainly noticed for seeds, whereas the leaves revealed the opposite effect at 250 °C. CO2 showed a higher ability to extract compounds from the Mo seed matrix, achieving a solubility of 786 μgext∙gCO2−1. The results may reflect the non-polar profile of the seed extract content and its affinity with the solvent [26,27,29].
The significant contribution of the pressure and temperature on the extractable amount of Mo biocompounds can be observed from the ANOVA results (Table 2) with . The highest extraction yields were obtained using pressures above 195 bar for all samples, and a similar increase was observed in their respective solubility values. The temperature influence was mainly noticed in the seed extractions. Temperatures below 55 °C promoted a significant increase in the extraction yields, explained by the solubility dominance of CO2 and its solvation power toward Mo seeds. For leaf and root extracts, no significant influence of the temperature alone was observed on the extraction yield; however, the correlation factors have shown some influence with .

Table 2.
Significant effects on SFE-CO2 experiments by ANOVA () for Mo seed, leaf, and root SFE-CO2 extracts.
Combining the data (Table 1 and Table 2), no crossover behaviour could be observed for the seed optimisation. The crossover results from an enhancement of extraction yield by increasing the temperature at constant pressure. Usually, this phenomenon occurs at pressures above 300 bar due to the balance between the CO2 density and the solute vapour pressure. Literature findings support the conclusions on the pressure range applied in this work [27,30,31].
The correlation between the density and compounds’ solubility can be observed by comparing experiments 7 and 8, both at 830 kg∙m−3 (Table 1). The extraction performed at 273 bar and 55 °C reached solubilities more than 1.7, 1.6, and 1.3 times higher than using 195 bar and 41 °C for seeds, leaves, and roots, respectively. This increase may correspond to the enhancement in the extracts’ solubility due to higher pressure and temperature [29]. These effects have also been indicated by the DOE results (Table 2), using 273 bar and 55 °C as the condition set to obtain the highest extraction yield of Mo plant parts by SFE-CO2. However, the extraction yield is not reflected in the efficiency to obtain target compounds useful for cosmetic applications, such as those belonging to the fatty acid, sterol, and tocopherol groups. Further discussion will address extraction yield and relative composition to define the best extraction condition for each sample material.
3.2. Optimisation Study of Mo SFE-CO2 Extracts by RSM
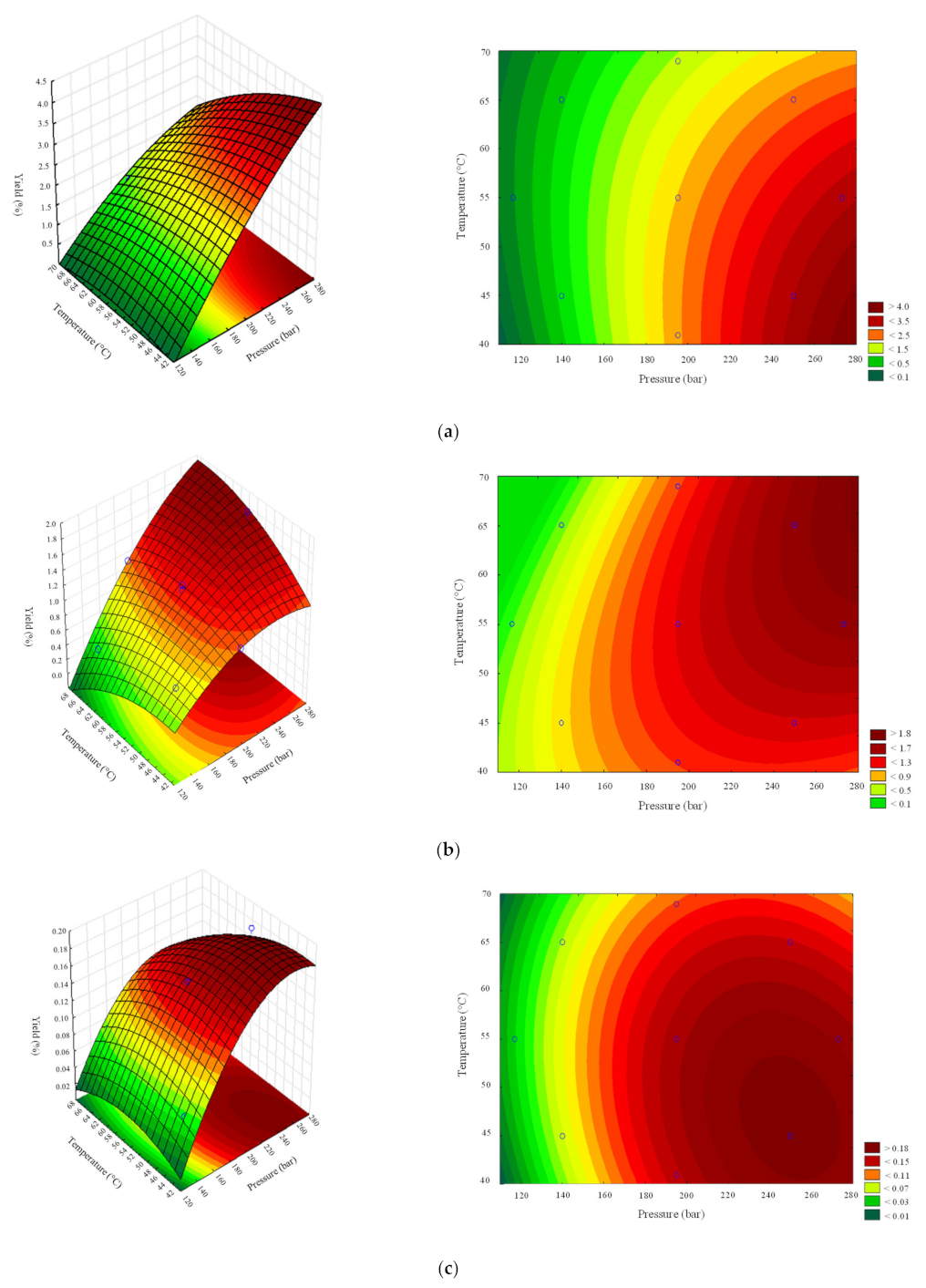
From the SFE-CO2 optimisation study, Figure 1 shows the RSM analysis obtained for each Mo part, seeds, leaves, and roots, for each experimental condition, where the empty circles represent the experimental data. Higher extraction yield has been shown to be achieved by increasing the pressure up to 273 bar for seed and leaves, with opposite responses regarding the temperature. Temperatures below 40 °C (Figure 1a) and above 55 °C (Figure 1b) have shown a tendency to improve the compounds’ solubility in CO2, respectively. Figure 1c evidences the range of pressures and temperatures applied in experiment E7 as the best condition to obtainroot’s extract.
Figure 1.
Response surface methodology (RSM) applied for (a) Mo seed, (b) leaf, and (c) root SFE-CO2 extracts, according to DOE analysis.
The following regression curves were obtained for each response surface model:
Table 2 exhibits the ANOVA analyses of the models considering the linear (L) and quadratic (Q) terms. A significant influence of pressure and temperature was observed on the seed extracts’ solubility, corroborating the previous results. The leaf extracts significantly differed in the pressure and correlated terms, while root SFE-CO2 extracts presented only a statistical influence due to the pressure. The statistical significance of each coefficient is indicated by p ≤ 0.05, and only the linear terms fit the response factor with significant influence. However, high F-value and low magnitude of p can also reveal influence on the coefficients (, , and ).
The predicted extraction yields () are shown in Table 1. All samples have presented errors below 1%, except the experiments carried out at 140 bar using Mo seeds. The conclusion is supported by the correlation between the observed and predicted data (Supplementary Material—Figure S1). The values fit well up to 99%, 98%, and 95% for seeds, leaves, and roots, respectively. Moreover, the three polynomial models showed MS residual , with 95% confidence. The quadratic model was previously chosen to describe the optimisation process of SFE-CO2 experiments using Mo seed samples by varying temperature, pressure, and particle size [29].
3.3. Chemical Profile of Mo SFE-CO2 Extracts Useful for Cosmetic Products
The most promising extracts obtained from Mo plant parts were characterised. The analysis criterion was based on the extraction yield, selecting one central point (E5) as a reference, and the two highest extraction yields in each optimisation process.
Figure 2 illustrates the chemical profile of Mo seed, leaf, and root SFE-CO2 extracts performed at 273 bar and 55 °C, for which the higher extraction yield was obtained. Table 3, Table 4 and Table 5 show the compound identification of Mo extracts for each plant part. Distinct chemical compositions were observed for the tested raw materials, requiring an individual interpretation of the extract potential as a cosmetic ingredient based on the identified compounds with reported interest in the field. The discussion focuses on the compounds with the most intense peaks, but also on target compounds in low relative concentrations.
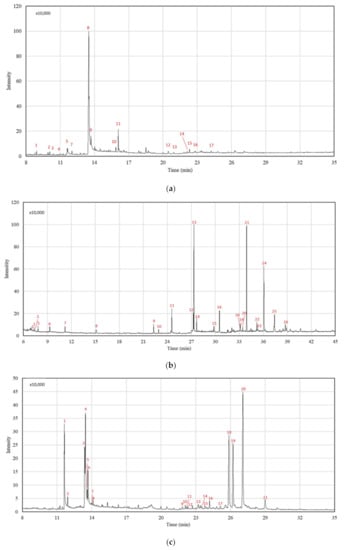

Table 3.
Chemical composition of SFE-CO2 extracts obtained from Mo seeds analysed by GC-MS.

Table 4.
Chemical composition of SFE-CO2 extracts obtained from Mo leaves analysed by GC-MS.

Table 5.
Chemical composition of SFE-CO2 extracts obtained from Mo roots and analysed by GC-MS.
3.3.1. Seed SFE-CO2 Extracts
GC-MS analyses of Mo seed extracts (experiments E3, E5 (CP) and E7) resulted in the identification of 17 compounds (Table 3, Figure 2a). Oleic acid is present with the highest relative concentration (82.04%) for all the tested experimental conditions, followed by its derivative oleic acid chloride (6.30–7.33%). These compounds belong to the monounsaturated fatty acid group (present with a total relative composition of 88.15–89.36%), and show antioxidant and emollient properties. Fatty acids are beneficial for reducing free radical skin production and preventing skin damage, such as photoaging. The effects of skin ageing and skin colour changes are observed due to reduced natural hydration (e.g., higher glycosylation process and lower sebum production) and increased melanin production, respectively [15]. Cosmetic formulations have included Mo seed oil to enrich their nourishing, moisturising, and lightening properties, as well as to maintain the natural skin pigmentation [15,40,41]. Stearic acid (2.33–2.43%), palmitic acid (1.54–2.11%), and methyl oleate compounds (0.29–0.32%) were also identified in Mo seed extracts, corroborating the extract’s potential for cosmetic applications, as these compounds have been shown to act as a functional barrier on the epidermal structure and skin formation [16]. Moreover, the absence of polyunsaturated fatty acids enhances the product’s thermal stability [40].
Hydrocarbons, with total relative composition of 2.66–3.90%, were also identified. Tetratriacontane and tetracosane were found with circa 1% of relative concentration, and 8-hexylpentadecane, heptadecane, and hexadecane at lower relative compositions. Hydrocarbon compounds present radical scavenging activity, i.e., antioxidant activity [2], while terpenes (phytol; <0.27%) and sterols (stigmasta-5,22-dien-3-β-ol, acetate and stigmast-5-en-ol, oleate; <0.82%) play a role in cosmetic applications as antibacterial, antioxidant, and anti-inflammatory active ingredients [17]. Studies have shown a contribution of phytosterols (sterols obtained from plants) regarding collagen synthesis. Collagen is a protein essential for skin formation and protection against environmental stressors, functioning as an anti-ageing response in topical application [16]. Other works have described the presence of various sterol molecules (e.g., stigmasterol, campesterol, β-sitosterol, and brassicasterol) in Mo seed extracts [15,26].
Finally, Table 3 shows that the chemical composition of seed SFE-CO2 extracts was slightly influenced by the tested extraction conditions.
3.3.2. Leaf SFE-CO2 Extracts
Experiments E4, E5 (CP), and E7 were selected for the GC-MS analyses. In total, 26 compounds were identified in Mo leaf SFE-CO2 extracts (Table 4, Figure 2b). Leaf extracts show the presence of similar chemical groups as seed extracts, but with significant differences in the compounds detected and their relative composition. The fatty acids group, with a total relative composition of 29.51–31.83%, include the linolenic (18.85–20.17%), palmitic (4.50–5.28%), and linoleic acids (3.28–3.47%). The higher fatty acid content in Mo leaf SFE-CO2 extracts obtained in this work compared to the other studies [25,36] may be a consequence of extraction conditions, environmental effects, and agriculture practices. The high unsaturated fatty acid content (linolenic and linoleic acids) makes the Mo leaf extracts a potential alternative for skin hydration and a protective barrier against pollution effects, mainly due to their antioxidant and antimicrobial activities [17].
Hydrocarbons are also present with a total relative composition of 34.46–36.73%, with the most relevant compound being nonacosane (17.74–22.89%). Other recent works on Mo SFE-CO2 extraction reported the presence of the nonacosane hydrocarbon varying from 19.10% [25] to 60.06% [36]. Nonacosane belongs to the waxy compound family located on the leaves’ surface, and shows reduced mass transfer resistance to the supercritical CO2 [36]. As a cosmetic ingredient, hydrocarbons have shown emollient activity and good spreadability on the skin surface due to their high interfacial tension with water, comparable to silicone derivatives [42]. In general, hydrocarbon-based products reduce the cosmetic product oiliness and intensify the freshness sensation, being the waxy ingredients used in lipstick, soft creams, and soaps [17,42]. Other hydrocarbons were also identified in Mo leaf extracts: heptacosane (10.04–11.73%), 8-hexylpentadecane (3.55–3.75%), tetradecane (1.31–1.46%), dodecane (0.93–1.26%), and squalane (0.37–0.52%).
Minor relative compositions were obtained for terpenoids (3.76–4.56%) and aldehydes molecules (3.37–4.09%). The monoterpenes α-pinene, p-cymene and D-limonene could be identified in the initial time analysis, followed by the diterpene cis-phytol and, finally, the triterpene β-amyrone. Usually, terpenes are incorporated into cosmetic products as essential oils to improve the odour and flavour characteristics [17]. As active ingredients, terpene compounds show antimicrobial, anti-inflammatory, and antioxidant activities regarding their nature and synergy with different molecules [25]. Their importance extends to the synthesis of aldehydes. Aldehydes are formed by the oxidation of terpenes and improve the antioxidant potential [17]. 2,4-Nonadienal, octanal, nonanal, cis, cis, cis-7, 10, 13-Hexadecatrienal, and pentadecanal were the aldehydes identified in Mo leaf extracts.
Finally, and very significantly, compounds on the tocopherols group are present in the Mo leaf extracts, namely α-tocopherol (16.41–21.51%) and γ-tocopherol (0.27–0.36%). Previous works on SFE-CO2 extractions also confirm the predominance of α-tocopherol in Mo leaf extracts [25,36], and this compound has been recognised for its benefits against skin oxidative stress (e.g., photoaging) [15]. The improvement in the epidermis appearance can be explained by the high antioxidant activity of the compound, higher than that observed in tocopherol isomers such as γ-tocopherol [25]. The highest values of α-tocopherol were found for experiments E5 and E7 (21.51% and 17.49%, respectively), both accomplished at 55 °C, and correlated to the negative influence of the temperature in experiment E4 (16.41%), carried out at 65 °C. This result may reflect that the solubility of α-tocopherol in CO2 decreases with increasing temperature below the crossover behaviour, usually observed at p > 300 bar [43].
3.3.3. Root SFE-CO2 Extracts
For the root SFE-CO2 extracts, 21 compounds were identified, mostly in the fatty acids (seven molecules, 25.70–36.11%) and sterols (nine molecules; 60.54–68.87%) (Table 5, Figure 2c). Experiment E5 showed the highest selectivity regarding the main compounds: γ-sitosterol (27.68%), campesterol (19.61%), and stigmasterol (14.08%). Previous works, using different solvent extraction methods, reported a higher overall number of identified compounds, but with lower selectivity for the main sterol compounds. Agboke and Attama (2016) [44], using cold hexane as solvent, identified 45 molecules, among which stigmasterol and campesterol reached 4.44% and 3.46%, respectively. The same behaviour can be observed for petroleum ether and dichloromethane extracts, which resulted in 39 and 63 compounds, respectively [45], but with a lower relative concentration of sterols such as campesterol (6.97%).
The highest relative concentration of fatty acids (36.11%) was obtained at the highest pressure, mainly due to the contribution of oleic acid (12.30%) and ethyl palmitate (8.08%). Although scarce, in previous works on Mo root phytochemicals, the presence of palmitic and linoleic acids has also been reported [44].
3.4. Selection of the Most Promising Extracts for Further Studies
Mo seed, leaf, and root were subjected to SFE-CO2 extraction, with the application of a DOE optimisation methodology (Table 1). Three experiments were selected from each plant part using the extraction yield criteria. The extracts’ characterisation was accomplished by GC-MS analysis (Table 3, Table 4 and Table 5), and more than six classes of molecules were identified. A high relative concentration of oleic acid, α-tocopherol, and γ-sitosterol were identified for seed, leaf, and root extracts, respectively. These compounds have been related to promising bioactivities, being useful to improve fine chemistry formulations, such as cosmetics. In addition, green solutions have been applied to extract natural ingredients from plants, therefore increasing their market value. Literature findings highlight extracts obtained by SFE-CO2 technology, mainly due to their highly appreciated characteristics for cosmetic applications (e.g., safe and thermal stable extracts) [17].
The previous results and analyses in this work suggest a low affinity between supercritical CO2 and root phytochemicals, reflected in the low extraction yield. Therefore, further mass quantification was limited to the Mo seed and leaf target main compounds, i.e., oleic acid, linolenic acid, nonacosane, and α-tocopherol (Table 6). Both central points did not present significant differences between them (data not shown), reflecting on the reliability of the data.

Table 6.
Mass composition of target compounds obtained by SFE-CO2 from Mo seed and leaf.
At 195 bar and 55 °C (E5, CP1), Mo seed extract reached 4.77 mg∙g−1 of oleic acid. The experiment resulted in a significant difference () compared to E3 and E7 (7.28 mg∙g−1 and 8.04 mg∙g−1, respectively). High pressures have indicated a higher solvation effect on oleic acid extraction, relating to the solubility of oleic acid in CO2 [46]. Increases in oleic acid solubility have been associated with higher CO2 densities. At the range of pressures and temperatures tested in this study, oleic acid presented solubility values from 0.18 to 43 g∙kg−1 of CO2 [46].
Linolenic acid (3.10 mg∙g−1) and nonacosane (0.46 mg∙g−1), identified in Mo leaf extract, also presented the highest extraction yield at 273 bar, with significant differences compared to E5. However, a distinct behaviour could be observed for the α-tocopherol mass composition. A significantly lower amount was obtained at 250 bar and 65 °C (E4) compared to the E5 and E7 experiments, both performed at 55 °C. The quantified values confirm the negative effect of high temperatures, possible correlated to the thermal degradation process and lower α-tocopherol solubility in CO2 [43]. No statistical differences were noticed between the E5 (central point) and E7, although the highest α-tocopherol mass composition (0.21 mg∙g−1) obtained at a higher pressure condition. According to the extraction conditions applied in this work, the solubility of α-tocopherol in supercritical CO2 was between 0.13 μg∙kg−1 and 1.83 μg∙kg−1 of CO2 [43].
Therefore, the mass composition and statistical analyses of oleic acid and α-tocopherol extracted by SFE-CO2 will be considered for further studies. The compounds have shown the best statistical efficiency for E3 (250 bar, 45 °C) and E5 (195 bar, 55 °C), using Mo seed and leaf samples, respectively.
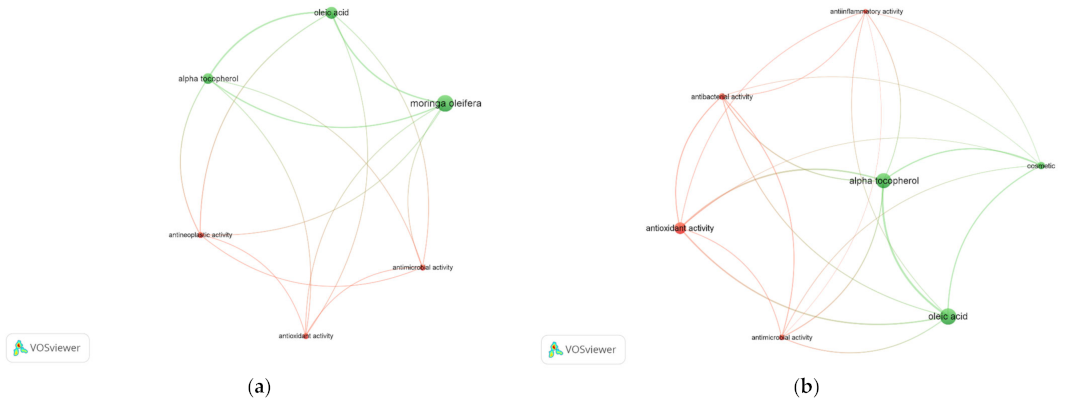
The potential use of the two target compounds as cosmetic ingredients were also investigated by correlating the compounds and bioactivities. The online Scopus database was used to export the literature occurrences of the selected keywords: Mo, oleic acid, and α-tocopherol (Figure 3a), as well as cosmetic, oleic acid, and α-tocopherol (Figure 3b). In both cases, the analyses resulted in two different clusters, suggesting a higher correlation among Mo, oleic acid, and α-tocopherol, although all connected to the antimicrobial, antioxidant, and antineoplastic activities. Cosmetic keyword has also evidenced the antioxidant capacity over the presence of the target compounds, increasing the prospect of using Mo extracts as ingredients for topic applications.
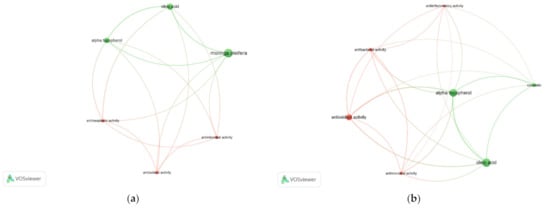
Figure 3.
Correlation among the relevant compounds—oleic acid, α-tocopherol—to use as cosmetic ingredients, identified in Mo seed and leaf SFE-CO2 extracts, and their bioactivities reported in scientific works during 2000 to 2022 (obtained on free online Scopus database, October 2022). Keywords: (a) Moringa oleifera, oleic acid, α-tocopherol, and (b) cosmetic, oleic acid, α-tocopherol. Clusters are represented by different colours using selected keywords in, at least, five occurrences. Larger circles represent higher keyword occurrences. Thicker lines represent higher correlation among keywords. Figure created in VOSviewer (version 1.6.18, Leiden, The Netherlands).
4. Conclusions
In this work, extracts obtained from the leaves, seeds, and roots of Moringa oleifera L. tree, using supercritical fluid extraction with CO2 as solvent, were studied as potential cosmetic ingredients. The extraction resulted in yields between 0.12–3.38%, 0.22–1.83%, and 0.02–0.18%, respectively. Seed extracts showed the presence of oleic acid, while the leaves presented a rich composition in linolenic acid, nonacosane, and α-tocopherol, and the roots were found to be composed mainly of γ-sitosterol, campesterol, and stigmasterol. The RSM optimisation revealed 250 bar at 45 °C and 195 bar at 55 °C as the optimal extraction conditions to improve the richness of the target compounds in the respective extracts—oleic acid in seeds and α-tocopherol in leaves—and the pressure significantly influenced the results. Moreover, temperature increase reduced the solvation power of CO2 upon the total extraction yield from seeds and the α-tocopherol content from the leaf extract. The results also suggested a low solubility of the recovered compounds regarding the supercritical CO2, mainly represented by sterol molecules. Therefore, the optimised Mo seed and leaf SFE-CO2 extracts have been selected for further studies as ingredients for cosmetic purposes, since they present a set of biocompounds useful to ensure photoprotective, moisturising, and skin-lightening properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10030210/s1, Figure S1: predicted and observed data fit for SFE-CO2 extracts obtained from Mo (a) seed, (b) leaf, and (c) root samples.
Author Contributions
Conceptualisation, J.C.K., Y.A.M., M.M.D. and M.F.B.; methodology, J.C.K., Y.A.M. and I.M.M.; validation, M.M.D., A.E.R. and M.F.B.; formal analysis, M.M.D., A.E.R. and M.F.B.; investigation, J.C.K., Y.A.M. and I.M.M.; writing—original draft preparation, J.C.K. and M.M.D.; writing—review and editing, I.M.M., M.M.D., A.E.R. and M.F.B.; supervision, Y.A.M., M.M.D. and M.F.B.; funding acquisition, M.M.D., A.E.R. and M.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by LA/P/0045/2020 (ALiCE), UIDB/50020/2020 and UIDP/50020/2020 (LSRE-LCM), UIDB/00690/2020 and UIDP/00690/2020 (CIMO) and LA/P/0007/2021 (SusTEC), funded by national funds through the Fundação para a Ciência e Tecnologia FCT/MCTES (PIDDAC). Júlia Cristiê Kessler acknowledges her PhD scholarship (ref. 2020.06656.BD) from FCT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank Moringa del Sur (Malaga, Spain, moringadelsur.com) for providing samples of Mo leaves, seeds, and roots.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.K.O.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications—A critical review. Crit. Rev. Biotechnol. 2021, 42, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Marrufo, T.; Nazzaro, F.; Mancini, E.; Fratianni, F.; Coppola, R.; De Martino, L.; Agostinho, A.B.; De Feo, V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. cultivated in Mozambique. Molecules 2013, 18, 10989–11000. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In Vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa Oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Fernandes, A.; Bancessi, A.; Pinela, J.; Dias, M.I.; Liberal, A.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.C.F.R.; et al. Nutritional and phytochemical profiles and biological activities of Moringa oleifera Lam. edible parts from Guinea-Bissau (West Africa). Food Chem. 2020, 341, 128229. [Google Scholar]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Rached, W.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Detailed chemical composition and functional properties of Ammodaucus leucotrichus Cross. & Dur. and Moringa oleifera Lamarck. J. Funct. Foods 2019, 53, 237–247. [Google Scholar]
- Ali, A.; Naveed, A.; Khan, M.S.; Rasool, F.; Iqbal, F.M.; Khan, M.T.; Din, M.U.; Elahi, E. Moisturizing effect of cream containing Moringa oleifera (Sohajana) leaf extract by biophysical techniques: In vivo evaluation. J. Med. Plant Res. 2013, 7, 386–391. [Google Scholar]
- Choi, E.J.; Debnath, T.; Tang, Y.; Ryu, Y.B.; Moon, S.H.; Kim, E.K. Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed. Pharmacother. 2016, 84, 870–877. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ndhlala, A.R.; Ncube, B.; Abdelgadir, H.A.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. S. Afr. J. Bot. 2020, 129, 106–112. [Google Scholar] [CrossRef]
- Pachauri, S.D.; Khandelwal, K.; Singh, S.P.; Sashidhara, K.V.; Dwivedi, A.K. HPLC method for identification and quantification of two potential anti-inflammatory and analgesic agents-1, 3-dibenzyl urea and aurantiamide acetate in the roots of Moringa oleifera. Med. Chem. Res. 2013, 22, 5284–5289. [Google Scholar] [CrossRef]
- Adeleye, S.A.; Braide, W.; Ibegbulem, C.R.; Nwigwe, V.N.; Ajunwa, O.M.; Korie, M.C. Phytochemistry and antifungal activity of root and seed extracts of Moringa oleifera. Int. J. Adv. Res. Biol. Sci. 2018, 5, 169–176. [Google Scholar]
- Athikomkulchai, S.; Tunit, P.; Tadtong, S.; Jantrawut, P.; Sommano, S.R.; Chittasupho, C. Moringa oleifera seed oil formulation physical stability and chemical constituents for enhancing skin hydration and antioxidant activity. Cosmetics 2021, 8, 2. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J. Modulating effect of fatty acids and sterols on skin aging. J. Funct. Foods 2019, 57, 135–140. [Google Scholar] [CrossRef]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 extracts in cosmetic industry: Current status and future perspectives. Sustain. Chem. Pharm. 2022, 27, 100688. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control. 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Bhutada, P.R.; Jadhav, A.J.; Pinjari, D.V.; Nemade, P.R.; Jain, R.D. Solvent assisted extraction of oil from Moringa oleifera Lam. seeds. Ind. Crops Prod. 2016, 82, 74–80. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Chemat, F.; Abert, V.M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Matshediso, P.G.; Cukrowska, E.; Chimuka, L. Development of pressurised hot water extraction (PHWE) for essential compounds from Moringa oleifera leaf extracts. Food Chem. 2015, 172, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibáñez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, Y.; Yang, R.; Liu, X.; Yang, Q.; Qin, X. The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind. Crops Prod. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- da Silva, M.; Trancoso, J.; Tormen, L.; Bombardelli, M.M.; Corazza, M.L.; Bainy, E.M. Extraction of compounds from Moringa oleifera leaves using supercritical CO2 plus ethanol as a cosolvent. J. Food Process. Eng. 2022, 45, e13979. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Siebenhandl-Ehn, S.; Schreiner, M.; Petrasch, A.M. Pilot-scale supercritical carbon dioxide extraction, physico-chemical properties and profile characterization of Moringa oleifera seed oil in comparison with conventional extraction methods. Ind. Crops Prod. 2014, 58, 68–77. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. A parametric study of supercritical carbon dioxide extraction of oil from Moringa oleifera seeds using a response surface methodology. SePurif. Techn. 2013, 113, 9–17. [Google Scholar] [CrossRef]
- Ngamprasertsith, S.; Sukaead, W.; Camy, S.; Condoret, J.S.; Sawangkeaw, R. Recovery of Moringa oleifera oil from seed cake by supercritical carbon dioxide extraction. Eng. J. 2021, 25, 67–74. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Gaspillo, P.D.; Maridable, J.B.; Malaluan, R.M.; Hinode, H.; Salim, C.; Huynh, H.K.P. Extraction of oil from Moringa oleifera kernels using supercritical carbon dioxide with ethanol for pretreatment: Optimization of the extraction process. Chem. Eng. Process. 2011, 50, 1207–1213. [Google Scholar] [CrossRef]
- Rai, A.; Mohanty, B.; Bhargava, R. Experimental modeling and simulation of supercritical fluid extraction of Moringa oleifera seed oil by carbon dioxide. Chem. Eng. Commun. 2017, 204, 957–964. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. An experimental investigation into the solubility of Moringa oleifera oil in supercritical carbon dioxide. J. Food Eng. 2014, 138, 1–10. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Petrasch, A. Oxidative susceptibility and thermal properties of Moringa Oliefera seed oil obtained by pilot-scale subcritical and supercritical carbon dioxide extraction. J. Food Process. Eng. 2016, 39, 226–236. [Google Scholar] [CrossRef]
- Dinesha, B.L.; Udaykumar, N.; Ramachandra, C.T.; Naik, N.; Sankalpa, K.B. Effect of supercritical carbon dioxide conditions on extraction of food phytochemical constituents from Moringa oleifera. Lam seed kernels. Int. J. Food Ferment. Technol. 2016, 6, 8. [Google Scholar] [CrossRef]
- Dinesha, B.L.; Udaykumar, N.; Ramachandra, C.T.; Naik, N.; Hugar, A. Optimization of supercritical extraction process for Moringa (PKM-1) seed kernel oil. Int. J. Agric. Sci. 2015, 5, 95–102. [Google Scholar]
- Rodríguez-Pérez, C.; Mendiola, J.A.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green downstream processing using supercritical carbon dioxide, CO2-expanded ethanol and pressurized hot water extractions for recovering bioactive compounds from Moringa oleifera leaves. J. Supercrit. Fluids 2016, 116, 90–100. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. SePurif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Manrique, Y.J.A. Supercritical Fluid Extraction and Fractionation of Bioactive Natural Products from Cork. Ph.D. Dissertation, Chemical Engineering, Faculty of Engineering of the University of Porto, Porto, Portugal, 2017. [Google Scholar]
- NIST—Isothermal Properties for Carbon Dioxide. Available online: https://webbook.nist.gov/ (accessed on 26 June 2022).
- Kessler, J.C.; Vieira, V.; Martins, I.M.; Manrique, Y.A.; Ferreira, P.C.; Calhelha, R.C.; Afonso, A.; Barros, L.; Rodrigues, A.E.; Dias, M.M. Chemical and organoleptic properties of bread enriched with Rosmarinus officinalis L.: The potential of natural extracts obtained through green extraction methodologies as food ingredients. Food Chem. 2022, 384, 132514. [Google Scholar] [CrossRef]
- Meireles, D.; Gomes, J.; Lopes, L.; Hinzmann, M.; Machado, J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Trad. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Zeitoun, H.; Michael-Jubeli, R.; El Khoury, R.; Baillet-Guffroy, A.; Tfayli, A.; Salameh, D.; Lteif, R. Skin lightening effect of natural extracts coming from Senegal botanical biodiversity. Int. J. Dermat. 2020, 59, 178–183. [Google Scholar] [CrossRef]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Len, C.; Pezron, I.; Luart, D.; van Hecke, E. Emollients for cosmetic formulations: Towards relationships between physico-chemical properties and sensory perceptions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Puah, C.W.; Choo, Y.M.; Ma, A.N.; Chuah, C.H. Solubility of tocopherol and tocotrienols from palm oil in supercritical carbon dioxide. J. Food Lipids 2007, 14, 377–385. [Google Scholar] [CrossRef]
- Agboke, A.; Attama, A. Bioactive components and antimicrobial activities of n-hexane extract of Moringa oleifera root bark on clinical isolates methicilin resistant Staphylococcus aureus. IJCRCPS 2016, 3, 1–9. [Google Scholar]
- Faizi, S.; Sumbul, S.; Versiani, M.A.; Saleem, R.; Sana, A.; Siddiqui, H. GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac. J. TroBiomed. 2014, 4, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Garmus, T.T.; de Oliveira, G.N.A.; Rammazzina Filho, W.A.; Queiroga, C.L.; Cabral, F.A. Solubility of oleic acid, triacylglycerol and their mixtures in supercritical carbon dioxide and thermodynamic modeling of phase equilibrium. J. Supercrit. Fluids 2019, 143, 275–285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).