Abstract
A simple, selective, rapid, sensitive and less costly green automated solid phase extraction bio-analytical high-performance liquid chromatographic-based technique with fluorescence detection (Aut-SPE-BA-HPLC-FL) for the quantification of levofloxacin in human serum samples has been developed and validated. The serum samples were loaded into the chromatographic system without prior treatment and then injected into short (20 mm × 4.6 mm, 20 µm) protein-coated (PC) µBondapak CN (µBCN) silica pre-column (PC-µBCN-pre-column). Levofloxacin was retained and pre-concentrated on the head of the PC-µBCN-pre-column, while proteins and other polar components were eliminated using phosphate buffer saline (PBS), pH 7.4, as the first mobile phase in the extraction step. Levofloxacin is then transferred to the analytical column; ZORBAX Eclipse XDB-C18 (150 mm × 46 mm, 5 µm), through the aid of a column-switching valve technique, on-throughs the elution mode using the second mobile phase containing a methanol and phosphate buffer (0.05 M, pH 5) in a ratio of 70:30 (v/v). Levofloxacin signals were detected using a fluorescence detector operated at excitation/emission wavelengths of 295/500 nm. The proposed Aut-SPE-BA-HPLC-FL methodology showed linearity over a levofloxacin concentration range of 10–10,000 ng/mL (r2 = 0.9992), with good recoveries ranging from 87.12 to 97.55%. Because of the validation qualities in terms of linearity, recovery, precision, accuracy, selectivity and robustness, the Aut-SPE-BA-HPLC-FL method has been used in some clinical trials for therapeutic drug monitoring and the pharmacokinetic study of levofloxacin in human serum.
1. Introduction
Levofloxacin is regarded as a safe antibiotic that is frequently used to treat various bacterial infections [1]. The introduction of new methods for analyzing levofloxacin in bio-fluid samples is crucial for two reasons. First, the drug’s bactericidal activity is highly dependent on its concentration, and mainly depends on the peak plasma concentration in relation to the minimum inhibitory concentration ratio [2,3]. Second, low serum concentration levels can result in the emergence of drug resistance, which is a serious issue that can result in treatment failure and negatively impact the patient’s quality of life. In this situation, levofloxacin serum drug monitoring is crucial to avoid reaching sub-therapeutic levels [4]. Thus, it is crucial to choose analytical methodologies that are selective, simple, speedy, accurate and affordable for the therapeutic drug monitoring (TDM) of levofloxacin in bio-fluid clinical samples.
The protein rich bio-fluid samples are considered to be complex matrices for analysis via high performance liquid chromatography (HPLC) as they might interfere with the target molecule or affect the efficacy and life span of the analytical column. It is therefore important to develop easy, fast, inexpensive and precise protocols to pre-treat these samples prior to injection into the conventional HPLC system, in order to avoid damage to the column, and to avoid any errors that might take place while handling the sample that could affect the final results [5,6,7]. The most employed protocols for pre-treating protein rich bio-fluid samples for levofloxacin measurement were liquid–liquid extraction (LLE) [3,8,9,10,11] solid-phase extraction (SPE) [12,13,14,15,16,17] and protein precipitation extraction (PPE) [2,18,19,20,21,22,23,24,25,26]. Depending on the polarity difference, LLE is carried out by employing various organic solvents for the extraction step. Unfortunately, this technique is time consuming, not environmentally green as it uses large amounts of unfavorable solvents and inaccurate as it may cause a loss of the analyte or contamination of the sample, which may ultimately give misleading results. SPE (off-line) was developed to minimize the problems of sample contamination and attain pre-concentration during the analysis of trace elements. However, it is still time consuming, and liable to error. Finally, PPE is employed to produce sticky colloid proteins that precipitate at the sample’s bottom after centrifugation, leaving a clear supernatant containing the relevant molecule that is ready for analysis. Although PPE is a quick and simple procedure, it still has some significant drawbacks, including the need to use a lot of non-green solvents, the possibility that the analyte will be lost during precipitation and the requirement that the solvents used for precipitation be compatible with HPLC systems, which may require more time and effort to optimize this step.
Researchers are currently working to develop and improve extraction protocols that are more accurate and reliable than the conventional extraction techniques. Direct liquid chromatography or automated sample pretreatment protocols are necessary for the analysis of protein-rich bio-fluid samples, especially if the sample is vulnerable to degradation and maintaining stable experimental conditions is thus critical, or if the concentration level of the target analyte is low. The chromatographic system, in which the extraction process is carried out online, is assembled with the aid of a multi-port switching valve, two different columns (pre-column and analytical column) and two isocratic pumps. Short pre-columns packed with conventional silica bonded reversed phase (RP) sorbents have been employed to implement the above mentioned on-line SPE approach. These short pre-columns have been used for the cleaning up of biological samples from endogenous proteins and other interfering components while analyzing different drugs [27,28,29]. Unfortunately, these pre-columns showed some limitations caused by the precipitation and denaturation of some proteinaceous materials on the solid supports that contribute to the clogging of the pre-column or increasing of the final back pressure. In such circumstances, these pre-columns must be renewed frequently while loading fewer samples.
In the current study, we utilized a µBondapak CN (µBCN) silica protein coated (PC) pre-column (PC-µBCN-pre-column) as an online SPE tool for removing proteins and trapping levofloxacin from the serum matrix. Using such pre-column of RP nature, levofloxacin can easily reach to the interior PC-pre-column’s surface, making its adsorption and desorption via different mobile phases straightforward. One of the distinguishing characteristics of the PC-µBCN-pre-column was its small surface area towards the macromolecular proteinaceous molecules due to its modification via immobilized protein layers. Thus, the developed pre-column helps in both protein removal and analyte enrichment from clinical serum samples before being transferred to the analytical columns [30,31,32,33]. In order to find an alternate method capable of providing excellent results without requiring a complicated and pricey instrument such as the LC-MS/MS [4,34,35,36,37,38,39,40], our study entailed a research effort aimed at replacing this sophisticated instrument with a simple HPLC with fluorescence (FL) detection as an easy and low-cost chromatographic approach. The automated SPE process was then optimized and used to measure lower concentration levels of levofloxacin in real bio-fluid samples with a high degree of precision and accuracy and applied for TDM of the analyte to study its pharmacokinetic profile in healthy participants.
2. Materials and Methods
2.1. Chemicals and Reagents
Pure standard levofloxacin (>99% purity) was kindly gifted by the Egyptian Drug Authority (EDA) Administration Center (Cairo, Egypt). Disodiumhydrogen phosphate, potassium monobasic phosphate, sodium chloride, potassium chloride, sodium hydroxide, disodium salt of ethylene diamine tetra acetic acid (Sigma-Aldrich, Inc., St. Louis, MO, USA), acetic acid (Riedel-de Haën Laboratory Chemicals, Seelze, Germany) and phosphoric acid (BDH Laboratory Supplies Poole, Dorset, UK) were analytical grade. Sigma-Aldrich provided the HPLC grade methanol (Darmstadt, Germany). The µBCN silica was obtained from Waters Associates (Milford, CT, USA) and manually packed into a stainless-steel column. All reagents were made in distilled deionized water, while methanol was employed to prepare the levofloxacin stock standard solution.
2.2. Mobile Phases
Two different isocratic elution systems were used in the Aut-SPE-BA-HPLC-FL methodology. The first one was formed of phosphate buffer saline (PBS) (pH 7.4) and used as a washing mobile phase (MI) in order to carry serum sample onto the PC-µBCN-pre-column for proteins removal and levofloxacin capture. The second one was 70% methanol in 0.05 M phosphate buffer of pH 5 and employed as an analytical mobile phase (MII) to assist in the measurement stage. Using a 0.45 µm regenerated cellulose membrane (Agilent Technologies, Santa Clara, CA, USA), freshly prepared MI and MII were filtered on the day of use followed by ultrasonic degassing for five minutes.
2.3. Instrumentation
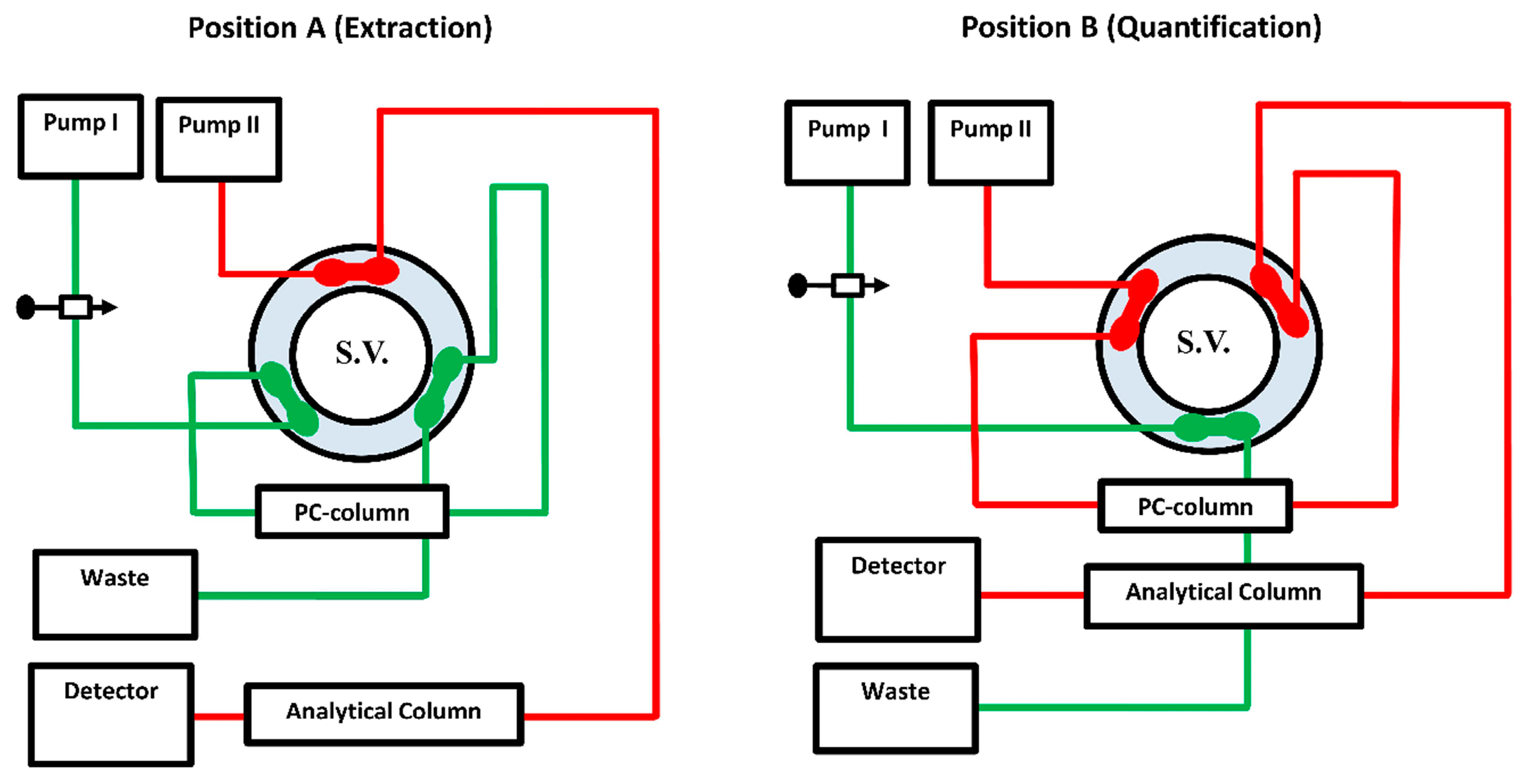
A green automated SPE bio-analytical HPLC-based technique with FL detection (Aut-SPE-BA-HPLC-FL) was employed (Figure 1). The configuration of the manifold has two pumps (Agilent 1100 Series Iso pump G1310A, Agilent Technologies, Santa Clara, CA, USA); one pump is utilized to load serum sample onto the laboratory-prepared PC-µBCN-pre-column (20 mm × 4.6 mm i.d., 20 µm) that was positioned in the sample preparation site, and the second pump is used to on-through flashing of levofloxacin from the PC-µBCN-pre-column to “ZORBAX Eclipse XDB-C18” as an analytical column (Agilent Technologies) (150 mm × 46 mm i.d., 5 µm). An injector, model 7725i coupled with a 100-µL sample loop and a high-pressure flow switching valve, model 7000 (Rheodyne, Berkeley, CA, USA), were used to load the bio-fluid sample and promote on-through flushing of levofloxacin from the sample preparation site onto the measurement site via the two mobile phases pumping at 1 mL/min. With an excitation wavelength of 295 nm and an emission wavelength of 500 nm, an Agilent 1200 series, G1321A FL detector was operated to monitor the eluents. Data acquisition was accomplished using Agilent LC ChemStation software. All experimental results were obtained under an ambient temperature (21 ± 1 °C).
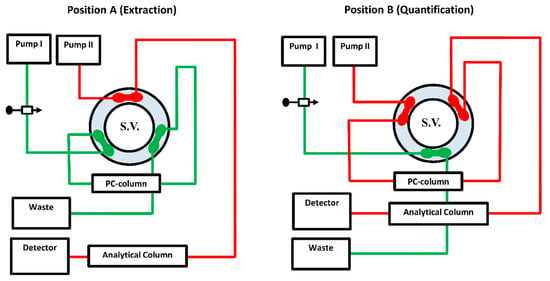
Figure 1.
The system manifold of the Aut-SPE-BA-HPLC-FL approach at position (A) was designed for loading, washing, trapping and pre-concentration of levofloxacin. Position (B), after being segregated from the extraction side, depicts the on-through elution mode of the target analyte followed by separation and quantification. A 6-port switching valve (S.V.) connects the PC-µBCN-pre-column to an ODS analytical column.
2.4. Aut-SPE-BA-HPLC-FL
The Aut-SPE-BA-HPLC-FL system is well illustrated in Figure 1. Firstly, the switching valve is initially adjusted over the extraction stage, and the MI is then pushed into the PC-µBCN-pre-column by the aid of pump I for equilibration, while the MII through pump II equilibrates the ODS analytical column. An aliquot of a 100-µL serum sample is then placed into the sample loop before being conveyed to the PC-µBCN-pre-column. To get rid of any macromolecular proteinaceous fragment or other weakly retained polar matrix ingredients, the PC-µBCN-pre-column was flushed with 2 mL of the MI flowing at 1 mL/min. Next, the switching valve is shifted to the quantification stage, allowing the analytical column and the PC-µBCN-pre-column to share the same channel. Throughout this configuration, the MII can pass the remaining clean portion that contains levofloxacin from the PC-µBCN-pre-column to the ODS analytical column at a flow rate of 1 mL/min, allowing levofloxacin to be separated and quantified. After a minute, the switching valve is finally moved back to the sample preparation stage, allowing the PC-µBCN-pre-column to regain its initial state via the MI and to be ready for further analysis.
2.5. Standard Solutions and Quality Control Samples
The levofloxacin stock standard solution (10 mg/mL) was prepared by mixing an adequate amount of the analyte with methanol. The stock standard solution was utilized to construct the matrix-matched calibration standards in the blank serum to achieve the calibration curve’s seven concentration levels ranging from 10 to 10,000 ng/mL. The serum calibration standards were frozen (−20 °C) until they were used for analysis. Similarly, the same stock solution was utilized to prepare quality control (QC) serum samples within the concentration range of 40–8000 ng/mL, which are then stored at −20 °C for method validation. Before chromatographic run, the frozen serum calibration standards and QCs were allowed to thaw at an ambient temperature (20 ± 1 °C), and then centrifuged at 2000× g for 5 min. The thawed serum samples should be filtered through 0.45 µm filters of regenerated cellulose polypropylene (Agilent Technologies, Santa Clara, CA, USA) before being loaded into the injector, since the thawed bio-fluid sample may contain tiny solid particles, which could cause a buildup of the pre-column back pressure. Finally, the clear portion containing levofloxacin was analyzed using the Aut-SPE-BA-HPLC-FL method.
2.6. Recovery and Precision
The concentration of the analyte measured from the spiked sample (found) to that stated to be presenting (nominal) were compared in order to determine the relative recovery of levofloxacin from the serum sample as follows:
Precision of the Aut-SPE-BA-HPLC-FL method was calculated by analyzing each of the four QC samples five times versus the standard calibration curve. The precision was estimated as the coefficient of variation percent (CV%) of the mean found concentrations acquired from intra-day validation (on the same day) and inter-day validation (over five consecutive days).
2.7. Application
The fully validated Aut-SPE-BA-HPLC-FL methodology was successfully employed for the TDM of levofloxacin in the blood samples from five Egyptian participants to study the pharmacokinetic profile of the drug. Through a medical history and physical examination, each participant was determined to be in good health. Their mean age ± SD was 28.46 ± 4.22 years. The average body weight was 81.53 ± 12.07 kg. After fasting for at least 8 h, all volunteers were given a single oral dose of 500 mg levofloxacin tablet with 250 mL of pure water. The venous blood samples for the levofloxacin pharmacokinetic study were collected in covered centrifuge tubes at 0, as well as 0.5, 1, 2, 3, 4, 6, 8, 10, 12 and 24 hours’ post-dose and centrifuged for 10 min at 2000× g (5 °C). Serum specimens were then transferred directly to Eppendorf polypropylene tubes and kept at −20 °C until analysis. The Ethics Committee of the Faculty of Pharmacy, Suez Canal University approved the research protocol (approval number: 202301RH1).
3. Results
3.1. Chromatographic Conditions
Firstly, the serum sample passes through PC-µBCN-pre-column via the aid of the MI, where macromolecules matrix ingredients as proteins are subjected to extraction from the column’s packing materials using the size exclusion liquid chromatographic (SE-LC) mode, while levofloxacin is retained on the PC-µBCN-pre-column’s head using the RP-LC mode for enrichment. In-order to avoid any problems with the PC-µBCN-pre-column, as protein precipitation or denaturation can cause blockage of the pre-column inlet, the MI should be compatible with the serum matrix nature. The PBS (pH 7.4) (MI) was chosen as an optimum MI condition to ensure extraction of proteins via the SE-LC mode and trapping of the analyte on the PC-µBCN-pre-column via the RP-LC mode. Secondly, by rotating the switching valve to the analytical site, the trapped levofloxacin is flushed in an on-through elution mode onto a ZORBAX Eclipse XDB-C18HPLC analytical column and becomes ready to be quantified using the aid of the MII. Thus, it was important to choose the MII in a way to achieve a high retention time for levofloxacin on the analytical column to prevent any interference from the co-eluted serum components. The selectivity of the Aut-SPE-BA-HPLC-FL method to quantify levofloxacin from the co-eluted serum components was dependent on choosing the optimum constituents for the MII. Since methanol has the dominant ability to affect the retention behavior of levofloxacin, a mixture of methanol and 0.05 M phosphate buffer (adjusted to pH 5 with ortho-phosphoric acid) in a ratio of 70:30 (v/v) was selected as the MII to achieve the best separation conditions for levofloxacin from the co-eluted, non-extracted matrix ingredients.
3.2. Properties of Columns and Switching Valve Timing
The dimensions and particle size of the two columns (pre-analytical and analytical columns) are important and were chosen carefully. When compared with the analytical column’s packing material, which has a particle size of 5 μm, the pre-column’s material has a particle size of 20 μm, which was thought to be a reasonable compromise between the adsorption capacity for small molecular analyte via RP-LC without clogging and the low surface area for macromolecular proteins to be extracted out via SE-LC. Also, the shortness of the PC-µBCN-pre-column (20 mm × 4.6 mm i.d.) packed with 20 µm µBCN particles made the on-line SPE of proteins more rapid and efficient. Moreover, the internal diameters of pre-analytical and analytical columns should be exactly the same (4.6 mm) to minimize the broadening of the peaks as much as possible. In addition, the peak broadening can be reduced by selecting a pre-column whose retention capacity is smaller than that of the analytical column under the experimental conditions. All of the aforementioned characteristics made it possible to inject the suitable volume of serum without having to worry about protein precipitation, denaturation as well as peak broadening.
To ensure appropriate extraction of proteins while trapping levofloxacin in the sample preparation stage, two minutes with a flow rate of 1 mL/min of MI were found to be enough. In order to avoid further dispersion and peak broadening, it was crucial for the transfer time (a period of time taken to transfer the clean portion of the injected untreated serum sample containing levofloxacin from the PC-µBCN-pre-column to the quantification stage) to be short. It takes less than one minute to completely elute levofloxacin from the PC-µBCN-pre-column into the quantification position by using 70% methanol in MII. To sum up, the sample is first injected into the extraction setup and attained for two minutes in this position. Second, the switching valve is turned into the quantification position and maintained in this configuration for one minute. The manifold is then rotated back to its starting position (extraction position) to prepare the PC-µBCN-pre-column for the subsequent injection.
3.3. On-Through Elution Mode
It is crucial to transfer the clean fraction of the sample containing levofloxacin completely from the extraction site to the quantification site, since the whole purpose of the current method is to extract the analyte on-line chromatographically from the serum and lower the interferences caused by the endogenous ingredients. Thus, it must be ensured that the retained portion of the clean sample containing the analyte is well-matched with the RP-HPLC post-separation at the measurement site. For this purpose, the RP µBCN silica packing materials of the pre-column can perform both the on-line SPE and RP-LC modes. These characteristics ensure the successful elution of levofloxacin from the PC-µBCN-pre-column’s head onto the analytical ODS column via the MII for the final separation and quantification, while the unwanted matrix proteins are pre-extracted with the MI to the waste without reaching the analytical column. In this case, an on-through elution mode is important to prolong the lifespan of the PC-pre-column.
3.4. Breakthrough Study of PC-µBCN-Pre-Column
To ensure that the optimum pre-column conditions are attained, a breakthrough study was conducted. Taking peak broadening into consideration, levofloxacin’s retention time during SE-LC for serum proteins should be high, and during its elution to the quantification position it should be low. This could be achieved by using silica sorbents with low hydrophobicity, but that still possess the ability to successfully trap the analyte via their RP performance. To do so, QC samples containing levofloxacin 40 and 8000 ng/mL were directly injected onto the PC-µBCN-pre-column via the MI, then a large volume of the same mobile phase was pumped before rotating the six-port switching valve to the quantification stage for separating and quantifying levofloxacin via the MII. The flushing of the PC-µBCN-pre-column with MI for 10 min showed levofloxacin recoveries ranging from 87.12 to 97.55%, indicating that the analyte was completely trapped on the pre-column till the extraction step was over.
3.5. PC-µBCN-Pre-Column Lifespan
In order to ensure the efficiency of the PC-µBCN-pre-column and its ability to fully trap the analyte and clean up the injected serum matrix from its endogenous proteinaceous components, the lifetime expectancy was tested as a function of the bio-fluid sample volumes. It was observed that the PC-µBCN-pre-column could be utilized to load up to 250 times the volume of 100 µL serum samples. Increasing the loaded volumes beyond these levels caused a gradual buildup of the PC-µBCN-pre-column back pressure, which eventually leads to clogging. Therefore, it is advised to routinely change the PC-µBCN-pre-column with a new one when the pre-column back-pressure starts to build up.
3.6. Greenness Assessment of the Developed On-Line SPE Protocol
In comparison to traditional sample preparation techniques such as LLE, PPE and off-line SPE, automated SPE utilizing PC-µBCN-pre-column and phosphate buffer saline, pH 7.4, is less expensive, less time-consuming, nondestructive and green. The Analytical Eco-Scale semi-quantitative approach [41] was used to assess how environmentally friendly the developed extraction protocol was. Based on the obtained results, the proposed on-line SPE process has an excellent green score of 93 points.
3.7. Method Validation
3.7.1. Linearity, Limit of Detection and Limit of Quantification
The calibration standards of levofloxacin were prepared with concentrations ranging from 10 to 10,000 ng/mL. This range was chosen based on the concentrations following administration of therapeutic doses of levofloxacin in order to mimic its therapeutic concentration levels in real biological samples. Plotting the peak areas measured using the Aut-SPE-BA-HPLC-FL method (n = 5) against the corresponding concentrations ranging from 10 to 1000 ng/mL of levofloxacin enabled the construction of a standard calibration curve. The linear equation of least square regression, Y = a + bx, was used to estimate the correlation coefficient, where Y represents the peak area of levofloxacin and x is its corresponding concentration. The characteristic parameters of the standard calibration curve are summarized in Table 1.

Table 1.
The Aut-SPE-BA-HPLC-FL method’s characteristic parameters for the regression equation used to measure levofloxacin concentrations in human serum.
One of the main goals of the current study was to increase the sensitivity as much as possible by using a FL detection system and large injected volume (100 µL). The limits of detection (LOD) and quantification (LOQ) of levofloxacin via the proposed method were experimentally determined to be 2.5 and 8.5 ng/mL, respectively.
3.7.2. Method of Absolute Recovery
The absolute extraction recovery using the Aut-SPE-BA-HPLC-FL methodology was estimated by comparing levofloxacin peak areas of directly injected serum specimens to that of standard analyte solutions with the same concentration levels. The Aut-SPE-BA-HPLC-FL technique was revealed to have high extraction recoveries ranging from 85.78 to 96.42%.
3.7.3. Precision
The relative recovery of levofloxacin via the proposed method was demonstrated by comparing the concentration of the analyte from measuring the spiked serum sample to the concentration stated to be present. The Aut-SPE-BA-HPLC-FL method, with its excellent online SPE achievement, showed good recoveries ranging from 87.12 to 97.55% (Table 2). As for precision, the intra-day (on the same day) and the inter-day results (over 5 consecutive days) were satisfactory, with CV% being in the ranges 3.72–8.98% and 4.19–9.54%, respectively.

Table 2.
Intra- and inter-day validation of the Aut-SPE-BA-HPLC-FL technique for measuring levofloxacin in human serum.
3.7.4. Selectivity
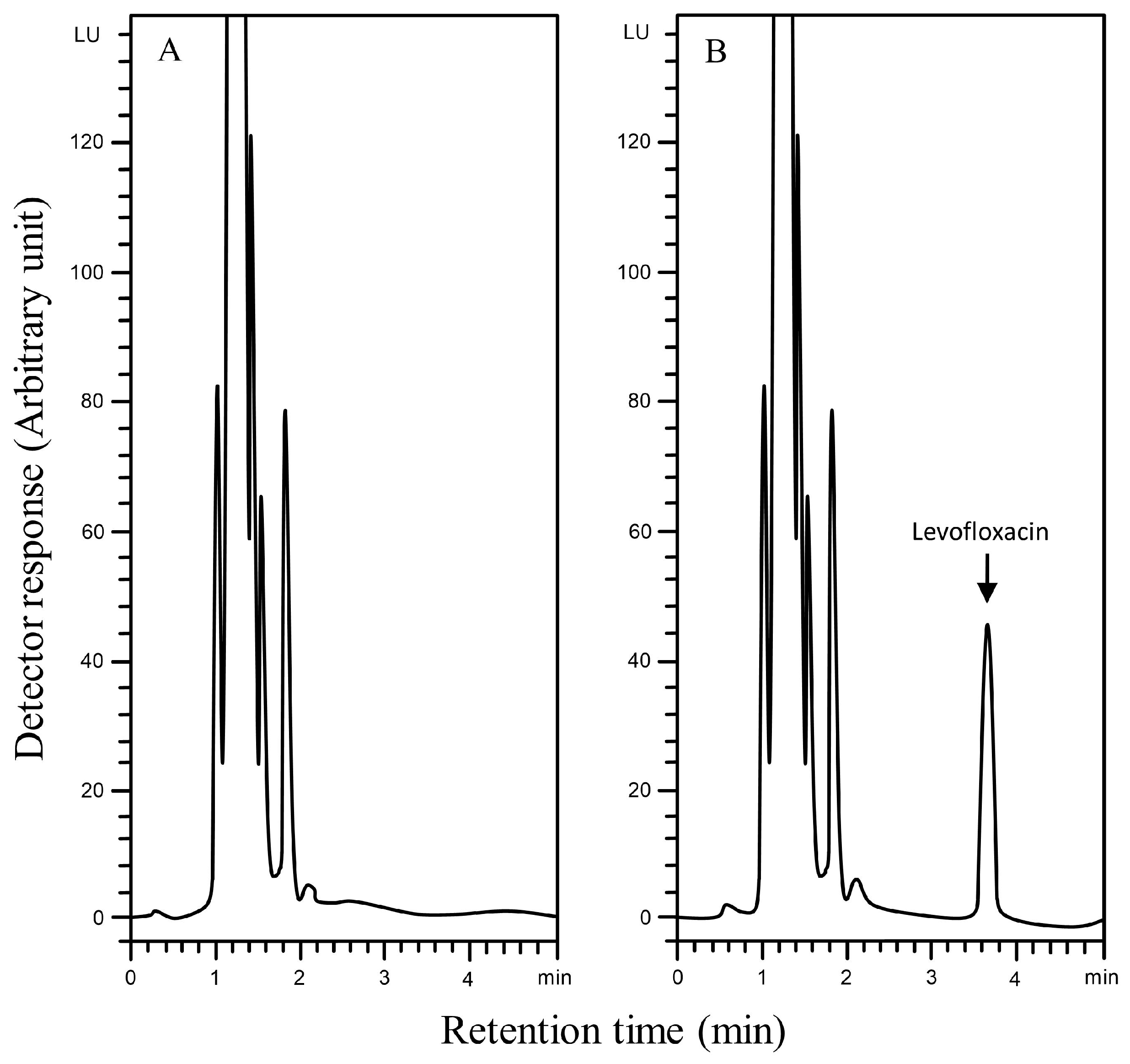
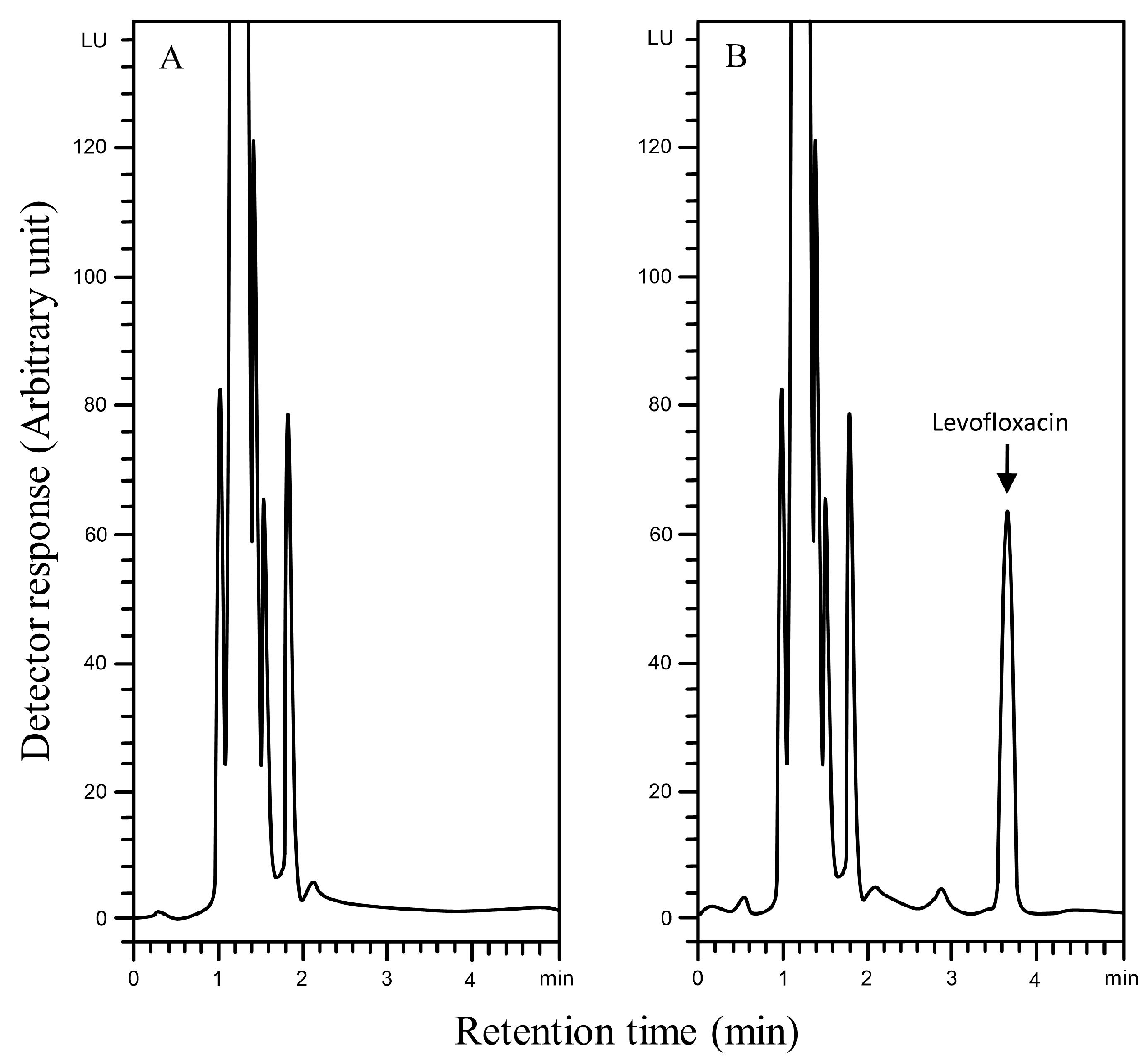
The selectivity of the Aut-SPE-BA-HPLC-FL technique was assessed by analyzing blank serum samples from different adult healthy donors to know if the endogenous matrix components will interfere with the peak of levofloxacin and hence affect the method’s accuracy and precision. Also, blank serum spiked with levofloxacin at low and high concentration levels were analyzed. The results did not show any significant interference near the detected levofloxacin peak, implying that the Aut-SPE-BA-HPLC-FL technique can achieve good selectivity towards the analyte without any interferences from matrix endogenous constituents (Figure 2A,B). The applicability of the Aut-SPE-BA-HPLC-FL technique for the TDM of levofloxacin was also assessed by measuring its concentration levels in human serum specimens following oral administration of a 500 mg therapeutic dose. Figure 3A,B exhibit the chromatograms of blank and clinical serum samples collected at 0 and 10 h, respectively, from the same volunteer after oral administration of a 500-mg levofloxacin tablet.
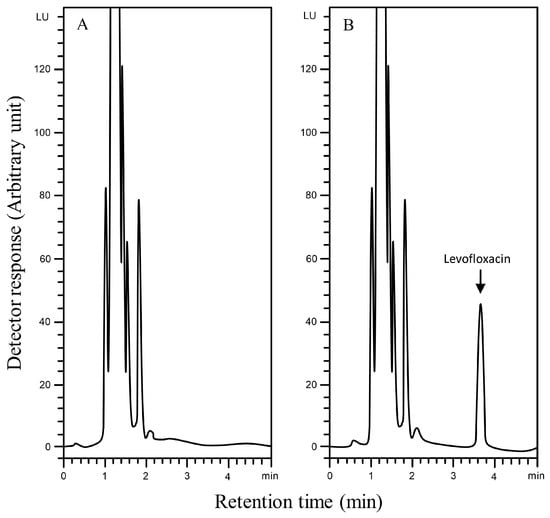
Figure 2.
Typical chromatograms using the Aut-SPE-BA-HPLC-FL technique for levofloxacin measurement in human serum, (A): blank serum; (B): blank serum spiked with 2000 ng/mL levofloxacin.
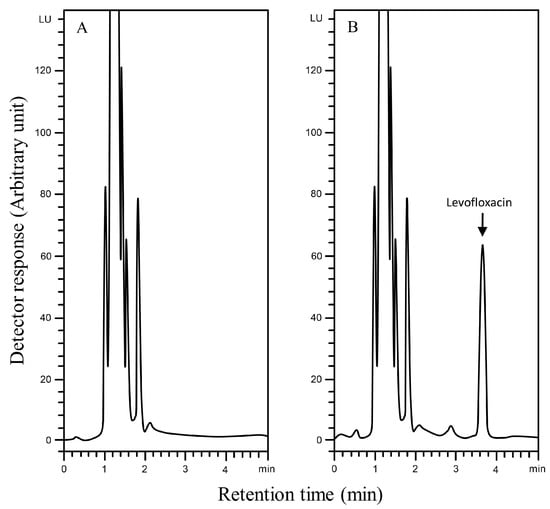
Figure 3.
Typical chromatograms using the Aut-SPE-BA-HPLC-FL technique for measuring levofloxacin in serum, (A): blank serum taken from a participant before the medication; (B): serum sample taken from the same participant 10 h after being given a single 500 mg tablet of levofloxacin.
3.7.5. Stability Studies
To ensure that the results of the Aut-SPE-BA-HPLC-FL methodology were reliable and valid, it was important to test for the stability of the serum specimens throughout handling and storage. Quality control samples with concentrations of 40 and 8000 ng/mL were subjected to stability tests. Three cycles of freezing and thawing were tested, as well as long-term stability at −20 °C for four weeks, short-term stability at room temperature for eight hours and 24-h stability at 5 °C. The levofloxacin concentration was determined following each storage period and compared to a newly produced sample containing the same concentration level. No significant differences in the mean peak areas of levofloxacin after three freeze–thaw cycles were observed. Moreover, there were no differences in the chromatograms after storing serum samples contain levofloxacin for one day and 4 weeks at 5 °C and −20 °C, respectively. Finally, the analyte remained stable at room temperature (21 ± 1 °C) for at least 8 h.
3.7.6. Robustness
The robustness of the proposed Aut-SPE-BA-HPLC-FL technique was examined to assess the capability of the whole method in terms of the extraction and separation of levofloxacin from the other endogenous components in serum matrices by intentionally causing small changes in the chromatographic conditions such as the methanol content in the MII, the pH, as well as the flow rate of both mobile phases. It was observed that changing the strength of methanol by ±2% did not have any significant effect on the chromatographic performance of the proposed technique. Additionally, the 2% variation in the flow rate as well as the pH of the MI and MII did not significantly affect the retention behavior of levofloxacin.
3.8. Application of the Aut-SPE-BA-HPLC-FL Technique
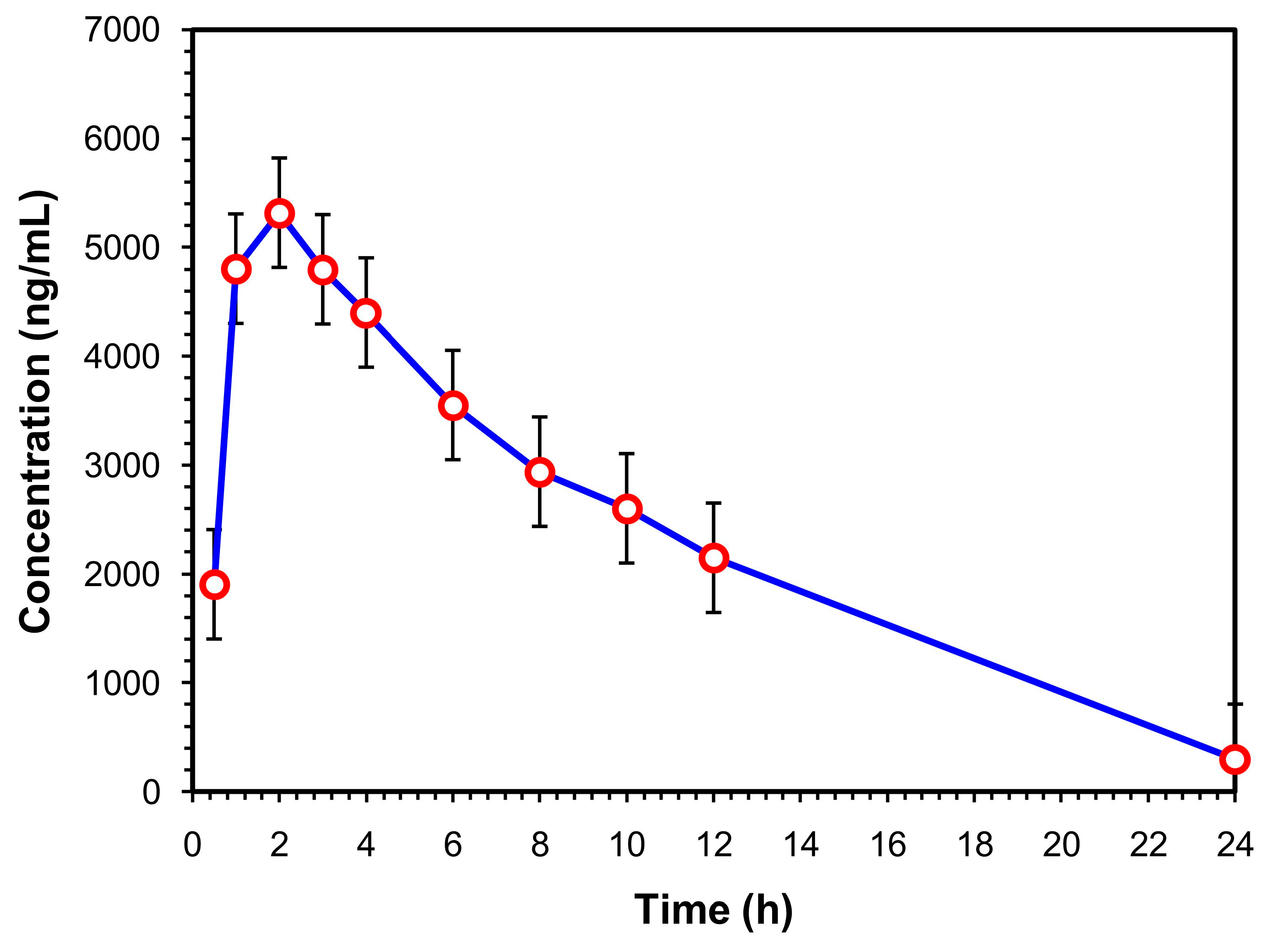
The current investigation’s goal is to confirm whether it is feasible to use the Aut-SPE-BA-HPLC-FL manifold to examine the pharmacokinetic profile of levofloxacin in healthy participants. The clinical serum specimens were donated by five Egyptian participants who were given a single 500-mg levofloxacin oral dose. Prior to administration as well as 0.5, 1, 2, 3, 4, 6, 8, 10, 12 and 24 h later, blood samples were obtained. The highest selectivity of the proposed technique was illustrated in the chromatograms of drug-free human serum samples and clinical serum samples taken at various intervals (Figures S1–S55). The fact that the measured levofloxacin concentrations fell within the reported concentration range (500–6000 ng/mL) [42] suggests that the Aut-SPE-BA-HPLC-FL approach can be employed for the TDM of levofloxacin in clinical serum samples. The estimated pharmacokinetic parameters included maximum serum concentration (Cmax), time to reach maximum concentration (Tmax), half-life of the drug (t1/2), mean residence time (MRT), area under the serum concentration–time curve from zero to the time of the last measured concentration (AUC0-t), area under serum concentration–time curve from zero to infinity (AUC0-inf), apparent volume of distribution after extravascular administration (V/F), apparent serum clearance of drug after extravascular administration (Cl/F) and finally terminal rate constant (λz). The non-compartmental pharmacokinetic analysis was performed using the PK solver add-in program for Microsoft Excel. The presentation of all data is as a mean ± standard deviation (SD) (Table 3). The average serum concentrations at various time intervals are given in Table S1 and graphically presented in Figure 4. Based on the obtained analysis data, the Cmax was reached after Tmax of 2.20 h with a value of 5453.20 ± 398.98 ng/mL. The estimated t1/2 was 4.33 ± 0.48 h, which corresponded to MRT of 8.19 ± 0.33 h. In addition, the data analysis showed an AUC0-t of 57,757.00 ± 2463.44 ng/mL·h, while the AUC0-inf was estimated to be 59,601.40 ± 2093.06 ng/mL·h. The obtained data provide a V/F and Cl/F of 0.05 ± 0.01 (mg)/(ng/mL) and 0.008 ± 0.00 (mg)/(ng/mL)/h, respectively, after extravascular administration. Based on the previously mentioned data, the TDM of levofloxacin using the developed Aut-SPE-BA-HPLC-FL technique provides accurate and comparable results to the other reported methods [40,43], and the obtained data when used in pharmacokinetic study are indicative of the administered analyte.

Table 3.
Estimates of levofloxacin’s pharmacokinetic characteristics following a single oral dosage of a 500-mg tablet (n = 5).
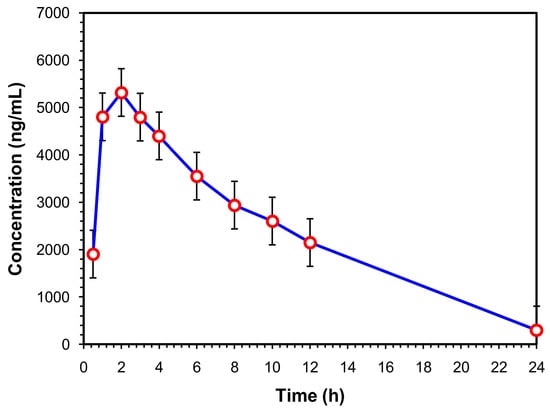
Figure 4.
Mean serum concentration–time curve of a single 500-mg levofloxacin tablet administered orally (n = 5).
4. Conclusions
Selection of the suitable sample preparation procedure and the use of selective sorbents that remove matrix interferences and boost selectivity are essential, since sample preparation is the most laborious phase of analysis, especially for complex human bio-fluid matrices. PC pre-column was prepared to overcome the problems of the traditional pre-treatment procedures prior to chromatographic analysis by the loss of adsorption capacity for matrix proteins via SE-LC while possessing noticeable RP characteristics for the retention of relatively small molecule analytes [44]. This mixed-mode feature is advantageous for the PC-µBCN-pre-column for the simultaneous cleanup, trapping and enrichment of levofloxacin directly from untreated serum specimens. Furthermore, the capacity of the PC-µBCN-pre-column is large enough to be loaded with a volume of 100 μL at least, with a subsequent increasing of the sensitivity of the whole technique. Since it is an automated extraction procedure, an internal standard, which was an additional step in the conventional HPLC methods, is no longer required. As for the economic aspect, the PC-µBCN-pre-column is prepared manually in the laboratory in a simple and reasonably priced process, which lowers the overall cost and time for the whole procedure [45]. Indeed, there is no need to employ an expensive instrument such as MS to trace levofloxacin in human serum, as it is easier to use an HPLC with an inexpensive FL detection system. Finally, this method is considered as a green extraction protocol, since there is no need to consume harmful solvents for sample preparation. As a result, the Aut-SPE-BA-HPLC-FL approach was suitable for the TDM of levofloxacin and could be a promising technique for measuring additional antibiotics directly from clinical samples due to the characteristics of the PC-µBCN-pre-column and the economic and environmental aspects of the sample preparation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10020136/s1.
Author Contributions
H.E.: resources, methodology, validation, and writing—original draft; H.S. and M.M.: writing—review and editing, formal analysis and validation; A.A. and Y.A.: methodology, resources and results interpretation; W.Z.: conceptualization, methodology, and results interpretation; G.H. and S.E.: conceptualization and supervision; L.B.: sample analysis, results interpretation, and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was authorized by the Ethics Committee of the Faculty of Pharmacy at Suez Canal University (approval number: 202301RH1), and it was carried out in accordance with the Declaration of Helsinki’s criteria.
Informed Consent Statement
Written informed consents to publish this information were obtained from the study participants.
Data Availability Statement
The data presented in this study are available in the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Gindy, A.; Emara, S.; Mostafa, A. UV partial least-squares calibration and liquid chromatographic methods for direct quantitation of levofloxacin in urine. J. AOAC Int. 2007, 90, 1258–1265. [Google Scholar] [PubMed]
- Czyrskia, A.; Szałekb, E. An HPLC method for levofloxacin determination and its application in biomedical analysis. J. Anal. Chem. 2016, 71, 840–843. [Google Scholar] [CrossRef]
- Schulte, S.; Ackermann, T.; Bertram, N.; Sauerbruch, T.; Paar, W.D. Determination of the newer quinolones levofloxacin and moxifloxacin in plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. Sci. 2006, 44, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Hateren, K.V.; Vrubleuskaya, N.; Koster, R.; Touw, D.; Alffenaar, J.C. Determination of levofloxacin in human serum using liquid chromatography tandem mass spectrometry. J. Appl. Bioanal. 2018, 4, 16–25. [Google Scholar] [CrossRef]
- Emara, S.; Morita, I.; Tamura, K.; Razee, S.; Masujima, T.; Mohamed, H.; Gizawy, S.; Rabbat, N. Utility of ion-pair chromatography for analysis of some anthracyclines in plasma and urine. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 681–692. [Google Scholar] [CrossRef]
- Hadad, G.; Salam, R.A.; Emara, S. Validated and optimized high-performance liquid chromatographic determination of tizoxanide, the main active metabolite of nitazoxanide in human urine, plasma and breast milk. J. Chromatogr. Sci. 2012, 50, 509–515. [Google Scholar] [CrossRef]
- Emara, S.; Razee, S.; Khedr, A.; Masujima, T. On-line precolumn derivatization for HPLC determination of methotrexate using a column packed oxidant. Biomed. Chromatogr. 1997, 11, 42–46. [Google Scholar] [CrossRef]
- Kumar, T.M.; Srikanth, G.; Rao, J.V.; Rao, K.S. Development and validation of HPLC-UV method for the estimation of levofloxacin in human plasma. Int. J. Pharm. Pharm. Sci. 2011, 3, 247–250. [Google Scholar]
- Wong, F.A.; Juzwin, S.J.; Flor, S.C. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J. Pharm. Biomed. Anal. 1997, 15, 765–771. [Google Scholar] [CrossRef]
- Srinivas, N.; Narasu, L.; Shankar, B.P.; Mullangi, R. Development and validation of a HPLC method for simultaneous quantitation of gatifloxacin, sparfloxacin and moxifloxacin using levofloxacin as internal standard in human plasma: Application to a clinical pharmacokinetic study. Biomed. Chromatogr. 2008, 22, 1288–1295. [Google Scholar] [CrossRef]
- Pea, F.; Di Qual, E.; Cusenza, A.; Brollo, L.; Baldassarre, M.; Furlanut, M. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin. Pharmacokinet. 2003, 42, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC applications of in-tube solid phase microextraction: A two-decade overview. Molecules 2020, 25, 2096. [Google Scholar] [CrossRef] [PubMed]
- Toi, P.V.; Pouplin, T.; Tho, N.D.K.; Phuong, P.N.; Chau, T.T.H.; Thuong, N.T.; Heemskerk, D.; Hien, T.T.; Thwaites, G.E. High-performance liquid chromatography with time-programmed fluorescence detection for the quantification of levofloxacin in human plasma and cerebrospinal fluid in adults with tuberculous meningitis. J. Chromatogr. B 2017, 1061, 256–262. [Google Scholar]
- Nemutlu, E.; Kır, S.; Özyüncü, Ö.; Beksaç, M.S. Simultaneous separation and determination of seven quinolones using HPLC: Analysis of levofloxacin and moxifloxacin in plasma and amniotic fluid. Chromatographia 2007, 66, 15–24. [Google Scholar] [CrossRef]
- Djabarouti, S.; Boselli, E.; Allaouchiche, B.; Ba, B.; Nguyen, A.T.; Gordien, J.B.; Bernadou, J.M.; Saux, M.C.; Breilh, D. Determination of levofloxacin in plasma, bronchoalveolar lavage and bone tissues by high-performance liquid chromatography with ultraviolet detection using a fully automated extraction method. J. Chromatogr. B 2004, 799, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ebers, A.; Stroup, S.; Mpagama, S.; Kisonga, R.; Lekule, I.; Liu, J.; Heysell, S. Determination of plasma concentrations of levofloxacin by high performance liquid chromatography for use at a multidrug-resistant tuberculosis hospital in Tanzania. PLoS ONE 2017, 12, e0170663. [Google Scholar] [CrossRef]
- Caufield, W.V.; Stewart, J.T. Determination of zidovudine and levofloxacin in human plasma by reversed phase and solid phase extraction. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 1791–1805. [Google Scholar] [CrossRef]
- Böttcher, S.; von Baum, H.; Hoppe-Tichy, T.; Benz, C.; Sonntag, H.G. An HPLC assay and a microbiological assay to determine levofloxacin in soft tissue, bone, bile and serum. J. Pharm. Biomed. Anal. 2001, 25, 197–203. [Google Scholar] [CrossRef]
- Aguilar-Carrasco, J.C.; Hernández-Pineda, J.; Jiménez-Andrade, J.M.; Flores-Murrieta, F.J.; Carrasco-Portugal, M.D.C.; López-Canales, J.S. Rapid and sensitive determination of levofloxacin in microsamples of human plasma by high-performance liquid chromatography and its application in a pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 341–345. [Google Scholar] [CrossRef]
- Szerkus, O.; Jacyna, J.; Wiczling, P.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Ultra-high performance liquid chromatographic determination of levofloxacin in human plasma and prostate tissue with use of experimental design optimization procedures. J. Chromatogr. B 2016, 1029, 48–59. [Google Scholar] [CrossRef]
- Notario, D.; Martono, S.; Ikawati, Z.; Hakim, A.R.; Jannah, F.; Lukitaningsih, E. A rapid and simple high-performance liquid chromatographic method for determination of levofloxacin in human plasma. Indones. J. Chem. 2017, 17, 54–62. [Google Scholar] [CrossRef]
- Siewert, S. Validation of a levofloxacin HPLC assay in plasma and dialysate for pharmacokinetic studies. J. Pharm. Biomed. Anal. 2006, 41, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Aldawsari, M.F.; Iqbal, Z.; Khuroo, A. Determination of levofloxacin in human plasma by HPLC: Method development, validation, stability evaluation and application to pharmacokinetics. Lat. Am. J. Pharm. 2020, 39, 1149–1158. [Google Scholar]
- Neckel, U.; Joukhadar, C.; Frossard, M.; Jäger, W.; Müller, M.; Mayer, B.X. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 2002, 463, 199–206. [Google Scholar] [CrossRef]
- Sousa, J.; Alves, G.; Campos, G.; Fortuna, A.; Falcão, A. First liquid chromatography method for the simultaneous determination of levofloxacin, pazufloxacin, gatifloxacin, moxifloxacin and trovafloxacin in human plasma. J. Chromatogr. B 2013, 930, 104–111. [Google Scholar] [CrossRef]
- Watabe, S.; Yokoyama, Y.; Nakazawa, K.; Shinozaki, K.; Hiraoka, R.; Takeshita, K.; Suzuki, Y. Simultaneous measurement of pazufloxacin, ciprofloxacin, and levofloxacin in human serum by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 2010, 878, 1555–1561. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Grellet, J.; Ba, B.B.; Quentin, C.; Saux, M.C. Simultaneous determination of levofloxacin, gatifloxacin and moxifloxacin in serum by liquid chromatography with column switching. J. Chromatogr. B 2004, 810, 77–83. [Google Scholar] [CrossRef]
- Martin, P.D.; Jones, G.R.; Stringer, F.; Wilson, I.D. Comparison of normal and reversed-phase solid phase extraction methods for extraction of β-blockers from plasma using molecularly imprinted polymers. Analyst 2003, 128, 345–350. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, I. Recent advances of online coupling of sample preparation techniques with ultrahigh performance liquid chromatography and supercritical fluid chromatography. J. Sep. Sci. 2019, 42, 226–242. [Google Scholar] [CrossRef]
- Emara, S.; Kamal, M.; Hadad, G.; Zaazaa, H.; Kawi, M.A. Back-flush column-switching technique for on-line sample cleanup and enrichment to determine guaiphenesin in human serum. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 15–27. [Google Scholar] [CrossRef]
- Emara, S.; Khedr, A.; Askal, H. Rapid and specific precolumn extraction high-performance liquid chromatographic assay for bupivacaine in human serum. Biomed. Chromatogr. 1996, 10, 131–134. [Google Scholar] [CrossRef]
- Emara, S. Simultaneous determination of caffeine, theophylline and theobromine in human plasma by on-line solid-phase extraction coupled to reversed-phase chromatography. Biomed. Chromatogr. 2004, 18, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, H.; Ebrahim, H.; Malak, M.; Ali, A.; Aboulella, Y.; Hadad, G.; Emara, S.; Shawky, A. Application of a small protein-coated column to trap, extract and enrich carbamazepine directly from human serum for direct chromatographic analysis. Separations 2023, 10, 71. [Google Scholar] [CrossRef]
- Szerkus, O.; Jacyna, J.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Robust HPLC-MS/MS method for levofloxacin and ciprofloxacin determination in human prostate tissue. J. Pharm. Biomed. Anal. 2017, 132, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Song, X.; Liu, Z.; Zhao, X.; Geng, L.; Bi, K.; Chen, X. Development of an LC-MS method for determination of three active constituents of Shuang-huang-lian injection in rat plasma and its application to the drug interaction study of Shuang-huang-lian freeze-dried powder combined with levofloxacin injection. J. Chromatogr. B 2012, 898, 130–135. [Google Scholar] [CrossRef]
- Rossmann, J.; Schubert, S.; Gurke, R.; Oertel, R.; Kirch, W. Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC-MS/MS. J. Chromatogr. B 2014, 969, 162–170. [Google Scholar] [CrossRef]
- Xu, H.; Chen, L.; Sun, L.; Sun, X.; Du, X.; Wang, J.; Wang, T.; Zeng, Q.; Wang, H.; Xu, Y.; et al. Microwave-assisted extraction and in situ clean-up for the determination of fluoroquinolone antibiotics in chicken breast muscle by LC-MS/MS. J. Sep. Sci. 2011, 34, 142–149. [Google Scholar] [CrossRef]
- Bao, D.; Truong, T.T.; Renick, P.J.; Pulse, M.E.; Weiss, W.J. Simultaneous determination of rifampicin and levofloxacin concentrations in catheter segments from a mouse model of a device-related infection by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008, 46, 723–727. [Google Scholar] [CrossRef]
- Ziarrusta, H.; Val, N.; Dominguez, H.; Mijangos, L.; Prieto, A.; Usobiaga, A.; Etxebarria, N.; Zuloaga, O.; Olivares, M. Determination of fluoroquinolones in fish tissues, biological fluids, and environmental waters by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 6359–6370. [Google Scholar] [CrossRef]
- Cao, G.; Zhu, Y.; Xie, X.; Chen, Y.; Yu, J.; Zhang, J.; Chen, Z.; Pang, L.; Zhang, Y.; Shi, Y. Pharmacokinetics and pharmacodynamics of levofloxacin in bronchial mucosa and lung tissue of patients undergoing pulmonary operation. Exp. Ther. Med. 2020, 20, 607–616. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Ternds Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Tilea, B.; Vlase, L.; Popa, D.-S.; Primejdie, D.; Muntean, D.L.; Tilea, I. Therapeutic monitoring of levofloxacin: A new LC-MS/MS method for quantification of levofloxacin in human plasma. Stud. Univ. Babes Bolyai Chem. 2013, 58, 105–115. [Google Scholar]
- Chien, S.C.; Chow, A.T.; Rogge, M.C.; Williams, R.R.; Hendrix, C.W. Pharmacokinetics and safety of oral levofloxacin in human immunodeficiency virus-infected individuals receiving concomitant zidovudine. Antimicrob. Agents Chemother. 1997, 41, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Emara, S.; El-Gindy, A.; Mesbah, M.K.; Hadad, G.M. Direct injection liquid chromatographic technique for simultaneous determination of two antihistaminic drugs and their main metabolites in serum. J. AOAC. Int. 2007, 90, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Emara, S.; Masujima, T.; Zarad, W.; Kamal, M.; Fouad, M.; El-Bagary, R. An Eco-friendly direct injection HPLC method for methyldopa determination in serum by mixed-mode chromatography using a single protein-coated column. J. Chromatogr. Sci. 2015, 53, 1353–1360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).