Abstract
The dyeing industry uses many chemicals and dyes. After the dying process is completed, they release a significant amount of dyes in wastewater. The dyes’ color emissions are extremely poisonous and dangerous for aquatic and terrestrial life. Due to the toxic nature of dyes, the current study was carried out to evaluate whether it would be effective to employ an adsorption procedure with leaves from the Adiantum capillus-veneris plant as an adsorbent to remove commonly used textile dyes from an aqueous dye solution and wastewater. The effect of pH, concentration, time and the adsorbent dose on the adsorption process was studied in order to determine the maximum adsorption under ideal conditions. The selected pH was 3; the optimum concentration was 30 ppm with a contact time of 90 min and the optimized adsorbent dose was 60 mg. The absorbent under study showed excellent results when compared with commercial adsorbents i.e., animal charcoal and silica gel. The leaves of the Adiantum capillus-veneris plant revealed a maximum removal of 90.36 percent crystal violet dye (adsorption capacity (Qe) 9.05 mg/g) without any treatment to activate or alter the surface chemistry of the biosorbent. Its effectiveness was also tested with water gathered from several sources, including canal water, tap water, distilled water, and saline water, to determine whether it was practical. In both the canal and the tap water, the adsorbent displayed good removal efficiency. From the results of the current study, it can be inferred that the leaves of the Adiantum capillus-veneris plant are a reasonably priced biosorbent that can be used to remove toxic dyes from wastewater to protect water bodies from toxic pollution and can be used to treat industrial wastewater directly.
1. Introduction
In the current day, water is one of the most in-demand and scarce resources. The health of people and the sustainability of the environment are now seriously at risk due to water body pollution brought on by discharges from residential, commercial, and industrial wastewater [1]. Food safety, human health, and soil and water pollution are all impacted by industrial and agricultural activities that emit dangerous chemicals into the environment [2]. Organic and inorganic materials are the most prevalent types of contaminants in aquatic environments. Organic compounds, such as volatile solvents, dyes, and other chemicals, make up the majority of industrial effluents [3]. The industries where dyes are most frequently used include textile, paper, plastic, food, leather, and paint [4]. The synthetic dye business produces more than 7∙105 tons worldwide each year, while the textile sector uses more than 10,000 different dyes. Up to 200,000 tons of these dyes are released into effluents each day as a result of improper dyeing methods. Triphenylmethane cationic dyes, the largest family of synthetic dyes, are used only to color proteinaceous fibers [5]. These dye molecules have complicated structures that make them difficult to degrade and refractory, which results in a decreased removal rate during primary and secondary wastewater treatment. Their discharge into the environment leads to severe environmental pollution [6,7]. In addition to harming the aquatic ecosystem, waste dyes that build up in water sources like rivers, lakes, or seas also have a negative impact on human health [8].
Malachite green and crystal violet are two of the triphenylmethane dyes that have been extensively used in the textile, food, aquaculture, and pharmaceutical industries. However, crystal violet and malachite green are exceedingly carcinogenic to a wide variety of organisms due to the existence of an aromatic chromophore group made up of delocalized p electrons, which causes biomagnification. It is very difficult to recycle and reuse industrial wastewater contaminated with such dyes, especially crystal violet dye. Due to its highly toxic nature and stable structure, it is extremely necessary to remove crystal violet from wastewater prior to its discharge to water bodies, and therefore, many researchers are working on the removal of crystal violet from wastewater using proper biosorbents [9,10,11,12,13,14,15,16,17]. For the purpose of removing Crystal Violet (CV) dye, the potential of powdered water hyacinth roots was investigated. At 500 ppm dye, 7.8 pH, and 27 °C, the biosorbent adsorbed 90% of CV at a dosage of 1.5 g L−1 [18]. In batch research, the leaf powder of Clerodendrum fragrans was used as a biosorbent to eliminate CV dye from textile effluent. At 19.44 ppm of CV, 30.9 °C, a solution pH of 5.03, and a biosorbent dosage of 1.27 g, the maximum removal efficiency of 96.72% was achieved [19]. Inactive biomass from Diaporthe schini was an intriguing biosorbent for colored effluents, with an effectiveness of 87% in the decolorization of a CV dye house effluent [14]. Red seaweed treated with citric acid showed a maximum CV biosorption performance of 93.40% from water under pH 7, 1.5 g L−1 of biosorbent, 20 ppm of CV concentration, and 90 min at 20 °C [20]. Crystal Violet Dye (CVD) removal from an artificial aqueous solution was investigated on the marine diatom alga Skeletonema costatum. The greatest adsorption efficiency (98%) was identified at 0.4 g of S. costatum, pH 3, 120 min of contact time, and 25 °C [21]. Modified rice bran (MRB) was investigated for its capacity to remove crystal violet (CV) from industrial wastewater. Chlorosulfonic acid was used for the modification of rice bran. At adsorbent dosage of 2 g L−1, initial CV concentration of 100 ppm, and contact time of 42.75 min, the highest removal effectiveness of 97.4% was achieved [22]. Maximum biosorption was produced by Punica granatum shell (PGS) in 2 h at 25 °C and a pH range of 5 to 10 by using 0.6 g of biosorbent [23].The most popular wastewater treatment techniques include membrane filtration, precipitation, flocculation, coagulation, biosorption, and electrochemical approaches [9,24]. These technologies don’t appear to be particularly effective or advantageous financially.
One of the most effective techniques for removing pollutants from wastewater is the biosorption process. It has been discovered that the design and efficient operation of the biosorption process is efficient and affordable. The biosorption approach, which has great selectivity and is simple to apply at large scales and low concentrations as is the case with textile effluents, appears to be effective at removing a variety of colors [25]. The materials to be employed as adsorbents are typically inexpensive and readily available, and they can be helpful to effluents having a mixture of dyes and have an easy subsequent separation. As a result, it is low-cost effective. Decolorizing textile effluent has been accomplished by using biosorbents made of carbonaceous materials (wood, rice), raw agricultural solid wastes (waste-orange-peels) [26] industrial solid waste (fly ash), wheat straw, apple pomace, natural materials (zeolites, silica), sugarcane bagasse, and biological materials (fungi, bacteria, yeast, and algae). Due to the importance of the topic, in this research work, we used a low-cost biosorbent i.e., powdered dried leaves of Adiantum capillus-veneris plant for the removal of crystal violet dye from aqueous medium without any treatment to activate or modify the surface of the biosorbent. The absorbent also showed good removal efficiency for crystal violet in the river and saline water and the performance is comparable with the commercially available adsorbents which need processing for their preparation and are expensive. Thus, the proposed adsorbent can be used to treat actual wastewater on a large scale without requiring any chemical processing to activate or modify the adsorbent, making the process affordable, straightforward, and efficient. Using the proposed bio-adsorbent also allows for faster wastewater treatment.
2. Materials and Methods
2.1. Materials
2.1.1. Adsorbent
In this study, leaves of Adiantum capillus-veneris plant were used as an adsorbent for the adsorption process to remove crystal violet dye from an aqueous solution (Figure 1a,b). Another part of this plant i.e., stem was also tested as an adsorbent; however, the leaves have shown the most promising results. Thus, the leaves have been chosen for the present study. The Adiantum capillus-veneris plant was collected from Swabi Khyber Pakhtunkhwa province of Pakistan.
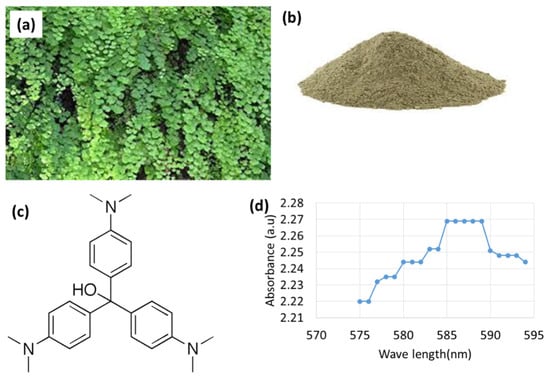
Figure 1.
(a). Leaves of Adiantum capillus-veneris. (b). The powder form of adsorbent. (c) The structural formula of crystal violet dye. (d) Spectral measurements of absorbance as a function of wavelength of crystal violet dye.
2.1.2. Adsorbate
Crystal violet dye (Figure 1c) was used as an adsorbate, which is a cationic dye, a member of the tri-phenyl methane group. It is mainly used in animal and veterinary medicines and as a protein-dye. It is also used in various commercial textile operations. It is a resistant chemical that is both carcinogenic and poorly digested by bacteria. It is also not biodegradable. Therefore, this dye must be removed from wastewater before its discharge for environmental safety.
2.1.3. Chemicals
Crystal violet dye was purchased from Sigma-Aldrich while distilled water was used for the preparation of the solution and ethanol was used for rinsing and cleaning the glass wares. During optimizing pH, HCl (37%) and NaOH pellet DAEJUNG (Gyeonggi-do, Republic of Korea) solutions were used to maintain the pH of the solution. For checking ionic strength, NaCl DEAJUNG (Gyeonggi-do, Republic of Korea) and MnCl2 salts were used. Tap and distilled water was collected from Chemistry Lab at the Women University Swabi, Swabi, Pakistan. However, the canal water was collected from Rashaka Dagai, a village in Swabi.
2.1.4. Instruments
In this study, the instruments used were a digital balance for taking the correct weight of adsorbent and adsorbate, a pH meter for maintaining pH, a UV-Visible spectrophotometer (range 3000, Hamburg, Germany) to find the absorbance, and a digital orbital shaker for shaking the solutions. Fourier transform infrared (FTIR) spectrometer (Affinity-1S FT-IR, Shimadzu, Columbia, MD, USA, 500–4000 cm−1) was used to study the functional groups present while Surface morphologies were investigated with a scanning electron microscope (JSM-5910, JEOL, Tokyo, Japan).
2.2. Methods
2.2.1. Preparation of Adsorbent
The leaves of Adiantum capillus-veneris plant were used as adsorbent. First, we removed leaves from the plant. The leaves were washed several times with tap water and then with distilled water to remove all the dust particles from the leaves. The leaves were dried in sunlight. Then, they were converted into powdered form with the help of mortar and pestle. These powders were sieved through a sieve to remove large particles (Figure 1b).
2.2.2. Determination of Wavelength Maximum of Crystal Violet
In order to determine the wavelength at which crystal violet dye shows maximum absorbance, a 50-ppm stock solution of crystal violet dye was prepared by mixing 50 mg of dye in one liter of distilled water. Then, absorbance was checked at different wavelengths i.e., range from 575 nm to 595 nm. The crystal violet dye shows maximum absorbance at a wavelength of 585 nm. As a result, 585 nm was selected as the wavelength maximum for crystal violet dye (Figure 1d).
According to Beer-Lambert law (Equation (1)), the absorption of light by a substance is directly proportional to its concentration in solution.
where A (absorbance of the solution), ε (constant), C (concentration of the solution), and l (path length of the cuvette).
A = εCl
Using the formula shown below, the value of Qe (mg/g), which represents the quantity of dye absorbed on the surface of the adsorbent, was determined (Equation (2)).
where Ci is the dye solution’s initial concentration before adsorption in ppm, Ce is the dye solution’s equilibrium concentration in ppm, V is the solution’s volume in L, and m denotes the mass of the adsorbent in g.
Similarly, percent removal was calculated using the following formula (Equation (3)) [27].
3. Results and Discussion
3.1. SEM and FTIR Spectra of Adiantum capillus-veneris Plant
The SEM images of Adiantum capillus-veneris plant leaves are given in Figure 2a,b. From SEM images it is clear that the porosity was decreased after the deposition of dye, indicating effective adsorption of dye. The FTIR Spectra of Adiantum capillus-veneris plant leaves before and after adsorption are presented in Figure 2c. The major peak at 1024.20 cm−1 is because of C-F stretch, which confirms the presence of aliphatic fluoro compounds in the Adiantum capillus-veneris plant. The peak for aliphatic fluoro compounds ranges from 1000–1400 cm−1. While the small peak at 1205 cm−1 peak shows the presence of C-O, which confirms the alcohols, ethers, carboxylic acid, and esters (the peak range for these compounds is from 1050–1300 cm−1). At 1516.35 cm−1 peak is due to the deprotonated carboxylate group and the small peaks at 1645 cm−1 and 1712 cm−1 are because of H-O-H bending mode. The peak at 1373 cm−1 represents C-H, which confirms the presence of alkanes; however, 2732.87 cm−1 peak is due to the presence of H-O [28]. Alkanes are once again confirmed by a sharp peak at 2920.17 cm−1 representing C-H, while phenols and H-bonded alcohols are also confirmed by a broad peak at 3432.33 cm−1, which may be caused by the presence of O-H. The peak range for these compounds is from 3200 to 3600 cm−1 [29]. The presence of various functional groups such as C-F, -C-O, C=O, -C-N, C=C, OH, C-H can effectively interact with crystal violet dye and can help in its effective removal. A slight shift in peak position and a small change in peak intensity in FTIR spectrum of Adiantum capillus-veneris plant leaves after adsorption of dye indicate effective deposition of dye at the surface of the adsorbent.
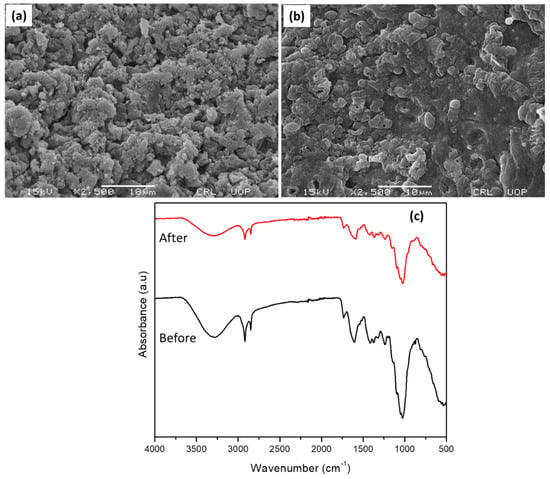
Figure 2.
SEM images of Adiantum capillus-veneris (a) before (b) after adsorption of dye. (c) FTIR analysis of Adiantum capillus-veneris.
3.2. Determination of Adsorption Efficiency of Various Parts of Adiantum capillus-veneris Plant
To check the adsorption efficiency of different parts of Adiantum capillus-veneris plant i.e., leaves and stem for crystal violet dye, a 50-ppm stock solution of crystal violet dye was prepared by mixing 50 mg of dye in 1 L of distilled water. In two 100 mL beakers, 20 mL solution was added using a graduated cylinder. A UV-visible spectrophotometer was then used to measure its absorbance. Following the addition of 0.1 g of adsorbent, both solutions were shaken on a digital orbital shaker for 80 min at 200 rpm. The absorbance of both solutions was then measured again after they had been filtered using filter paper. Finally, the dye solution’s final concentration was estimated. From Figure 3, the percent removal and Qe values of leaves were higher than that for a stem, so leaves were used as adsorbent for further study.
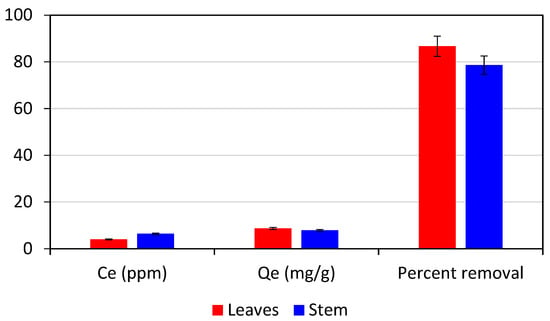
Figure 3.
Comparison of adsorption performance of different parts of Adiantum capillus-veneris for 50 ppm crystal violet (20 mL) with adsorbent dose 0.1 g, contact time 80 min, and shaker speed 200 rpm.
3.3. Determination of Adsorption Efficiency of Adiantum capillus-veneris Plant Leaves Prepared Using Direct Sunlight, Room Temperature, and Oven at 150 °C for Drying
To determine the effect of drying conditions on the adsorption performance, we dried leaves under three different conditions such as at room temperature, under direct sunlight, and oven at 150 °C. After drying, the leaves were ground into a powder with the help of a mortar and pestle. A 20 mL of 30 ppm solutions were added to three 100 mL beakers and then 60 mg of adsorbent was added to each of these solutions. The solutions were placed on a digital orbital shaker for 90 min at 200 rpm. Afterward, following filtration, the absorbance of the filtrate was checked to calculate the final concentration of the dye.
The result shows that absorbent prepared under different conditions showed different performances. From Figure 4, the performance of leaves dried under direct sunlight was the highest, while the lowest performance was shown by the leaves that were dried at room temperature. This might be because the leaves dried in direct sunlight were well dried and yielded uniform powder with small particles size that provide a large surface area, and consequently, the highest removal performance was observed. Thus, for further study, the leaves were dried under direct sunlight.

Figure 4.
The efficiency of adsorbent dried under different experimental conditions (adsorbent dose 0.06 g, contact time 90 min, concentration 30 ppm, shaker speed 200 rpm, the volume of solution 20 mL).
3.4. Optimization of Initial Dye Concentration
In order to determine the effect of the initial dye concentration of crystal violet on adsorption by Adiantum capillus-veneris plant leaves powder, a 100-ppm stock solution of crystal violet dye was prepared by mixing 100 mg of dye in 1 L of distilled water. This stock solution was then diluted with distilled water to form solutions having a concentration range from 10 to 100 ppm. The absorbance was recorded using UV-visible spectrophotometer and then 100 mg of adsorbent was added to all the solutions (total volume of each solution = 10 mL), which were then placed on a digital orbital shaker at 200 rpm for 80 min. After shaking, all the solutions were filtered using filter paper and again absorbance was recorded to calculate the percent removal of the adsorbate. Figure 5 it is revealed that a maximum percent removal was achieved for 30 ppm dye concentration that was decreased gradually upon a further increase in the initial dye concentration. This may be because of the availability of more binding sites at the surface of the adsorbent when the dye concentration is comparatively low [30]. Therefore, up to 30 ppm the percent removal increased, and then, it decreased with a further increase in the dye concentration. The same dye concentration was also found appropriate by Ilyasse Loulidi using agricultural waste residue for the adsorption of crystal violet dye [31].
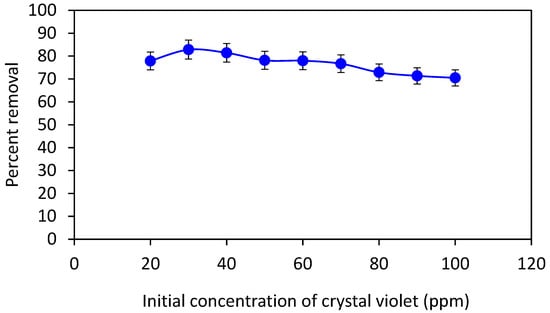
Figure 5.
Plot of percent removal of crystal violet as a function of initial dye concentration at 0.1 g of adsorbent dose, 80 min contact time, 200 rpm shaker speed, and the volume of solution, 10 mL.
3.5. Selection of Adsorbent Dosage
To select the optimum adsorbent dose, a 30-ppm stock solution of crystal violet dye was prepared in 250 mL of distilled water. The absorbance of the stock solution was recorded using a UV-visible spectrophotometer. In 10 (100 mL) beakers, 20 mL volume of the dye was taken using a graduated cylinder and each solution was loaded with a different dose of the adsorbent i.e., 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 mg. These solutions were then placed on a digital orbital shaker at 200 rpm for 90 min. After shaking, all these solutions were filtered using filter paper. Figure 6 shows a rapid increase in the uptake of crystal violet dye as a function of the adsorbent dose from 10 to 60 mg. Further increase in the adsorbent dose, however, did not result in any appreciable increase in the adsorption capacity; therefore, 60 mg is the optimum adsorbent dose. It might be since at lower adsorbent dosages (less than 60 mg) not enough sites were available for dye molecules. When the adsorbent dose was increased, the percentage removal was also increased which might be because of an increase in the adsorbent surface area. Further increase in adsorbent dose has no significant effect on percent removal. This may be because of numerous interactions, such as aggregation of adsorbent particles at increased dosage [32]. Such aggregation would result in a reduction in the adsorbent’s overall surface area and an increase in the diffusional path length which leads to decreased adsorption capacity [33].
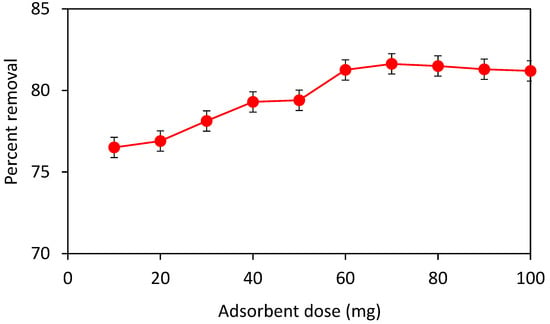
Figure 6.
Effect of adsorbent dose on percent removal of crystal violet at 30 ppm dye concentration, contact time 90 min, shaker speed 200 rpm and volume of solution 20 mL.
3.6. Determination of Contact Time for Adsorption
Time is one of the most important parameters that can affect the adsorption process. To optimize the contact time, a 30 ppm stock solution of crystal violet was prepared. After then ten 100 mL beakers were filled with 20 mL of 30 ppm dye solution with the help of a graduated cylinder. Each solution was loaded with 60 mg of adsorbent following shaking on a digital orbital shaker (200 rpm) at different contact times. For example, the first solution was shaken for 30 min, the second solution for 60 min, the third one for 90 min, and so on up to 300 min. The absorbance of each solution was monitored following filtration by using filter paper. The maximum percent removal of crystal violet dye was achieved at a contact time of 90 min which is presumed to be the equilibrium time; the amount of dye adsorbed does not show time-dependent change once equilibrated (Figure 7). Thus, within 90 min adsorbent active sites get occupied with dye molecules, and therefore, no further increase in percent removal was observed after 90 min. However, a small decrease in the percent removal of crystal violet might be due to desorption.
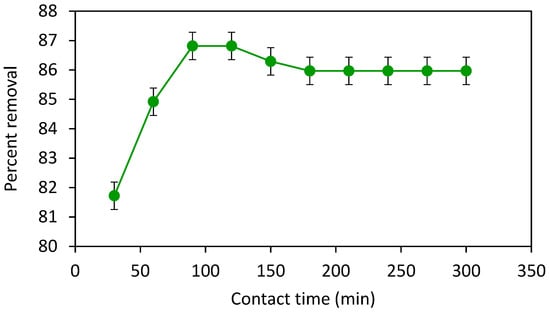
Figure 7.
Effect of contact time on percent removal of crystal violet at 0.06 g of adsorbent dose, 30 ppm of crystal violet, shaker speed 200 rpm and the volume of solution 20 mL.
3.7. Determination of the Effect of pH on Dye Adsorption
This experiment was performed to check and find the pH at which maximum adsorption will occur. For this, a 30-ppm solution of crystal violet dye was prepared and 10 solutions portions, having 20 mL volume, were taken in ten 100 mL beakers using a graduated cylinder. The pH of the solutions was maintained from 2–11 using 0.2 M HCl and NaOH solutions. Their absorbance was recorded. Each of the solutions was loaded with a 60 mg adsorbent, which was then placed on a digital orbital shaker at 200 rpm for 90 min. Following filtration after shaking for 90 min, the absorbance of the filtrate was recorded. At pH 3, the maximum percent removal was achieved as represented by Figure 8. The structure of the adsorbate, the charge on the adsorbent surface, and the ionization and dissociation of various surface charges on the adsorbent are all controlled by pH, which is crucial for dye adsorption [34]. The pH of the medium affects the strength of the electric interaction between ionized dye molecules. In solutions with varied pH levels, both the adsorbent and the adsorbatefunctional groups that are presentcan be protonated or deprotonated to produce different surface charges, resulting in electrostatic attraction or repulsion between the charged adsorbates and adsorbents [35].
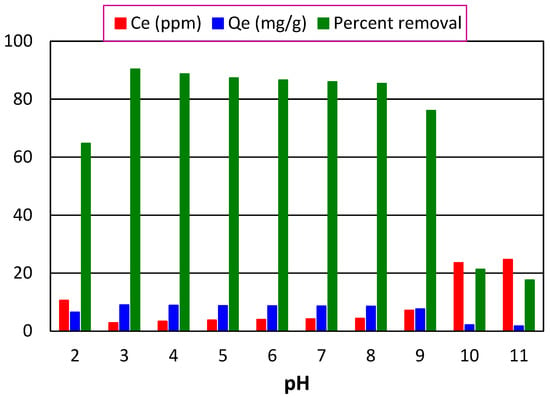
Figure 8.
Effect of pH of the dye solution on its percent removal when 0.06 g of adsorbent stayed in contact with 30 ppm dye (20 mL) for 90 min at 200 rpm.
3.8. Comparison of Adsorption Efficiency of Adiantum capillus-veneris Plant Leaves with Animal Charcoal and Silica Gel
A 30 ppm solution of crystal violet dye was divided into three 100 mL beakers with 20 mL of the dye solution in each to compare the adsorption capacity of the leaves powder of the Adiantum capillus-veneris plant with animal charcoal and silica gel. With the help of a UV-visible spectrophotometer, the absorbance was evaluated. Then, 60 mg each of animal charcoal, silica gel, and the powdered leaves of the Adiantum capillus-veneris plant were added to the beakers, which were then shaken on a digital orbital shaker for 90 min at 200 rpm. Following that, the filter paper was used to filter each solution, and absorbance was recorded. Figure 9 shows that the leaf powder of Adiantum capillus-veneris plant exhibits promising results when compared to commercial adsorbents such as animal charcoal and silica gel. The Adiantum capillus-veneris plant leaves are a low-cost adsorbent when compared to animal charcoal and silica gel because these leaves can be directly used for the removal of dye without any treatment.
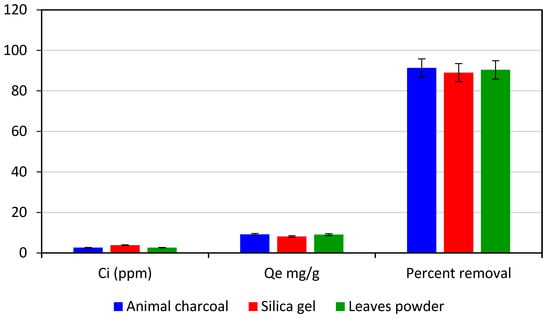
Figure 9.
Comparison of adsorption performance of Adiantum capillus-veneris plant leaves, silica gel, and animal charcoal.
3.9. Comparison of Adsorption Performance of Adiantum capillus-veneris Plant Leaves in Water Collected from Different Sources
Water can dissolve any substance so the water contains various substances which are different from each other in their behavior towards adsorption. To check the adsorption efficiency of adsorbent in different types of water and its real-world application, experiments were carried out in water collected from various sources such as lab tap water, canal water, and distilled water. For this purpose, a 20 mL stock solution of 30 ppm of crystal violet dye was prepared in all these different types of water, and pH was maintained at 3. A 60 mg of adsorbent was added to all three samples and placed on a digital orbital shaker and shaken for 90 min at 200 rpm. Following shaking, all these solutions were filtered and then the absorbance of the filtrate was noted. The adsorbent showed high adsorption efficiency even in tap water and canal water, 85.46%, and 81.13%, respectively (Figure 10), thus it can be used for the practical removal of toxic dyes from industrial wastewater without any special treatment of Adiantum capillus-veneris plant leaves to convert them into an adsorbent. When the performance of our biosorbent was compared with the reported literature, it has high removal efficiency (Table 1).
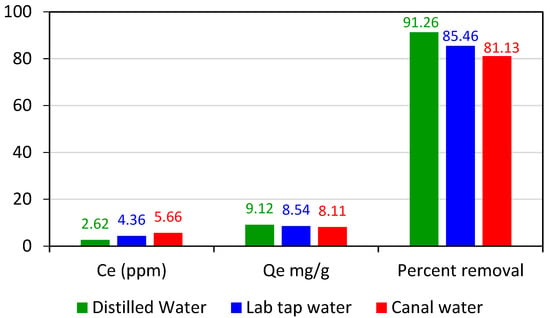
Figure 10.
Adsorption efficiency of Adiantum capillus-veneris plant leaves in water collected from various sources (adsorbent dose 0.06 g, time 90 min, concentration 30 ppm, pH 3, shaker speed 200 rpm, the volume of solution 20 mL).

Table 1.
Comparison of our adsorbent with other low-cost biosorbents for removal of Crystal Violet dye.
3.10. Effect of Ionic Strength on Adsorption Efficiency of the Leaves Powder of Adiantum capillus-veneris
The effect of the presence of salt on the process of adsorption was also checked. A 30-ppm solution of the adsorbate (dye) was divided into eight 100 mL beakers having 20 mL dye solution and 60 mg of Adiantum capillus-veneris plant leaves powder (adsorbent) in each at constant pH 3. The concentration of NaCl was varied in each solution such as from 0.1 M to 0.8 M. Similarly, solutions were prepared for MnCl2 with the same salt concentration i.e., 0.1 M–0.8 M. All the solutions were placed on a digital orbital shaker at 200 rpm for 90 min. After 90 min of shaking, all these solutions were filtered using filter paper and their absorbance was noted. In addition, the absorbance was recorded for the solution without adding adsorbent because wastewater discharged from various industries contains a variety of salts; therefore, this study aimed to examine the impact of the presence of different ions on the process of adsorption [41]. Figure 11 reveals that an increase in salt concentration decreases the percent removal of crystal violet. This might be due to the electrostatic interaction of salt with adsorbate and various functional groups present at the surface of adsorbent as indicated by FTIR analysis, which results in low adsorption of dye molecules at the surface of the adsorbent. However, the adsorption efficiency of adsorbent was comparatively high even at high salt concentrations and thus can be used in saline water and real industrial wastewater.
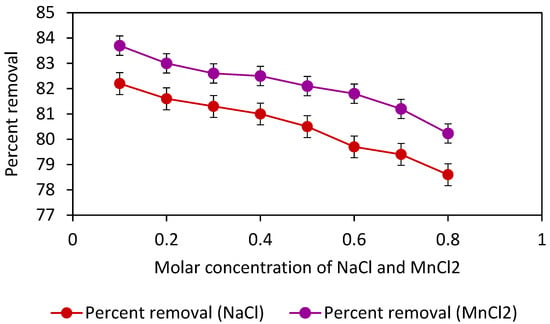
Figure 11.
Removal efficiency of absorbent in the presence of NaCl and MnCl2 at adsorbent dose 0.06 g, contact time 90 min, dye concentration 30 ppm, shaker speed 200 rpm, the volume of the solution 20 mL, and pH 3.
3.11. Adsorption Isotherms
“Adsorption isotherm” refers to the relation between a substance’s equilibrium concentration and the amount of that substance adsorbed at a particular temperature. The parameters derived from the various models provide important clues about the adsorption mechanism, as well as the surface characteristics and affinities of the adsorbent. The most commonly usedsurface adsorption models for single-solute systems are the Langmuir and Freundlich adsorption models [27,42,43]. Adsorption isotherms are essential for explaining how the adsorbate molecules or ions interact with the adsorbent surface active sites. These isotherms are explained below.
3.11.1. Langmuir Adsorption Isotherm
The Langmuir isotherm is based on homogeneous adsorption, in which the adsorbate can only bind to a fixed number of small, localized sites and cannot move across the plane of the surface. Equation (4) represents the linear form of the Langmuir model.
Qe is the adsorption capacity (mg/g), Ce (mg/L) is the equilibrium concentration of adsorbate, KL is the Langmuir isotherm constant, and Qmax represents the maximum adsorption capacity of the biosorbent.
3.11.2. Freundlich Adsorption Isotherm
Freundlich isotherm was proposed to explain heterogeneous surface, reversible, multilayer and non-ideal adsorption [44]. The Freundlich isotherm model’s linear form is depicted by Equation (5) [45].
where 1/n (slope) and KF (intercept, log KF) are Freundlich isotherm constants and are related to adsorption capacity, while the adsorption capacity (mg/g) is Qe, and the equilibrium concentration of adsorbate (ppm) is Ce.
Table 2 lists several parameters that were acquired from the Langmuir and Freundlich isotherms after fitting the experimental data (Figure 12a,b) [46,47]. When comparing both of the plots i.e., Langmuir linear plot and Freundlich linear plot, the results reveal the Freundlich isotherm’s regression coefficient/constant (R2) higher than that of the Langmuir isotherm model, indicating non-ideal, reversible, multilayer, and heterogeneous adsorbent surfaces.

Table 2.
Parameters calculated from adsorption isotherm for the adsorption of crystal violet at the leaves powder of Adiantum capillus-veneris plant.
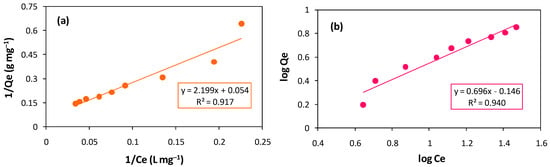
Figure 12.
(a) Linear plot of Langmuir isotherm. (b) Linear plot of Freundlich isotherm.
3.12. Kinetic Study of the Crystal Violet Adsorption
The kinetics of the adsorption of crystal violet was studied by implementing pseudo-first-order (PFO) [48,49], and pseudo-second-order (PSO) kinetic Equations (6) and (7) [50]. Important details about the chemical route, dye’s adsorption mass transfer mechanism, and diffusion rate can be revealed via kinetic studies [51].
3.12.1. Pseudo-First-Order Kinetic Model
The linear form of the PFO model is mentioned in Equation (6) [46]. Wherein, k1 is the PFO rate constant (min−1), Qt and Qe are the amounts of dye adsorbed (mg/g) at contact time t (min) and equilibrium on the adsorbent, respectively.
The plot of ln(Qe − Qt) vs. t should give a straight line with a slope of k1 and an intercept of lnQe, allowing for the calculation of the adsorption rate constant, k1 and the equilibrium adsorption capacity [52].
3.12.2. Pseudo-Second-Order Kinetic Model
For PSO model, the equation is given below (Equation (7)).
Wherein, k2 is the PSO model’s equilibrium rate constant [27]. The plot of t/Qt vs. t should be linear and should have a slope equal to 1/Qe and an intercept 1/k2Qe.
When we implemented both of the kinetic models on our data, we observed a linear plot for the PSO kinetic model with a linear regression (R2) value of 0.999. However, the linearity was poor in the case of the PFO kinetic model as depicted in Figure 13a. From the results, it can be seen that the adsorption of crystal violet on our selected biosorbent follows PSO kinetics (Figure 13b), wherein the rate-determining phase involves chemisorption, depicting that the electrons are shared or exchanged among cationic dye molecules and Adiantum capillus-veneris plant leaves surface active groups [53]. The parameters determined from both models are mentioned in Table 3.
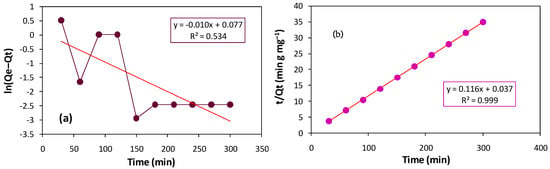
Figure 13.
(a) Pseudo-first order kinetic plot. (b) Pseudo-second order kinetic plot.

Table 3.
The kinetic parameter for the adsorption of crystal violet dye on the leaves powder of Adiantum capillus-veneris plant.
3.13. Mechanism of Adsorption of Crystal Violet Dye on Leaves Powder of Adiantum capillus-veneris
In the present work, the leaves of Adiantum capillus-veneris plant were used as an adsorbent for the adsorption of crystal violet (CV) dye (Figure 14). FTIR analysis indicated the presence of many functional groups, including C-F, C-O, C=O, -C-N, C=C, and OH, which were actively involved in the adsorption of crystal violet dye. The functional groups at the surface of the adsorbent are all electron-rich, which causes the cationic dye (CV), which carries a positive charge in solution, to have strong electrostatic interaction with the surface of the adsorbent and aid in the dye’s adsorption. The catalytic effect of low pH, such as 3, for the greatest adsorption was revealed by studying the effect of pH on the adsorption process. Compared to very acidic (pH 2) and highly basic (pH 9–11) media, pH 3–8 had a higher percent elimination of the CV dye. The electrostatic repulsive forces between positively charged CV dye and protons, as well as between electron-rich hydroxyl ions and the surface of the adsorbent, accelerated the adsorption of CV dye in the acidic and mildly basic pH range. Meanwhile, the charge neutralization of CV causes a decrease in its percentage removal and, thus, adsorption in a strongly basic media. Similar to this, in a very acidic media, protons compete with the CV dye to electrostatically interact with the electron-rich active sites of the adsorbent, leading to ion exchange and, consequently, a reduction in the percent removal of CV. If we compare the FTIR results before and after adsorption, the leaves of the Adiantum capillus-veneris plant showed a little shift in peak position and a slight change in peak intensity. This change suggests that the dye was effectively deposited at the surface of the adsorbent via strong electrostatic bonding. The results are compatible with the Freundlich isotherm and PSO kinetic model because non-ideal, reversible, multilayer adsorption occurs at heterogeneous sites at the adsorbent. Figure 14 depicts the adsorption mechanism.
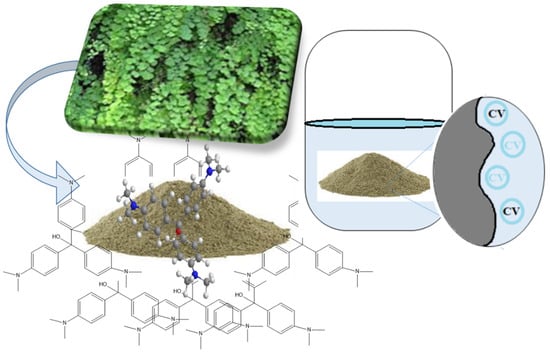
Figure 14.
Mechanism of adsorption of crystal violet dye on leaves powder of Adiantum capillus-veneris as an efficient biosorbent.
4. Conclusions
The leaves of the Adiantum capillus-veneris plant, which are inexpensive and widely accessible, have proven to be an extremely efficient way to remove crystal violet from aqueous solutions. This study unequivocally demonstrates the viability, effectiveness, and affordability of crystal violet removal using Adiantum capillus-veneris plant leaves. The biosorbent under study showed compatible results with commercially available biosorbents and is of low cost. Its performance was also tested in saline water, canal water, and tap water besides distilled water and the removal efficiency achieved in tap water and canal water, were 85.46%, and 81.13%, respectively. Thus, it can be concluded that the Adiantum capillus-veneris plant leaves can be used without any chemical surface treatment and modification to effectively remove dyes from wastewater on a commercial scale.
Author Contributions
Conceptualization, S.G. (Salma Gul), S.G. (Shehla Gul) and H.G.; methodology, S.G. (Salma Gul), H.G. and R.K.; software, S.G. (Shehla Gul), M.S.K. and A.E.K.; validation, S.G. (Salma Gul), S.G. (Shehla Gul), H.G., F.K., R.K., M.S.K., R.U. (Rizwan Ullah), R.U. (Rooh Ullah) and Z.W.; formal analysis, S.G. (Shehla Gul), M.S.K., R.U. (Rizwan Ullah), R.U. (Rooh Ullah) and Z.W.; investigation, S.G. (Salma Gul), S.G. (Shehla Gul), H.G. and R.K.; resources, S.G. (Salma Gul), H.G., R.K., A.E.K. and I.Z.; data curation, S.G. (Salma Gul) and S.G. (Shehla Gul); writing—original draft preparation, S.G. (Salma Gul), F.K. and H.G.; writing—review and editing, R.K., M.S.K., A.E.K. and I.Z.; visualization, H.G. and R.K.; supervision, S.G. (Salma Gul); project administration, S.G. (Salma Gul). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
S.G. (Salma Gul), H.G. and R.K. acknowledge the Department of Chemistry, Women University Swabi, Pakistan and the Department of Chemistry, Shaheed Benazir Bhutto Women University, Peshawar, Pakistan for financial and technical support. A.E.K.’s contribution was supported by the LZP Project Nr. lzp-2021/1-0090 CircleP.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dey, P.; Mahapatra, B.; Juyal, V.; Pramanick, B.; Negi, M.; Paul, J.; Singh, S. Flax processing waste–a low-cost, potential biosorbent for treatment of heavy metal, dye and organic matter contaminated industrial wastewater. Ind. Crop Prod. 2021, 174, 114195. [Google Scholar] [CrossRef]
- Sintakindi, A.; Ankamwar, B. Fungal biosorption as an alternative for the treatment of dyes in waste waters: A review. Environ. Technol. Rev. 2021, 10, 26–43. [Google Scholar] [CrossRef]
- Chatla, A.; Almanassra, I.W.; Kochkodan, V.; Laoui, T.; Alawadhi, H.; Atieh, M.A. Efficient removal of eriochrome black T (EBT) dye and chromium (Cr) by hydrotalcite-derived Mg-Ca-Al mixed metal oxide composite. Catalysts 2022, 12, 1247. [Google Scholar] [CrossRef]
- Ali, N.S.; Jabbar, N.M.; Alardhi, S.M.; Majdi, H.S.; Albayati, T.M. Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: Isotherm, kinetics, and thermodynamic studies. Heliyon 2022, 8, e10276. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Karthik, K.; Veerabagu, U.; Hari, A.; Swaminathan, K.; Al-Kheraif, A.A.; Whangchai, K. Bi-model cationic dye adsorption by native and surface-modified Trichoderma asperellum BPL MBT1 biomass: From fermentation waste to value-added biosorbent. Chemosphere 2021, 277, 130311. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.; Zhang, Q.; Sun, S.; Zhou, X.; Xu, Y. A novel natural lignocellulosic biosorbent of sunflower stem-pith for textile cationic dyes adsorption. J. Clean. Prod. 2022, 331, 129878. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Shah, M.U.H.; Reddy, A.V.B.; Aminabhavi, T.M. Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef]
- Isik, B.; Ugraskan, V.; Cankurtaran, O. Effective biosorption of methylene blue dye from aqueous solution using wild macrofungus (Lactarius piperatus). Sep. Sci. Technol. 2022, 57, 854–871. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmad, R. Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 2011, 265, 112–118. [Google Scholar] [CrossRef]
- Ali, H.; Muhammad, S.K. Biosorption of crystal violet from water on leaf biomass of Calotropis procera. J. Environ. Sci. Technol 2008, 1, 143–150. [Google Scholar] [CrossRef]
- Putri, K.N.A.; Keereerak, A.; Chinpa, W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int. J. Biol. Macromol. 2020, 156, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.B.; Priyantha, N.; Mansor, N.H.M. Artocarpus altilis (breadfruit) skin as a potential low-cost biosorbent for the removal of crystal violet dye: Equilibrium, thermodynamics and kinetics studies. Environ. Earth Sci. 2015, 73, 3239–3247. [Google Scholar] [CrossRef]
- Rammel, R.; Zatiti, S.; El Jamal, M. Biosorption of crystal violet by chaetophora elegans alga. J. Univ. Chem. Technol. Metall. 2011, 46, 283–292. [Google Scholar]
- Grassi, P.; Reis, C.; Drumm, F.C.; Georgin, J.; Tonato, D.; Escudero, L.B.; Kuhn, R.; Jahn, S.L.; Dotto, G.L. Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci. Technol. 2019, 79, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.; Nasar, A. Utilization of Cucumis sativus peel as an eco-friendly biosorbent for the confiscation of crystal violet dye from artificially contaminated wastewater. Anal. Chem. Lett. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Elsherif, K.; El-Dali, A.; Alkarewi, A.; Ewlad-Ahmed, A.; Treban, A. Adsorption of crystal violet dye onto olive leaves powder: Equilibrium and kinetic studies. Chem. Int. 2021, 7, 79–89. [Google Scholar]
- Lin, Y.; He, X.; Han, G.; Tian, Q.; Hu, W. Removal of Crystal Violet from aqueous solution using powdered mycelial biomass of Ceriporia lacerata P2. J. Environ. Sci. 2011, 23, 2055–2062. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. -Effic. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Kumar, M.R.; King, P.; Wolde, Z.; Mulu, M. Application of optimization response surface for the biosorption of crystal violet dye from textile wastewater onto Clerodendrum fragrans leaves. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Essekri, A.; Aarab, N.; Hsini, A.; Ajmal, Z.; Laabd, M.; El Ouardi, M.; Ait Addi, A.; Lakhmiri, R.; Albourine, A. Enhanced adsorptive removal of crystal violet dye from aqueous media using citric acid modified red-seaweed: Experimental study combined with RSM process optimization. J. Dispers. Sci. Technol. 2022, 43, 1359–1372. [Google Scholar] [CrossRef]
- Ashour, M.; Alprol, A.E.; Khedawy, M.; Abualnaja, K.M.; Mansour, A.T. Equilibrium and Kinetic Modeling of Crystal Violet Dye Adsorption by a Marine Diatom, Skeletonema costatum. Materials 2022, 15, 6375. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Baghdadi, M.; Mehrdadi, N.; Ali Abdoli, M. Adsorption of crystal violet dye by agricultural rice bran waste: Isotherms, kinetics, modeling and influencing factors. Environ. Eng. Res. 2021, 26, 200128. [Google Scholar] [CrossRef]
- Silveira, M.B.; Pavan, F.A.; Gelos, N.F.; Lima, E.C.; Dias, S.L.P. Punica granatum shell preparation, characterization, and use for Crystal Violet removal from aqueous solution. Clean Soil Air Water 2014, 42, 939–946. [Google Scholar] [CrossRef]
- Yadav, M.; Thakore, S.; Jadeja, R. Removal of organic dyes using Fucus vesiculosus seaweed bioadsorbent an ecofriendly approach: Equilibrium, kinetics and thermodynamic studies. Environ. Chem. Ecotoxicol. 2022, 4, 67–77. [Google Scholar] [CrossRef]
- Ruscasso, F.; Bezus, B.; Garmendia, G.; Vero, S.; Curutchet, G.; Cavello, I.; Cavalitto, S. Debaryomyces hansenii F39A as biosorbent for textile dye removal. Rev. Argent. Microbiol. 2021, 53, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Karaman, C.; Karaman, O.; Show, P.-L.; Karimi-Maleh, H.; Zare, N. Congo red dye removal from aqueous environment by cationic surfactant modified-biomass derived carbon: Equilibrium, kinetic, and thermodynamic modeling, and forecasting via artificial neural network approach. Chemosphere 2022, 290, 133346. [Google Scholar] [CrossRef]
- Gul, S.; Kanwal, M.; Qazi, R.A.; Gul, H.; Khattak, R.; Khan, M.S.; Khitab, F.; Krauklis, A.E. Efficient removal of Methyl Red dye by using bark of Hopbush. Water 2022, 14, 2831. [Google Scholar] [CrossRef]
- Omidi, S.; Sedaghat, S.; Tahvildari, K.; Derakhshi, P.; Motiee, F. Biosynthesis of silver nanocomposite with Tarragon leaf extract and assessment of antibacterial activity. J. Nanostructure Chem. 2018, 8, 171–178. [Google Scholar] [CrossRef]
- Hadi, I.; Hussein, H.M. Antimicrobial Activity and spectral chemical analysis of methanolic leaves extract of Adiantum capillus-veneris using GC-MS and FT-IR spectroscopy. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 369–385. [Google Scholar]
- Tripathi, N. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. J. Appl. Chem. 2013, 5, 91–108. [Google Scholar] [CrossRef]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M.; Amar, A.; Jabri, M.; Kali, A.; Chraibi, S.; Hadey, C.; Aziz, F. Adsorption of crystal violet onto an agricultural waste residue: Kinetics, isotherm, thermodynamics, and mechanism of adsorption. Sci. World J. 2020, 2020, 5873521. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Bano, S.; Fahim, A.; Parveen, R.; Khurshid, S. Efficient removal of crystal violet and eosin B from aqueous solution using Syzygium cumini leaves: A comparative study of acidic and basic dyes on a single adsorbent. Korean J. Chem. Eng. 2015, 32, 882–895. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Alshabanat, M.; Alsenani, G.; Almufarij, R. Removal of crystal violet dye from aqueous solutions onto date palm fiber by adsorption technique. J. Chem. 2013, 2013, 210239. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S. Optimization of crystal violet adsorption on common lilac tree leaf powder as natural adsorbent material. Glob. NEST J. 2022, 24, 87–96. [Google Scholar]
- Sultana, S.; Islam, K.; Hasan, M.A.; Khan, H.J.; Khan, M.A.R.; Deb, A.; Al Raihan, M.; Rahman, M.W. Adsorption of crystal violet dye by coconut husk powder: Isotherm, kinetics and thermodynamics perspectives. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100651. [Google Scholar] [CrossRef]
- Smitha, T.; Santhi, T.; Prasad, A.L.; Manonmani, S. Cucumis sativus used as adsorbent for the removal of dyes from aqueous solution. Arab. J. Chem. 2017, 10, S244–S251. [Google Scholar] [CrossRef]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet adsorption: A mechanistic study. Microporous Mesoporous Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef]
- Wang, X.S.; Liu, X.; Wen, L.; Zhou, Y.; Jiang, Y.; Li, Z. Comparison of basic dye crystal violet removal from aqueous solution by low-cost biosorbents. Sep. Sci. Technol. 2008, 43, 3712–3731. [Google Scholar] [CrossRef]
- Santhi, T.; Manonmani, S.; Smitha, T. Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of Ricinus communis by adsorption. J. Hazard. Mater. 2010, 179, 178–186. [Google Scholar] [CrossRef]
- Nasuha, N.; Zurainan, H.; Maarof, H.; Zubir, N.; Amri, N. Effect of Cationic and Anionic Dye Adsorption from Aqueous Solution by Using Chemically Modified Papaya Seed. In Proceedings of the International Conference on Environment Science and Engineering, Bali Island, Indonesia, 1–3 April 2011; pp. 50–54. [Google Scholar]
- Aboelenin, R.M.; Kheder, S.; Farag, H.K.; El Nabarawy, T. Removal of cationic neutral red dye from aqueous solutions using natural and modified rice straw. Egypt. J. Chem. 2017, 60, 577–589. [Google Scholar] [CrossRef]
- Gul, S.; Gul, H.; Gul, M.; Khattak, R.; Rukh, G.; Khan, M.S.; Aouissi, H.A. Enhanced adsorption of Rhodamine B on biomass of Cypress/False Cypress (Chamaecyparis lawsoniana) fruit: Optimization and kinetic study. Water 2022, 14, 2987. [Google Scholar] [CrossRef]
- Karimulla, S.; Ravindhranath, K. Removal of Brilliant Green dye from polluted waters using bio-sorbents derived from some plant materials. Asian J. Res. Chem. 2012, 5, 1350–1359. [Google Scholar]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Adsorption characteristics of brilliant green dye on kaolin. J. Hazard. Mater. 2009, 161, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Application of low-cost adsorbents for dye removal–a review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.F. Pseudo first-order kinetics. J. Chem. Educ. 1972, 49, 663. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Mahmood, T.; Noreen, U.; Ali, R.; Ullah, A.; Naeem, A.; Aslam, M. Adsorptive removal of Congo red from aqueous phase using graphene–tin oxide composite as a novel adsorbent. Int. J. Environ. Sci.Technol. 2022, 19, 10275–10290. [Google Scholar] [CrossRef]
- Ullah, T.; Gul, H.; Khitab, F.; Khattak, R.; Ali, Y.; Rasool, S.; Khan, M.S.; Zekker, I. Adsorption of Remazol Brilliant Violet-5R from aqueous solution using sugarcane bagasse as biosorbent: Kinetic and thermodynamic studies. Water 2022, 14, 3014. [Google Scholar] [CrossRef]
- Tay, C.-I.; Ong, S.-T. Guava leaves as adsorbent for the removal of emerging pollutant: Ciprofloxacin from aqueous solution. J. Phys. Sci. 2019, 30, 137–156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).