Abstract
Triamcinolone acetonide (TA) is a synthetic corticosteroid commonly used in medical practice to treat various skin conditions, including eczema, dermatitis, and allergies. It is a highly potent derivative of triamcinolone, with a strength that is about eight times greater than prednisone. Although it is sometimes used by athletes, it is important to note that the World Anti-Doping Agency (WADA) prohibits the use of glucocorticoids in competition when administered via injection, oral (including oromucosal, such as buccal, gingival, or sublingual), or rectal routes. However, they are allowed if administered otherwise, such as via inhalation or topical application to the skin. Anti-doping laboratories generally report Adverse Analytical Findings (AAF) for glucocorticoid group substances when their estimated concentration exceeds 30 ng/mL, with some exceptions such as triamcinolone acetonide, which has a reporting limit of 15 ng/mL. It is important to note that this only applies to the parent compound of specified metabolites. To address interpretation issues that can arise with other glucocorticoids, such as budesonide, the authors of this study investigated whether similar issues occur with triamcinolone acetonide. Specifically, they examined whether therapeutic doses of the commonly used medication Previsone could result in anti-doping rule violations due to the presence of triamcinolone acetonide and its metabolites in urine. The study involved ten healthy volunteers, and the analytical procedure was developed using liquid/liquid extraction, hydrolysis, and LC/MS/MS analysis. The results of the study showed that topical administration of therapeutic doses of Previsone does not pose a threat of anti-doping rules violation, as the excretion of the parent compound does not exceed the reporting limit in urine. Additionally, the concentration of 6β-hydroxy Triamcinolone acetonide was also well below the reporting limit.
1. Introduction
Triamcinolone acetonide (TA) is a synthetic glucocorticoid (GC) used commonly as an anti-inflammatory agent. For therapeutic purposes it may be administered via a variety of routes: inhaled, intramuscularly, nasally, intra-articularly and locally. It is used to treat a wide array of conditions, such as: allergic disorders (e.g., asthma, rhinitis, atopic dermatitis), skin diseases (e.g., erythema multiforme), gastrointestinal diseases (e.g., ulcerative colitis), rheumatic conditions (gout, psoriatic arthritis, systemic lupus erythematosus). In most countries, medications containing triamcinolone acetonide are issued on prescription. These include medications containing only triamcinolone acetonide as the active substance, as well as complex preparations (Table 1).

Table 1.
Examples of medications containing triamcinolone acetonide [1,2,3,4].
Triamcinolone acetonide (TA) was deemed prohibited substance in sports in 2004, when the WADA added a new group of substances—glucocorticoids—to the List of Prohibited Substances and Methods in Sports [5].
WADA prohibited TA and other glucocorticoids if used in competition if applied by any injectable, oral [including oromucosal (e.g., buccal, gingival, sublingual)] or rectal route [6]. Additionally, according to WADA Technical Document—TD2022MRPL—“Minimum Required Performance Levels For Detection And Identification Of Non-Threshold Substances”, anti-doping laboratories report Adverse Analytical Finding (AAF) for glucocorticoids if their estimated concentrations exceed 30 ng/mL with some exceptions as triamcinolone acetonide with a reporting limit of 15 ng/mL. This applies solely to the parent compound [7,8].
In respect of results interpretation issues for another glucocorticoid, budesonide, which is now prohibited with a reporting limit of 45 ng/mL only for its minor metabolite 6β-hydroxy-budesonide—as it was proved that the detection of major budesonide metabolite 6α-hydroxyprednisolone in a concentration exceeding its reporting limit could have resulted from the administration of the substance by allowed routs e.g., inhalation. The authors undertook to investigate whether a similar problem may occur in the case of triamcinolone acetonide [9,10] and to verify whether 6β-hydroxy Triamcinolone acetonide as major metabolite would exceed the reporting level for parent compound when administered by the allowed route. As part of the investigation, an analytical procedure designed to detect the substance of interest and its metabolite 6β-hydroxy triamcinolone acetonide was developed and validated.
The result of our work significantly complements Triamcinolone Acetonide metabolism studies for doping purposes as other administration protocols of the medication were used [11,12,13,14,15,16,17].
2. Materials and Methods
2.1. Study Design and Doses
For the preliminary study, 18 test urine samples delivered to the Polish Anti-Doping Laboratory were tested; worth to notice that TA and its metabolite 6β-hydroxy triamcinolone acetonide were found to be present in those samples in the course of routine tests. Those findings were considered as motivation to conduct further excretion studies.
Therefore the second part of the project was designed to involve testing of urine collected from 10 volunteers (5 women and 5 men) after topical application of Pevisone in the form of cream (10 mg triamcinolone acetonide + 1.1 mg Econazole)/g.
The medication contains active substances—econazole—in the form of econazole nitrate and triamcinolone in the form of triamcinolone acetonide. Triamcinolone acetonide applied topically has anti-inflammatory and antipruritic properties, and also acts as a vasoconstrictor. Pevisone cream is indicated for the treatment of skin mycoses caused by dermatophytes and yeasts with apparent inflammation symptoms.
Volunteers were required to apply the medication in accordance with the following routine: once daily for 3 days (in the morning), then twice daily for 3 days (in the morning and at night before sleep).
2.2. Sample Collection before, during and after Substance Administration
Each participant collected three urine samples before the application of the preparation, including one, on the first day of the study. Samples were collected using sterile urine containers and then stored at −20 °C.
As it was described above, Pevisone was administered percutaneously once daily for the first 3 days and twice daily for the following 3 days. One portion of the preparation applied to the skin weighed 4 g, delivering a single dose of 4.4 mg of the active substance acetonide triamcinolone. All participants applied the preparation to the sacrolumbar part of the spine. 3 samples were collected every day at ca. 4 h intervals from the application, with the exception of night-time.
After the end of the administration, participants were required to collect samples every day in the morning (1 sample) for 5 successive days.
2.3. Chemicals and Reagents
Reference substances: Triamcinolone acetonide and 6β-hydroxy Triamcinolone acetonide purchased from SIGMA and Toronto Research Chemicals, respectively; and D3-Testosterone and D3-Epitestosterone from the National Measurement Institute (North Ryde, Australia). Other substances used: b-glucuronidase (Escherichia coli) from Roche Diagnostics GmbH (Mannheim, Germany), disodium phosphate and sodium phosphate from Honeywell Fluka (Seelze, Germany), potassium carbonate and potassium hydrogen carbonate (both from POCH Gliwice, Poland), methyl tert-butyl ether (Rathburn, Walkerbum, Scotland). To prepare the mobile phase, the following were used: acetonitrile (Fisher Chemicals, Hampton, NH, USA), formic acid (98–100%) (Honeywell, Charlotte, NC, USA) and water prepared in-house using Millipore DirectQ UV3 system (Darmstadt, Germany—R > 18 MΩcm).
Standard solutions were prepared at the concentration of 1 mg/mL in methanol and stored at −20 °C.
2.4. Urine Samples Preparation
Internal standards (ISTD) composed of 19-D3-testosterone and D3-epitestosterone were added to urine samples. Next, 1M phosphate buffer and B-glucuronidase (Escherichia coli) were added. Samples were hydrolyzed at 50 °C for 1 h. Next, 1 mL of potassium carbonate and potassium hydrogen carbonate mixture was added. Samples were undergoes extraction (in portions, two times 3 mL) using methyl tert-butyl ether. After decanting the layer of ethyl and vaporizing the ether samples were dissolved in 100 µL of acetonitrile: water phase (1:1, v/v).
2.5. Instrument Setups
2.5.1. Liquid Chromatography
Chromatographic separation was conducted using Waters Acquity UPLC System liquid chromatograph with BEH C18 1.7 µm, 100 mm × 2.1 mm from Waters.
Mobile phase flowing through the column at 0.3 mL/min consisted of a mixture containing 0.1% formic acid in water and acetonitrile (v/v). The gradient programme are presented in Table 2.

Table 2.
Gradient programme. 11 min gradient programme was used (in reference to acetonitrile content).
2.5.2. Mass Spectrometry
The mass spectrometer operated in the positive ionisation mode (ESI+). In order to optimise conditions for mass detection, the parent substance and its metabolite were fragmented at the collision chamber of the mass spectrometer. On the basis of fragmentation patterns, the most intensive daughter ions were selected. Selected precursor ions and corresponding daughter ions used for tracking selected fragmentation reactions including collision energy values and cone voltage are listed in Table 3.

Table 3.
Analysed ion passages of the tested substances and of internal standards used in the method with corresponding collision energy and cone voltage values.
In addition, mass detector operated in the electron ionisation mode ESI+ with reaction monitoring mode MRM and scanning mode. Desolvation gas flow was set at 643 L/h at 450 °C with ion source temperature at 120 °C. Capillary voltage was 3.0 kV.
3. Results
In this chapter the validation of the analytical procedure is described, with a special attention to mass spectrometry measurements towards monitoring of TA and its metabolites.
3.1. Method Validation
3.1.1. Linearity
Selected urine samples with varying properties (pH, specific gravity) were used for drawing the calibration curves, each based on 12-point standards within concentration range 0.5–100 ng/mL. The pH of the urines ranged from acidic (4.93) through neutral to alkaline (7.42) with specific gravity between 1.005 ÷ 1.022. The respective data are presented in Table 4.

Table 4.
Characteristic of urine samples used for creating calibration curves: pH and specific gravity.
The results of calibration curves showed that the method is resistant to pH and specific gravity in the tested ranges. The obtained results show that the linearity has been maintained in the concentration range of 0.5 ng/mL ÷ 100 ng/mL.
3.1.2. Selectivity and Limit of Detection (LOD)
In order to determine selectivity and limit of detection of the method under selected experimental conditions, six different blank urine (blank urine—urine obtained from volunteers who did not use the tested drugs) were tested with and without the addition of a mixture of standards containing TA and 6β- hydroxy TA. Final concentrations for each of those two compounds were as follows: 2 ng/mL, 5 ng/mL, 10 ng/mL, 20 ng/mL, 30 ng/mL, 40 ng/mL, 50 ng/mL, 60 ng/mL, 70 ng/mL, 80 ng/mL, 90 ng/mL and 100 ng/mL. Internal standards (ISTD) containing D3-testosterone and D3-epitestosterone were added to all tested samples. Urines without the addition of the mixture of standards with the addition of internal standards were also tested. The registered chromatograms of 20 blank urine samples from different individuals were examined, finally no signals were found to interfere from the matrix for each MRM of the determined substances which is the evidence of the method being highly selective (Figure 1).
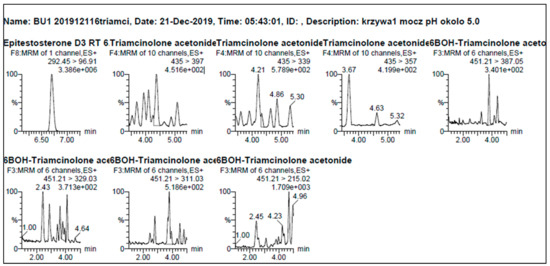
Figure 1.
Example of chromatograms of blank urine with the addition of an internal standards.
Calibration curve parameters for triamcinolone acetonide and its metabolite were collected in Table 5.

Table 5.
Triamcinolone acetonide and 6β-hydroxy-triamcinolone acetonide—calibration curve parameters a.
To determine the limit of detection (LOD) of the method the criterion was adopted that it is the lowest concentration at which the ratio of the analyte’s signal to noise determined based on the surface area of peak is ≤3 with the same shape and resolution of peak and relative retention time (±2% of real RT). Thus, LOD for triamcinolone acetonide and its metabolite was 0.5 ng/mL, which was found to be sufficient for the given purpose.
3.1.3. Carry—Over Effect
Carry-over effect was evaluated based on three subsequent injections of urine samples with the addition of standards: triamcinolone acetonide and 6β-hydroxy triamcinolone acetonide at 200 ng/mL concentration for both analytes. Six different urine’s samples were analysed. Result were assessed on the basis of the chromatograms obtained. The ratio of peak surface areas of the blank urine to those of the sample containing analytes was deemed satisfactory when it was below 0.1%. No sample carry-over effect was found in the examined analytical procedure.
3.1.4. Repeatability of Retention Times
Six different urine samples with the addition of standards were analysed: triamcinolone acetonide and 6β-hydroxy triamcinolone acetonide at 30 ng/mL final concentration for both analytes. The test was conducted for two series, over two consecutive days. Retention time precision was checked between those two days. Based on the results obtained, relative retention time was determined as a ratio of retention times of the observed compounds and of the retention time of the internal standard in each sample and for each of the two compounds. Next, standard deviation of the relative retention time (RSD) was calculated. Results are presented in Table 6.

Table 6.
Average retention times for both substances and standard deviation of the relative retention time.
Data collected in the table show that the method is characterised by stable retention times.
Below, example chromatograms were presented of selected MRM ion passages (m/z) used for analysis (Figure 2) and mass spectra of daughter ions [M-H]+ in positive ionisation mode (ESI+) (Figure 3).
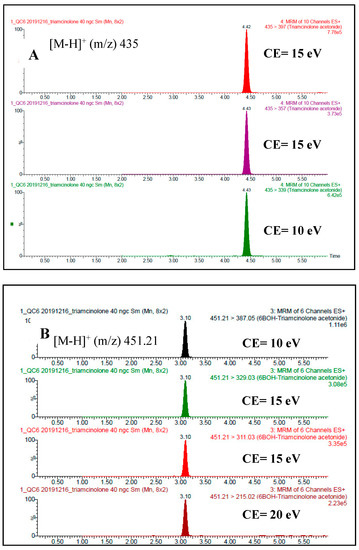
Figure 2.
Example chromatograms of monitored MRM ion passages (m/z). (A) Triamcinolone acetonide at concentration 40 ng/mL (B) 6β-hydroxy triamcinolone acetonide at concentration 40 ng/mL.
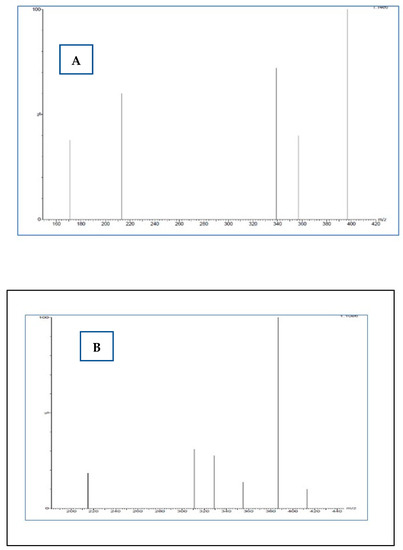
Figure 3.
Mass spectra of daughter ions [M-H]+ in positive ionisation mode (ESI+). (A) Daughter ions for m/z 451.21 (B) Daughter ions for m/z 435.
3.1.5. Real Anti-Doping Samples Testing
18 urine samples, delivered to the Polish Anti-Doping Laboratory, were tested and found to contain triamcinolone acetonide and its metabolite in course of routine tests.
Tests conducted confirmed the presence of triamcinolone acetonide and its metabolite 6β-hydroxy triamcinolone acetonide, in some cases close to or above (6β-hydroxy TA) 15 ng/mL (reporting limit of TA parent compound). Concentrations of these compounds were in the range of 1.89–29.63 ng/mL for TA and 2.16–145.30 ng/mL for 6β-hydroxy TA; results were presented in Table 7. In addition, pH and specific gravity of samples were measured and presented in Table 7 below.

Table 7.
Determined concentrations of triamcinolone acetonide and its metabolite 6β-hydroxy triamcinolone acetonide.
The results indicate that triamcinolone acetonide and its metabolite 6β-hydroxy triamcinolone acetonide were detected more frequently in male athletes, than in female athletes. The high concentrations of the metabolite of triamcinolone acetonide were detected in four samples—2 male and 2 female athletes, respectively. In two cases with a high concentration of target compounds other substances from the groups of stimulants and β-2-agonists were found. Samples 8 and 11 were collected from the same athlete in July and August of the same year. The concentrations of TA and its metabolite were lower than 30 ng/mL but higher than 15 ng/mL in one case: 2.73 ng/mL and 29.43 ng/mL, 2.45 ng/mL, and 11.14 ng/mL, respectively. Any possible trends related to the sport/discipline were not observed. For most cases, the concentration of triamcinolone acetonide was lower 2–4.5 times than 6β-hydroxy triamcinolone acetonide indicating that 6β-hydroxy triamcinolone is a much more sensitive marker of TA administration.
3.1.6. Excretion Study after Transdermal TA Administration
Samples from volunteers who used Previsone in a controlled mode in accordance with the abovementioned scheme were tested. The elimination study of TA and its metabolite were presented in Figure 4 and Figure 5 (5 male volunteers were aged between 28 and 60; 5 female volunteers were aged between 29 and 48).
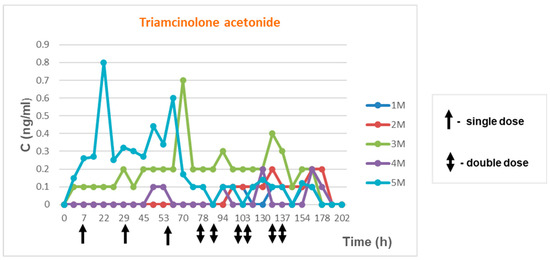
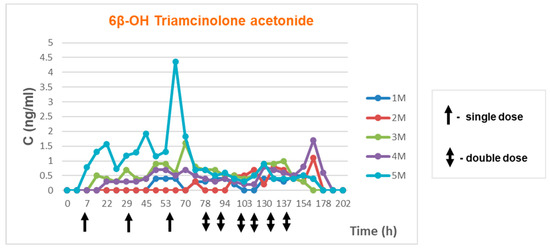
Figure 4.
Elimination of triamcinolone acetonide and its metabolite in time—men.
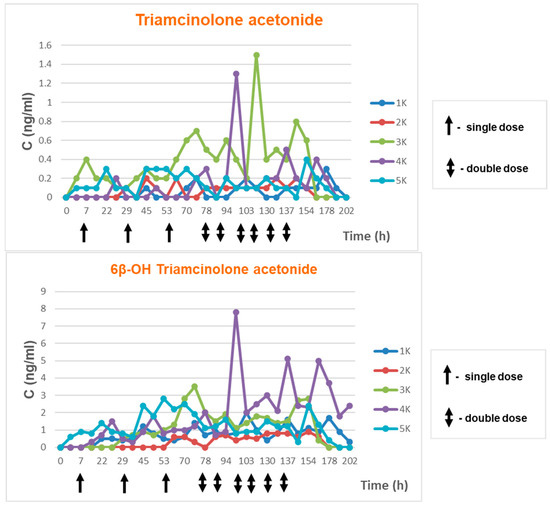
Figure 5.
Elimination of triamcinolone acetonide and its metabolite in time—women.
It has been hypothesized that women, by virtue of having greater subcutaneous lipid content, receive different doses of transdermally administered drugs so we decided to analyse genders separately and compare the results. In the publication by Kwiatkowska at all it was noticed that transdermal absorption of Ecdysterone was different between genders [18]
Urine samples marked as 01 and 02 were collected on the day preceding testing, while sample 03 on the day of testing before the first dose of the preparation.
Following samples marked as 1 ÷ 23 were collected during the course of the experiment at specified intervals. Samples 24 ÷ 28 consisted of morning urine collected on successive days following discontinuation of cream use.
Age, body weight, height and calculated Body Mass Index are presented in the Table 8.

Table 8.
Volunteers—men.
Triamcinolone acetonide concentrations observed in the tests ranged from 0.1 ng/mL to 0.8 mg/mL. Triamcinolone acetonide metabolite concentrations ranged from 0.2 ng/mL to 4.36 ng/mL. Elimination of both compounds in time are presented in Figure 4.
Age, body weight, height and calculated Body Mass Index are presented in the Table 9.

Table 9.
Volunteers—women.
Triamcinolone acetonide concentrations observed in the tests ranged from 0.1 ng/mL to 1.5 mg/mL. Triamcinolone acetonide metabolite concentrations ranged from 0.3 ng/mL to 7.80 ng/mL. Elimination of both compounds in time are presented in the Figure 5.
Elimination profile monitoring for triamcinolone acetonide showed that concentrations during the experiment ranged from 0.1 ng/mL to 1.5 ng/mL. In the case of its metabolite 6β-hydroxy triamcinolone acetonide, concentrations ranged from 0.1 ng/mL to 7.8 ng/mL. We did not see the cumulative effect at the time of the multi-dose application for concentration TA or its metabolite in urine.
Triamcinolone acetonide concentrations in all volunteers of both sexes did not exceed 1.5 ng/mL regardless of age and BMI. Meanwhile, metabolite concentrations were higher in female volunteers.
Based on data collected from all participants it may be stated that none of the concentration results exceeded 15 ng/mL, reporting limit set by WADA (in accordance with the WADA technical document).
4. Discussion
The careful evaluation of the results, namely the concentration of triamcinolone acetonide and its metabolite in urine samples collected during anti-doping controls, indicates that in cases with the estimation concentration of parent compound (triamcinolone acetonide) alone is concerned, none of the samples tested would constitute an AAF result per WADA requirements. The 15 ng/mL reporting limit stipulated in the WADA document [WADA2022MRPL] [8] was not exceeded. However, hypothetically focusing on the presence of triamcinolone acetonide metabolite 6β-hydroxy triamcinolone acetonide, the threshold would have been exceeded in four cases. The publication by Coll S and all [14] conclude that reporting limit of 5 ng/mL of TA would allow distinguishing forbidden intramuscular i.m. from allowed intranasal TA administrations as the majority of i.m. applications would not cause exceeding reporting limit of 15 ng/mL. On the other hand, intranasal application leads to the appearance of TA at much lower concentrations than reporting limit and would not cause false adverse analytical findings.
When examining WADA statistics (Table 10), one might consider whether the higher frequency of samples found to contain triamcinolone acetonide in recent years (not taking into account the initial years, where the typical effect of higher detectability of a newly listed substance was observed) is the result of increased use of the compound of interest and not reflecting whole picture because as mentioned above systemic i.m. application would not exceed reporting limit in many cases [14].

Table 10.
WADA statistics for 2004–2020 for triamcinolone acetonide when AAF was above 30 ng/mL [19].
The study confirms observations of a group of researchers from Barcelona [11,12,13] where they tested other topical routes of application of TA that the reporting limit of 15 ng/mL for TA is very safe and would not cause false adverse analytical findings with the exception for intraarticular application, which is prohibited route (according to WADA 2023 prohibited list any injectable routes are prohibited). After intraarticular application majority of samples collected from males are exceeding reporting limit for TA up to 96 h after application. In the case of females, no more than 50% would excrete TA at a concentration higher than 15 ng/mL [12]. Moreover, even single local injection of triamcinolone acetonide would lead to adverse analytical findings [13].
5. Conclusions
As regards the elimination process, it may be stated that triamcinolone acetonide is not affected by the problem occurring in the case of budesonide at least for the transdermal application., namely the observation of its major metabolite at concentrations in excess of 15 ng/mL when therapeutic doses are used via allowed routes supporting the hypothesis that lower reporting limit could be established. Nevertheless, other studies are required to test its application via other allowed routes e.g., inhalation, intranasal by recruiting a much bigger sample size and comparing the results with at least the same sample size of volunteers which administrated TA by prohibited route to be able to achieve reporting limit that would discriminate TA application by routes of administration.
Author Contributions
Conceptualization, D.K.; Methodology, D.K. and M.W.; Validation, M.W. and D.K.; Formal Analysis, M.W.; Investigation, D.K. and M.W.; Resources, D.K. and P.K.; Data Curation, D.K. and E.B.; Writing—Original Draft Preparation, D.K. and M.W.; Writing—Review & Editing, D.K. and E.B.; Visualization, D.K. and M.W.; Supervision, D.K and E.B.; Funding Acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Sport and Tourism of the Republic of Poland grant number 2019.0432/1575/UDot/BM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The presented excretion study involved ten human participants. The ethical approval from the Research Ethics Committee established at the Institute of Sport in Warsaw, Poland (KEBN-19-49-DK) was obtained. Furthermore, the written consents were received from the volunteers allowing the performance of triamcinolone acetonide excretion study and the use of urine samples for research purposes.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Pharmindex. Available online: https://pharmindex.pl/listalekow# (accessed on 15 December 2022).
- Service of Professional Information about Medicines (Serwis Fachowej Informacji o Lekach). Available online: https://www.lekinfo24.pl/wyniki-wyszukiwania?qh=triamcinolone+aceton&q=triamcinolone+aceton (accessed on 15 December 2022).
- Encyclopedia of Medicines (Encyklopedia Leków). Available online: http://medycyna.anauk.net/101-0-1443-Triamcinolone+acetonide.Encyklopedia.Lekow.htmL (accessed on 15 December 2022).
- Practical Medicine for Doctors (Medycyna Praktyczna dla Lekarzy). Available online: https://www.mp.pl/szukaj?q=triamcinolone+acetonide (accessed on 15 December 2022).
- World Anti-Doping Agency. The 2004 Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/resources/files/WADA_Prohibited_List_2004_EN.pdf (accessed on 15 December 2022).
- World Anti-Doping Agency. The 2023 Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/2022-09/2023list_en_final_9_september_2022.pdf (accessed on 3 February 2023).
- World Anti-Doping Agency. WADA Technical Document-TD2019MRPL-Minimum Required Performance Levels For Detection and Identification of Non-Threshold Substances. Available online: https://www.wada-ama.org/sites/default/files/resources/files/td2019mrpl_eng.pdf (accessed on 15 December 2022).
- World Anti-Doping Agency. WADA Technical Document-TD2022MRPL-Minimum Required Performance Levels For Detection and Identification of Non-Threshold Substances. Available online: https://www.wada-ama.org/sites/default/files/2022-01/td2022mrpl_v1.1_eng_0.pdf (accessed on 15 December 2022).
- Kaliszewski, P.; Kończak, D.; Chołbiński, P.; Wicka, M.; Michalak, D.; Kwiatkowska, D.; Lewandowska-Pachecka, S.; Namieśnik, J.; Pokrywka, A. Budesonide treatment of professional athletes and anti-doping testing-case studies. Acta Pol. Pharm.-Drug Res. 2016, 73, 229–237. [Google Scholar]
- Matabosch, X.; Pozo, O.J.; Pérez-Mña, C.; Farré, M.; Marcos, J.; Segura, J.; Ventura, R. Discrimination of prohibited oral use from authorized inhaled treatment of budesonide in sports. Ther. Drug Monit. 2013, 35, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Matabosch, X.; Pozo, O.J.; Papaseit, E.; Farré, M.; Marcos, J.; Segura, J.; Ventura, R. Detection and characterization of triamcinolone acetonide metabolites in human urine by liquid chromatography/tandem mass spectrometry after intramuscular administration. Rapid Commun. Mass Spectrom. 2014, 28, 1829–1839. [Google Scholar] [CrossRef]
- Matabosch, X.; Pozo, O.J.; Pérez-Mañá, C.; Papaseit, E.; Marcos, J.; Segura, J.; Ventura, R. Evaluation of the reporting level to detect triamcinolone acetonide misuse in sports. J. Steroid Biochem. Mol. Biol. 2015, 145, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Huang, T.Y.; Tseng, Y.C.; Chang-Chien, G.P.; Lin, S.F.; Hsu, M.C. Positive doping results caused by the single-dose local injection of triamcinolone acetonide. Forensic Sci. Int. 2014, 244, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Coll, S.; Monfort, N.; Alechaga, É.; Matabosch, X.; Pérez-Mañá, C.; Ventura, R. Additional studies on triamcinolone acetonide use and misuse in sports: Elimination profile after intranasal and high-dose intramuscular administrations. Steroids 2019, 151, 108464. [Google Scholar] [CrossRef] [PubMed]
- Coll, S.; Matabosch, X.; Llorente-Onaindia, J.; Carbó, M.L.; Pérez-Mañá, C.; Monfort, N.; Monfort, J.; Ventura, R. Elimination profile of triamcinolone hexacetonide and its metabolites in human urine and plasma after a single intra-articular administration. Drug Test Anal. 2019, 11, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Knych, H.K.; Vidal, M.A.; Casbeer, H.C.; McKemie, D.S. Pharmacokinetics of triamcinolone acetonide following intramuscular and intra-articular administration to exercised Thoroughbred horse. Equine Vet J. 2013, 45, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Allado, E.; Poussel, M.; Gambier, N.; Saunier, V.; Starck, M.; Buisson, C.; Cinquetti, G.; Albuisson, E.; Chenuel, B. SporTRIA study-a multicentre trial protocol for excretion kinetics of triamcinolone acetonide following sport-related intra-articular injections in knees: Definitions of the washout periods. BMJ Open 2021, 11, e047548. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, D.; Grucza, K.; Chajewska, K.; Konarski, P.; Wojtkowiak, K.; Drapała, A.; Wicka, M. Ecdysterone: Possible sources of origin in urine. Drug Test Anal. 2022. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency Anti-Doping Testing Figures Report. Available online: https://www.wada-ama.org/en/resources/laboratories/anti-doping-testing-figures-report (accessed on 15 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).