Development and Validation of a Bioanalytical Method for the Quantification of Nitrated Fatty Acids in Plasma Using LC-MS/MS: Application to Cardiovascular Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Consumables
2.2. Bioanalytical Method Development
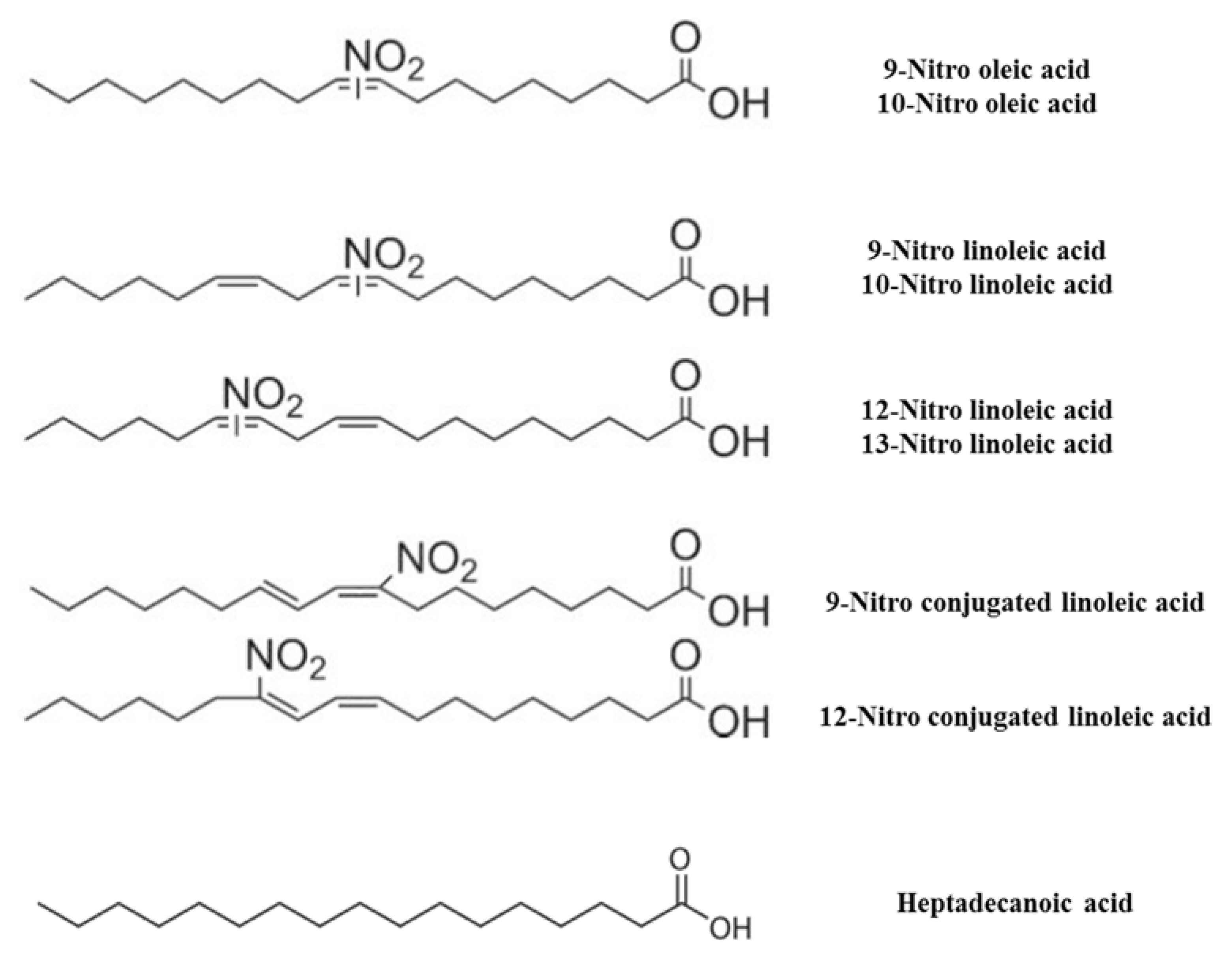
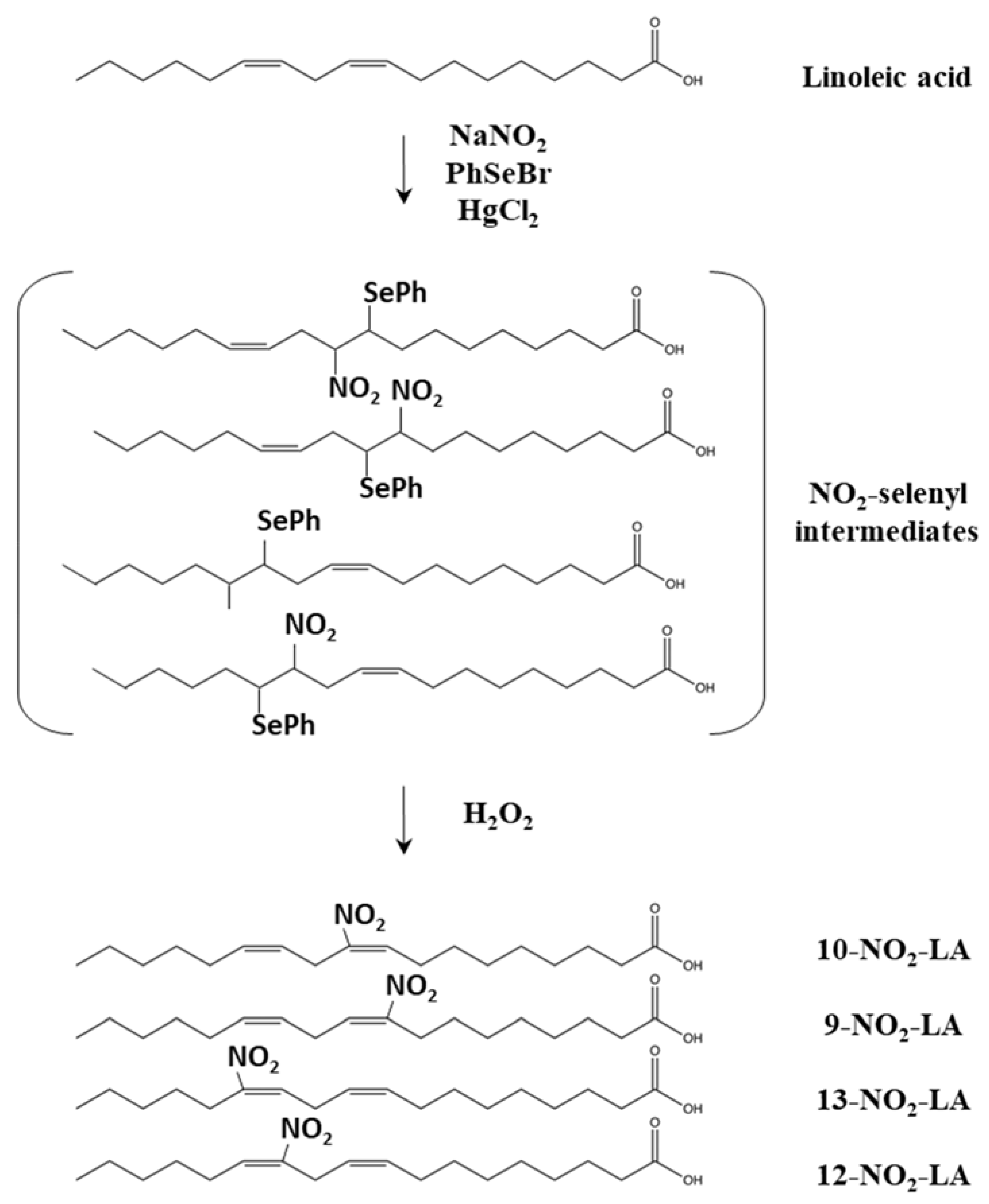
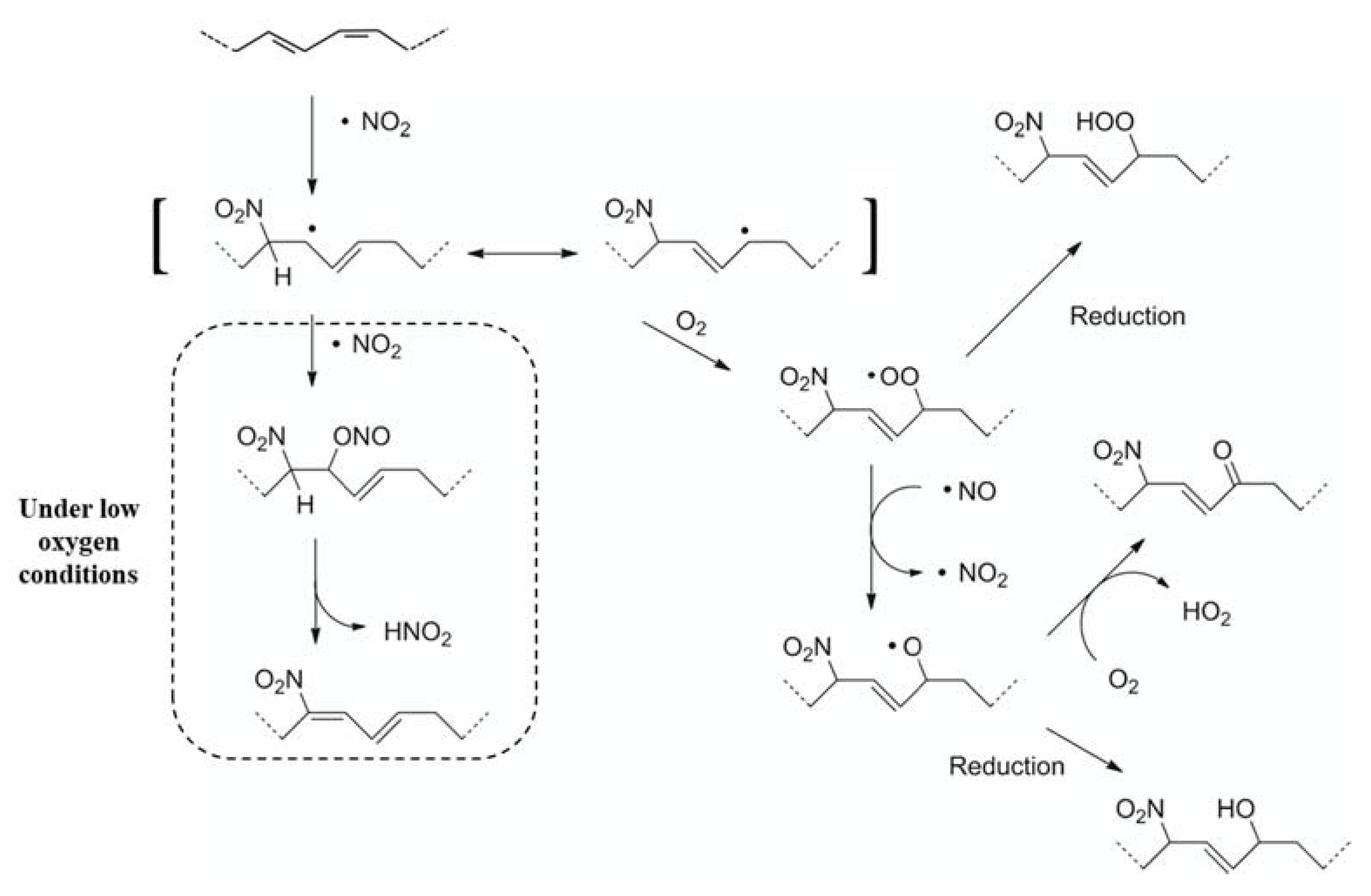
2.2.1. Synthesis of NO2-FAs
2.2.2. Optimization of MS Parameters
2.2.3. Liquid Chromatography
2.3. Blood Samples Collection
2.4. Plasma Samples Preparation
2.5. Method Validation
2.6. Quantification of NO2-FAs
2.7. Statistical Analysis
3. Results & Discussion
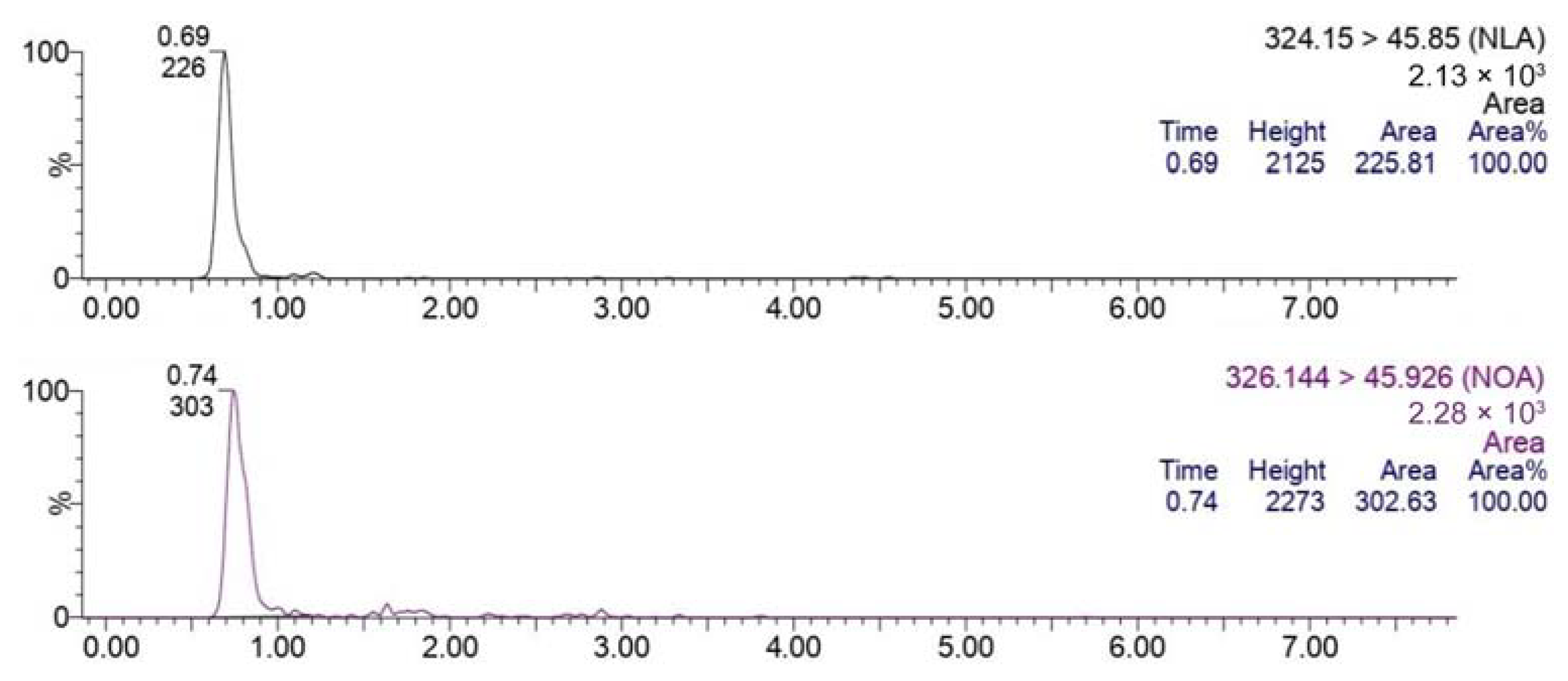
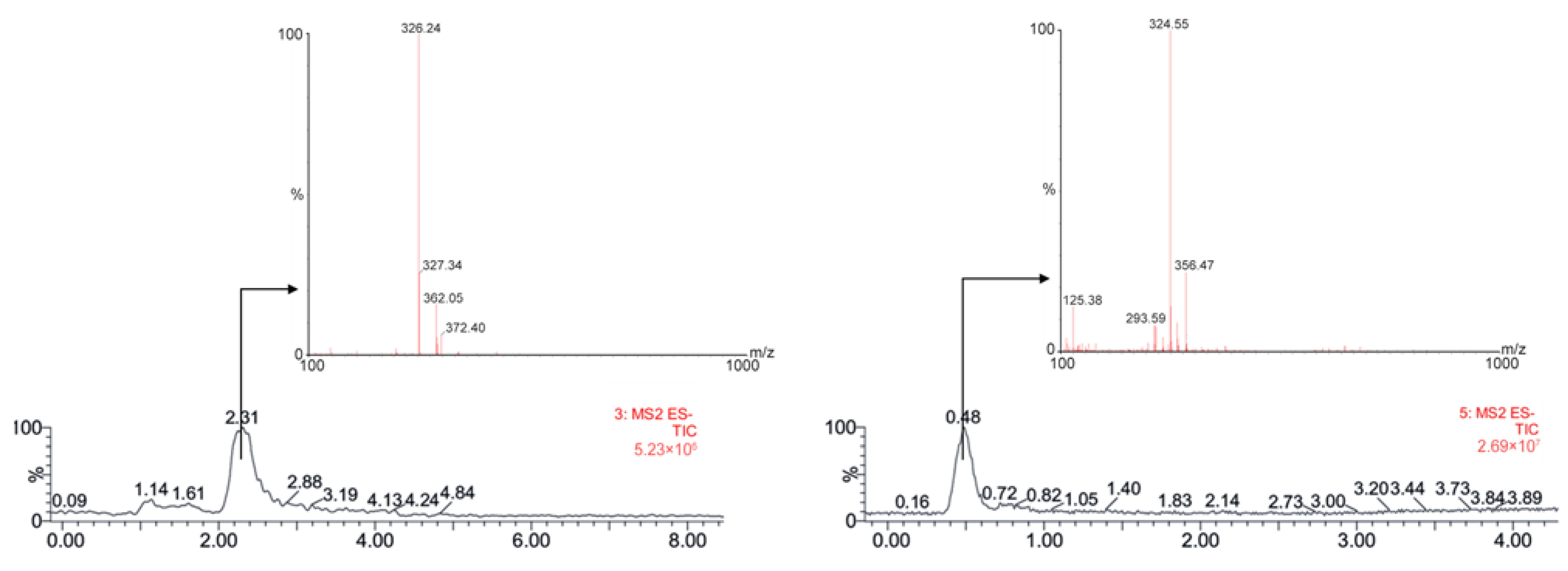
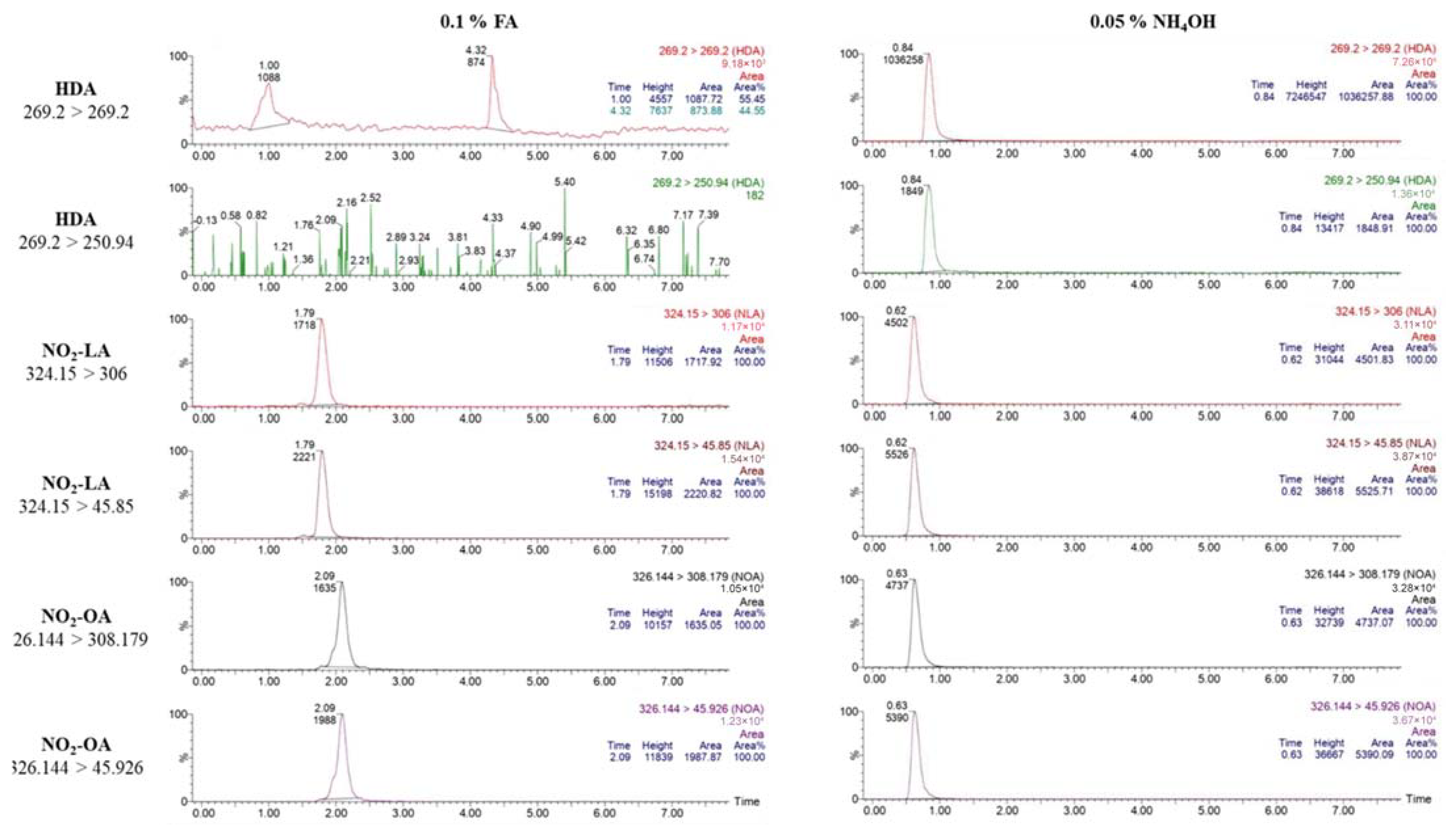
3.1. Bioanalytical Method Development and Optimization
3.2. Bioanalytical Method Validation
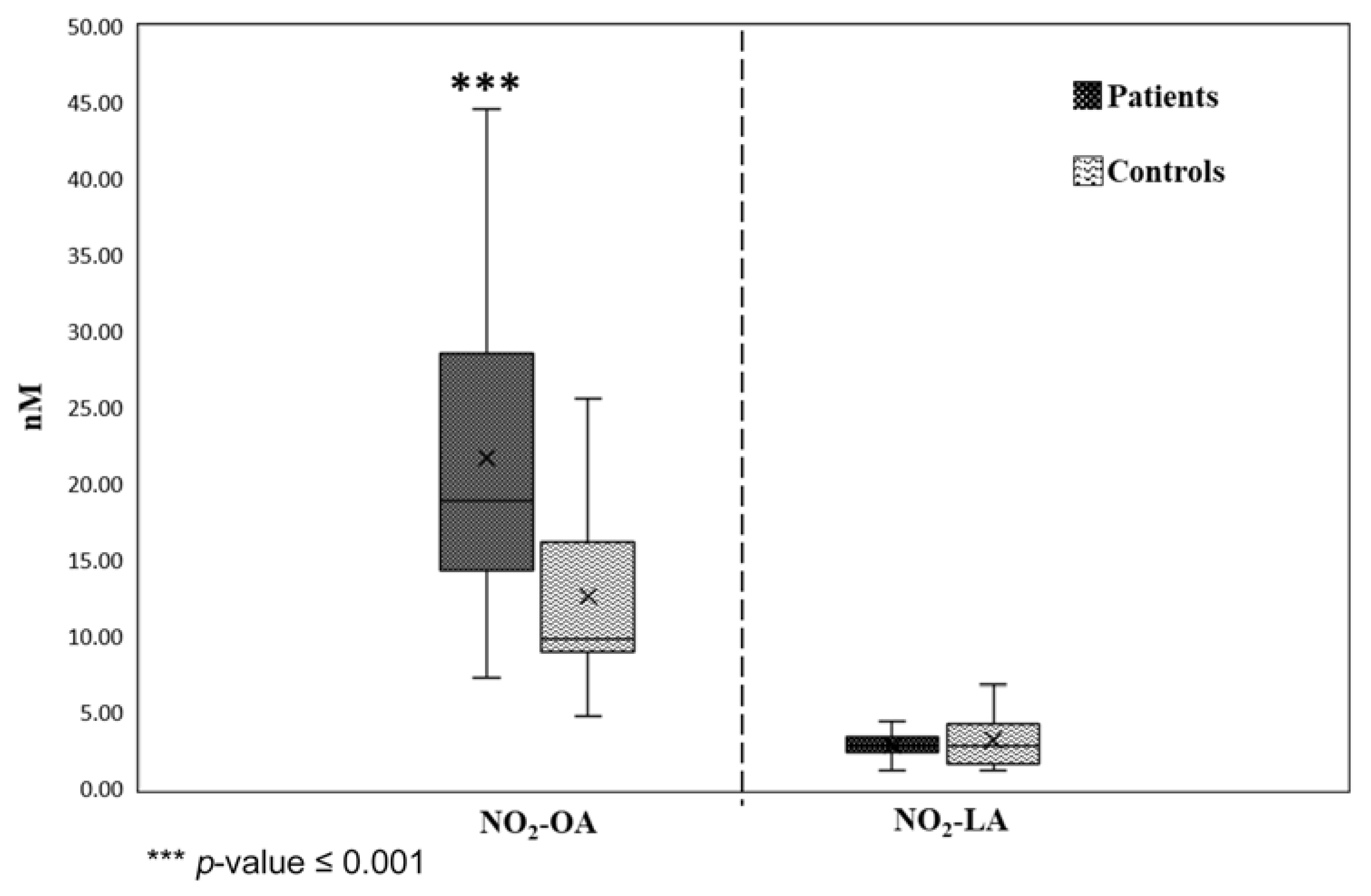
3.3. Application of Validated Method in Plasma Samples of IHD Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melo, T.; Montero-Bullón, J.F.; Domingues, P.; Domingues, M.R. Discovery of Bioactive Nitrated Lipids and Nitro-Lipid-Protein Adducts Using Mass Spectrometry-Based Approaches. Redox Biol. 2019, 23, 101106. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, V.; Rudolph, T.K.; Schopfer, F.J.; Bonacci, G.; Woodcock, S.R.; Cole, M.P.; Baker, P.R.S.; Ramani, R.; Freeman, B.A. Endogenous Generation and Protective Effects of Nitro-Fatty Acids in a Murine Model of Focal Cardiac Ischaemia and Reperfusion. Cardiovasc. Res. 2010, 85, 155–166. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Khoo, N.K.H. Nitro-Fatty Acid Logistics: Formation, Biodistribution, Signaling, and Pharmacology. Trends Endocrinol. Metab. 2019, 30, 505–519. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Cipollina, C.; Freeman, B.A. Formation and Signaling Actions of Electrophilic Lipids. Chem. Rev. 2011, 111, 5997–6021. [Google Scholar] [CrossRef] [PubMed]
- Grippo, V.; Mojovic, M.; Pavicevic, A.; Kabelac, M.; Hubatka, F.; Turanek, J.; Zatloukalova, M.; Freeman, B.A.; Vacek, J. Electrophilic Characteristics and Aqueous Behavior of Fatty Acid Nitroalkenes. Redox Biol. 2021, 38, 101756. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, S.R.; Bonacci, G.; Gelhaus, S.L.; Schopfer, F.J. Nitrated Fatty Acids: Synthesis and Measurement. Free Radic. Biol. Med. 2013, 59, 14–26. [Google Scholar] [CrossRef]
- Khoo, N.K.; Schopfer, F.J. Nitrated Fatty Acids: From Diet to Disease. Curr. Opin. Physiol. 2019, 9, 67–72. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Vitturi, D.A.; Jorkasky, D.K.; Freeman, B.A. Nitro-Fatty Acids: New Drug Candidates for Chronic Inflammatory and Fibrotic Diseases. Nitric Oxide 2018, 79, 31–37. [Google Scholar] [CrossRef]
- Kelley, E.E.; Baust, J.; Bonacci, G.; Golin-Bisello, F.; Devlin, J.E.; Croix, C.M.S.; Watkins, S.C.; Gor, S.; Cantu-Medellin, N.; Weidert, E.R.; et al. Fatty Acid Nitroalkenes Ameliorate Glucose Intolerance and Pulmonary Hypertension in High-Fat Diet-Induced Obesity. Cardiovasc. Res. 2014, 101, 352–363. [Google Scholar] [CrossRef]
- Jobbagy, S.; Tan, R.J. Nitrolipids in Kidney Physiology and Disease. Nitric Oxide Biol. Chem. 2018, 78, 121–126. [Google Scholar] [CrossRef]
- Rom, O.; Xu, G.; Guo, Y.; Zhu, Y.; Wang, H.; Zhang, J.; Fan, Y.; Liang, W.; Lu, H.; Liu, Y.; et al. Nitro-Fatty Acids Protect against Steatosis and Fibrosis during Development of Nonalcoholic Fatty Liver Disease in Mice. EBioMedicine 2019, 41, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.K.; Rudolph, V.; Edreira, M.M.; Cole, M.P.; Bonacci, G.; Schopfer, F.J.; Woodcock, S.R.; Franek, A.; Pekarova, M.; Khoo, N.K.H.; et al. Nitro-Fatty Acids Reduce Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Villacorta, L.; Chang, L.; Fan, Z.; Hamblin, M.; Zhu, T.; Chen, C.S.; Cole, M.P.; Schopfer, F.J.; Deng, C.X.; et al. Nitro-Oleic Acid Inhibits Angiotensin II-Induced Hypertension. Circ. Res. 2010, 107, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Rubbo, H. Nitrated Fatty Acids: Mechanisms of Formation, Chemical Characterization, and Biological Properties. Free Radic. Biol. Med. 2008, 44, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.A.; Baker, P.R.S.; Schopfer, F.J.; Woodcock, S.R.; Napolitano, A.; D’Ischia, M. Nitro-Fatty Acid Formation and Signaling. J. Biol. Chem. 2008, 283, 15515–15519. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Mehrkens, D.; Rudolph, V. Nitrated Fatty Acids in Cardiovascular Diseases. Nitric Oxide Biol. Chem. 2018, 78, 146–153. [Google Scholar] [CrossRef]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Dalos, D.; Spinka, G.; Schneider, M.; Wernly, B.; Paar, V.; Hoppe, U.; Litschauer, B.; Strametz-Juranek, J.; Sponder, M. New Cardiovascular Biomarkers in Ischemic Heart Disease—GDF-15, a Probable Predictor for Ejection Fraction. J. Clin. Med. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Hanafi, R.S.; Lämmerhofer, M. Quality-by-Design Approach for Development of Aqueous Headspace Microextraction GC-MS Method for Targeted Metabolomics of Small Aldehydes in Plasma of Cardiovascular Patients. Anal. Chim. Acta 2022, 1221, 340176. [Google Scholar] [CrossRef]
- Ali, S.E.; Farag, M.A.; Holvoet, P.; Hanafi, R.S.; Gad, M.Z. A Comparative Metabolomics Approach Reveals Early Biomarkers for Metabolic Response to Acute Myocardial Infarction. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Lima, É.S.; Di Mascio, P.; Rubbo, H.; Abdalla, D.S.P. Characterization of Linoleic Acid Nitration in Human Blood Plasma by Mass Spectrometry. Biochemistry 2002, 41, 10717–10722. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R.S.; Lin, Y.; Schopfer, F.J.; Woodcock, S.R.; Groeger, A.L.; Batthyany, C.; Sweeney, S.; Long, M.H.; Iles, K.E.; Baker, L.M.S.; et al. Fatty Acid Transduction of Nitric Oxide Signaling: Multiple Nitrated Unsaturated Fatty Acid Derivatives Exist in Human Blood and Urine and Serve as Endogenous Peroxisome Proliferator-Activated Receptor Ligands. J. Biol. Chem. 2005, 280, 42464–42475. [Google Scholar] [CrossRef]
- Baker, P.R.S.; Schopfer, F.J.; Sweeney, S.; Freeman, B.A. Red Cell Membrane and Plasma Linoleic Acid Nitration Products: Synthesis, Clinical Identification, and Quantitation. Proc. Natl. Acad. Sci. USA 2004, 101, 11577–11582. [Google Scholar] [CrossRef]
- Rudolph, V.; Schopfer, F.J.; Khoo, N.K.H.; Rudolph, T.K.; Cole, M.P.; Woodcock, S.R.; Bonacci, G.; Groeger, A.L.; Golin-Bisello, F.; Chen, C.S.; et al. Nitro-Fatty Acid Metabolome: Saturation, Desaturation, β-Oxidation, and Protein Adduction. J. Biol. Chem. 2009, 284, 1461–1473. [Google Scholar] [CrossRef]
- Tsikas, D.; Zoerner, A.; Mitschke, A.; Homsi, Y.; Gutzki, F.M.; Jordan, J. Specific GC-MS/MS Stable-Isotope Dilution Methodology for Free 9- and 10-Nitro-Oleic Acid in Human Plasma Challenges Previous LC-MS/MS Reports. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2895–2908. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Zoerner, A.A.; Mitschke, A.; Gutzki, F.M. Nitro-Fatty Acids Occur in Human Plasma in the Picomolar Range: A Targeted Nitro-Lipidomics GC-MS/MS Study. Lipids 2009, 44, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. I 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Li, M.J.; Xiao, H.; Qiu, Y.X.; Huang, J.H.; Man, R.Y.; Qin, Y.; Xiong, G.H.; Peng, Q.H.; Jian, Y.Q.; Peng, C.Y.; et al. Identification of Potential Diagnostic Biomarkers of Cerebral Infarction Using Gas Chromatography-Mass Spectrometry and Chemometrics. RSC Adv. 2018, 8, 22866–22875. [Google Scholar] [CrossRef] [PubMed]
- Persson, X.M.T.; Błachnio-Zabielska, A.U.; Jensen, M.D. Rapid Measurement of Plasma Free Fatty Acid Concentration and Isotopic Enrichment Using LC/MS. J. Lipid Res. 2010, 51, 2761–2765. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Pranger, I.G.; Corpeleijn, E.; Muskiet, F.A.J.; Kema, I.P.; Singh-Povel, C.; Bakker, S.J.L. Circulating Fatty Acids as Biomarkers of Dairy Fat Intake: Data from the Lifelines Biobank and Cohort Study. Biomarkers 2019, 24, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Willett, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating Biomarkers of Dairy Fat and Risk of Incident Diabetes Mellitus Among Men and Women in the United States in Two Large Prospective Cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Slim, M.; Ha, C.; Vanstone, C.A.; Morin, S.N.; Rahme, E.; Weiler, H.A. Evaluation of Plasma and Erythrocyte Fatty Acids C15:0, t-C16:1n-7 and C17:0 as Biomarkers of Dairy Fat Consumption in Adolescents. Prostaglandins Leukot. Essent. Fat. Acids 2019, 149, 24–29. [Google Scholar] [CrossRef]
- Shibata, R.; Gotoh, N.; Kubo, A.; Kanda, J.; Nagai, T.; Mizobe, H.; Yoshinaga, K.; Kojima, K.; Watanabe, H.; Wada, S. Comparison of Catabolism Rate of Fatty Acids to Carbon Dioxide in Mice. Eur. J. Lipid Sci. Technol. 2012, 114, 1340–1344. [Google Scholar] [CrossRef]
- Jenkins, B.; Aoun, M.; Feillet-Coudray, C.; Coudray, C.; Ronis, M.; Koulman, A. The Dietary Total-Fat Content Affects the In Vivo Circulating C15:0 and C17:0 Fatty Acid Levels Independently. Nutrients 2018, 10, 1646. [Google Scholar] [CrossRef]
- Strassburg, K.; Huijbrechts, A.M.L.; Kortekaas, K.A.; Lindeman, J.H.; Pedersen, T.L.; Dane, A.; Berger, R.; Brenkman, A.; Hankemeier, T.; Van Duynhoven, J.; et al. Quantitative Profiling of Oxylipins through Comprehensive LC-MS/MS Analysis: Application in Cardiac Surgery. Anal. Bioanal. Chem. 2012, 404, 1413–1426. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Baker, P.R.S.; Freeman, B.A.; Brookes, P.S. Mitochondrial Nitroalkene Formation and Mild Uncoupling in Ischaemic Preconditioning: Implications for Cardioprotection. Cardiovasc. Res. 2009, 82, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Padilla, M.N.; Mata-Pérez, C.; Melguizo, M.; Barroso, J.B. In Vitro Nitro-Fatty Acid Release from Cys-NO2-Fatty Acid Adducts under Nitro-Oxidative Conditions. Nitric Oxide Biol. Chem. 2017, 68, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, G.; Baker, P.R.S.; Salvatore, S.R.; Shores, D.; Khoo, N.K.H.; Koenitzer, J.R.; Vitturi, D.A.; Woodcock, S.R.; Golin-Bisello, F.; Cole, M.P.; et al. Conjugated Linoleic Acid Is a Preferential Substrate for Fatty Acid Nitration. J. Biol. Chem. 2012, 287, 44071–44082. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. 2013, 4, 311. [Google Scholar] [CrossRef] [PubMed]
- Granado-Casas, M.; Mauricio, D. Oleic Acid in the diet and what it does: Implications for diabetes and its complications. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: New York, NY, USA, 2019; pp. 211–229. [Google Scholar]

| Compound | Parent (m/z) | Daughter (m/z) | Cone Voltage (V) | Collision Energy (V) |
|---|---|---|---|---|
| NO2-OA | 326.1443 | 45.9263 | 25 | 10 |
| 326.1443 | 308.1792 | 25 | 8 | |
| NO2-LA | 324.1500 | 45.8500 | 25 | 10 |
| 324.1500 | 306.0000 | 25 | 8 | |
| HDA | 269.2000 | 269.2000 | 45 | 5 |
| 269.2000 | 250.9400 | 45 | 22 |
| Time (Min) | Flow Rate (ml/Min) | Mobile Phase A (%) H2O + 0.05% NH4OH | Mobile Phase B (%) ACN + 0.05% NH4OH |
|---|---|---|---|
| Initial | 0.300 | 15.0 | 85.0 |
| 0.50 | 0.300 | 15.0 | 85.0 |
| 3.50 | 0.300 | 0.0 | 100.0 |
| 4.00 | 0.300 | 0.0 | 100.0 |
| 4.50 | 0.300 | 15.0 | 85.0 |
| 8.00 | 0.300 | 15.0 | 85.0 |
| Aliquot 1 (0 nM) | Aliquot 2 (20 nM) | Aliquot 3 (40 nM) | |
|---|---|---|---|
| Plasma | 150 μL | 150 μL | 150 μL |
| IS (1 μg/mL) | 3 μL | 3 μL | 3 μL |
| Vortex | 30 s | 30 s | 30 s |
| NO2-FAs (200 nM of NO2-OA and NO2-LA) | 0 μL | 3 μL | 6 μL |
| Vortex | 30 s | 30 s | 30 s |
| Acidified MeOH | 150 μL | 147 μL | 144 μL |
| Vortex | 1 min | 1 min | 1 min |
| Incubate (4 °C) | 20 min | 20 min | 20 min |
| 0.02% BHT in DEE | 1500 μL | 1500 μL | 1500 μL |
| Vortex | 3 min | 3 min | 3 min |
| Centrifuge (4 °C, 4000 rpm) | 15 min | 15 min | 15 min |
| Filter | 1275 μL | 1275 μL | 1275 μL |
| Concentrate under vacuum, no heat | |||
| Reconstitute in 30 μL of MeOH/IPA | |||
| NO2-OA | NO2-LA | |||
|---|---|---|---|---|
| Spiked conc. (nM) | 0 | 10 | 0 | 10 |
| Peak area ratio * | 0.170 ± 0.022 | 0.224 ± 0.012 | 0.030 ± 0.001 | 0.064 ± 0.012 |
| T-test (p-value) | 0.00997 | 0.01671 | ||
| LOQ (nM) | 10 nM | 10 nM | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herz, M.M.; Gad, M.Z.; Hanafi, R.S. Development and Validation of a Bioanalytical Method for the Quantification of Nitrated Fatty Acids in Plasma Using LC-MS/MS: Application to Cardiovascular Patients. Separations 2023, 10, 87. https://doi.org/10.3390/separations10020087
Herz MM, Gad MZ, Hanafi RS. Development and Validation of a Bioanalytical Method for the Quantification of Nitrated Fatty Acids in Plasma Using LC-MS/MS: Application to Cardiovascular Patients. Separations. 2023; 10(2):87. https://doi.org/10.3390/separations10020087
Chicago/Turabian StyleHerz, Magy Maged, Mohamed Zakaria Gad, and Rasha Sayed Hanafi. 2023. "Development and Validation of a Bioanalytical Method for the Quantification of Nitrated Fatty Acids in Plasma Using LC-MS/MS: Application to Cardiovascular Patients" Separations 10, no. 2: 87. https://doi.org/10.3390/separations10020087
APA StyleHerz, M. M., Gad, M. Z., & Hanafi, R. S. (2023). Development and Validation of a Bioanalytical Method for the Quantification of Nitrated Fatty Acids in Plasma Using LC-MS/MS: Application to Cardiovascular Patients. Separations, 10(2), 87. https://doi.org/10.3390/separations10020087









