Abstract
Coriander (Coriandrum sativum L.) is among the most widely used medicinal and aromatic plants. It is well known for its multiple health benefits, most of which are correlated with its phenolic composition. Four phenolic compounds were identified in the extracts of aerial parts of coriander extracts, including caffeic acid, isoquercitrin, quercetin-3-O-glucuronide, and rutin. Caffeic acid was the major compound in the extracts. A Box–Behnken Design (BBD) was employed in conjunction with the response surface methodology (RSM) to develop an ultrasound-assisted extraction method for the determination of phenolic compounds in the aerial parts of coriander using the level of caffeic acid as the target response. The following working variables were evaluated: methanol level in the extraction solvent, temperature, sonication time, and liquid-to-solvent ratio. It was found that the methanol concentration is the most significant factor that influences the recovery of caffeic acid. The optimal extraction conditions were: 10 min as the extraction time, 70 °C as the temperature, 50% for methanol in water as the solvent, and 6.51 mL of solvent per gram of sample. The repeatability and reproducibility were calculated and RSD values below 6% were obtained in both cases. The new method was employed for the extraction of real coriander samples and it is suggested that this method could potentially be applied for quality control analyses.
1. Introduction
In the Mediterranean diet, the consumption of spices is common, and there is a high demand for fresh spices due to their various health benefits. Natural phenolic compounds found in spices play an important role as antioxidant substances with multiple medicinal properties [1,2,3]. They are a widely distributed group of secondary metabolites in the plant kingdom and are ubiquitous in all plant organs [4].
Hydroxycinnamic acids, including caffeic acid, are non-flavonoids that belong to the phenolic acid family. They are found in abundance in fruits and vegetables [5]. Caffeic acid is widely distributed in plant tissues, and it is mainly present in food sources that are mostly esterified with quinic acid [6], including coffee drinks, blueberries, apples, and cider. It has been found with the highest level in coriander.
Coriander (Coriandrum sativum L.) is a medicinal and aromatic plant that belongs to the Apiaceae family; it has been used since ancient times for cooking, medication, and flavoring [7,8,9,10]. It is indigenous to Mediterranean countries, and it has spread to the rest of the world [11]. It is an annual herb that is usually used as a condiment or a spice and it provides two types of herbal material, namely the leaves and fruit. Coriander seeds are a popular seasoning agent used in liqueurs, tea, meat products, and pickles. Leaves are a good source of vitamins (vitamin C and vitamin A) and minerals [12,13]. Owing to the presence of a multitude of bioactive substances, including phenolic compounds, several pharmacological activities have been ascribed to some parts of this herb [8,14]. It is also used in the prevention of food spoilage and diseases [15]. According to Middleton et al. [16] and Barros et al. [17], coriander extracts avoid the formation of atherosclerotic plaque, increase anti-inflammatory responses, present good antihypertensive and antiarrhythmic effects, and act as antiviral and anti-carcinogenic agents. These effects are correlated with the presence of caffeic acid [18]. Therefore, an analytical method for the determination of phenolic compounds in coriander samples, and specifically the levels of caffeic acid, would be interesting for the development of additional studies on the biological effects of coriander included in the human diet.
As with most solid food samples, phenolic compounds must be extracted before a final analytical determination can be made. In fact, extraction is a critical step in both the analysis and exploitation of phenolic compounds [19,20]. An inappropriate extraction technique can produce chemical and enzymatic reactions that produce changes in the phenolic compounds from the plant samples [19]. Hence, it is important to select the best extraction method, which should be simple and rapid for analytical and industrial applications [19,21]. Ultrasound-assisted extraction (UAE) is an easy and simple technique based on the transmission of ultrasound through liquids. An ultrasound can lead to the disruption of plant cell walls by the ultrasonic waves, thereby enhancing the mass transfer and accelerating the release of extractable compounds. Hence, it is one of the most suitable methods for the extraction of bioactive compounds from plants [22,23].
Several parameters condition the recovery efficiency of an extraction method. First, these include the chemical nature of the sample, the solvent used, and the working experimental variables, including agitation, extraction time, sample/solvent ratio, and temperature [3,24,25]. It must be noted, however, that some degradation reactions, including oxidation and hydrolysis, can appear during the extraction procedures. Therefore, short extraction times are recommended to avoid reductions in the yield of the extraction due to degradation reactions. [26]. UAE-based methods for natural products must be carefully developed and evaluated [27,28,29].
The optimization of an extraction method allows the performance to be improved and is also an indispensable means to achieve an accurate analysis and to increase the extraction recovery of bioactive compounds [30]. The response surface methodology (RSM) is an effective multivariable statistical tool that can be used to optimize such processes [30]. RSM enables the evaluation of the effects of each factor and the interaction effects between factors [31,32].
It has been reported that the antioxidant properties are more potent in coriander extracts obtained from aerial parts rather than seeds [33]. Furthermore, reports on the extraction of caffeic acid from the aerial parts of coriander using UAE have not been published to date. However, there is a study about the recovery of phenolic acids from coriander using an alkaline hydrolysis clean-up step with a solid-phase extraction method [34] and several studies about the recovery of phenolic compounds from coriander seeds, with most of them using polar solvents, including methanol [35] and ethanol [36], or specific extraction conditions, including subcritical water extraction [37] and supercritical CO2 extraction [38]. UAE has been applied on the raffinate after the supercritical fluid extraction (SFE) of coriander seeds to obtain some phenolics not recovered in the SFE [39].
The work described here aimed to identify the phenolic compounds of coriander aerial parts, and then to develop and validate a new UAE method that can provide a full recovery of the main phenolics, specifically the caffeic acid, from coriander samples. The Box–Behnken design (BBD) and RSM were employed to optimize and study the interaction effects of the independent variables.
2. Materials and Methods
2.1. Chemicals and Reagents
HPLC-grade methanol, acetonitrile, and acetic acid were purchased from Merck (Darmstadt, Germany). Caffeic acid, isoquercitrin, quercetin-3-O-glucuronide, and rutin were obtained from Sigma–Aldrich (St. Louis, MO, USA). Water was purified with a Milli-Q purification system (Millipore, Billerica, MA, USA). Calibration curves (8 points) were prepared for the phenolic compounds. The standards were dissolved in methanol at 1000 mg L−1 and then diluted with MilliQ water to reach values between 1 and 200 mg L−1. The square coefficients of regression ranged from 0.997 for rutin to 0.999 for caffeic acid. LOD ranged from 0.10 to 0.17 mg L−1 for caffeic acid and rutin, respectively. The LOQ was 0.30 and 0.53 mg L−1 for the same compounds.
2.2. Coriander Sample Preparation
The samples were fresh vegetative parts of coriander obtained from a supermarket in Spain. Fresh coriander material was ground using a kitchen mixer and the resulting material was homogenized and stored in a closed bottle in the fridge prior to use. The developed extraction method was applied to four different Spanish coriander samples.
2.3. Extraction of Phenolic Compounds
The extraction was performed using an ultrasound apparatus (UP200S ultrasonic system, Hielscher Ultrasonics GmbH, Teltow, Germany) composed of an ultrasound generator and a water bath coupled to a temperature controller.
The sample was prepared by mixing 10 mL of solvent with the required amount of coriander in an extraction tube according to the experimental design. The mixture was subjected to sonication for 10 min at different temperatures. The different independent variables and their ranges were as follows: liquid-to-solid ratio (10:2–10:1 mL g−1), methanol concentration (100–50%), and temperature (70–10 °C). At the end of the extraction, the mixture was centrifuged at 6000 rpm for 5 min and the supernatant was removed and adjusted to 25 mL with the appropriate solvent. The extract was stored at 4 °C and filtered through a nylon filter (0.22 µm) prior to injection into a UPLC-PDA system.
2.4. Ultra-High Performance Liquid Chromatography (UPLC) Analysis
The identification and quantification of phenolic compounds were carried out using an ACQUITY UPLC H-Class system with a UV-Visible detector and controlled by Empower TM 3 Chromatography Data Software. The injection volume was 3 µL. The separation was performed on a reverse-phase C 18 column (Acquity UPLC® BEH, 2.1 × 100 mm, 1.7 μm, Waters Corporation, Wexford, Ireland). The solvent gradient was as follows (time, B %): 0–1 min, 0%; 3 min, 5%; 3–4.5 min, 5–10%; 4.5–7 min, 10–20%; 7–10 min, 20–30%; 10–15 min, 30–100%; and 15–20 min, 100–0%. All the analyses were performed at a temperature of 47 °C and the wavelength used for the quantification was 320 nm.
2.5. Experimental Design
To evaluate the extraction parameters and optimize the conditions for coriander extraction using UAE, the response surface methodology was used in conjunction with a Box–Behnken Design (BBD). The methanol concentration (% in water) (X1), extraction temperature (°C) (X2), and liquid-to-solid (LS) ratio (mL g−1) (X3) were the three independent variables tested in 15 combinations. The independent variables were coded at three levels: −1, 0, and +1 for low, intermediate, and high values, respectively. Table 1 shows the coded values for the experimental factors, as well as the specific levels for the BBD.

Table 1.
Independent variables and their coded levels.
The response variables were fitted to the following polynomial model equation:
which allowed the relationship between the response and independent variables to be described along with the interaction effects between factors. In the above equation, y is the predicted response; β0 is the model constant; βi, βii, and βij are the model coefficients for the intercept, linear, quadratic, and interaction terms, respectively; xi and xj are the independent variables; and ԑ is the error.
2.6. Data Analysis
The experimental data were analyzed using the statistical software Minitab 17 (Minitab LLC, State College, PA, USA). The significance of the difference between all the terms was determined by an evaluation of variance (ANOVA). A second-order polynomial regressed equation was established by a Box–Behnken Design (BBD) analysis of the experimental data, and the adequacy of the model was verified through the coefficient of determination R2 and the p-value of the F-test.
3. Results
3.1. Identification of Phenolic Compounds from Coriander
The phenolic compounds were identified by a comparison of the UV-Vis spectra and retention times with available standards. Four phenolic compounds were identified in the coriander extracts: caffeic acid, isoquercitrin (quercetin-3-O-glucoside), quercetin-3-O-glucuronide, and rutin (quercetin-3-O-rutinoside). Caffeic acid and quercetin-3-O-glucuronide represent the main phenolic compounds of coriander aerial parts in the studied samples.
3.2. Optimization of Extraction Conditions by Experimental Design
The extraction conditions were optimized to obtain the highest recovery of phenolic compounds. The extraction process was optimized by applying a BBD. The analyzed variables were the methanol concentration (% in water) (X1), the extraction temperature (°C) (X2), and the liquid-to-solid ratio (mL∙g−1) (X3), as shown in Table 1. Values corresponding to the area reported for the four phenolic compounds at 320 nm were used as the response. The BBD used 15 runs, including 3 center points. The results were used to obtain a mathematical model. The total area ranged from 19821.3 (µv×sec) to 36889.7 (µv×sec), as illustrated in Table 2. The agreement between the experimental area was very high and the relative error of prediction ranged from −4.3% to 6.9%, with an average error of prediction (absolute value) of 3.8%.

Table 2.
BBD with 3 variables at 3 levels for the UAE of phenolic compounds and the observed responses.
3.3. Effect of Experimental Parameters on the Recovery of Phenolic Compounds
The results were used to obtain the surface response by fitting the data to a polynomial model and to evaluate the effects of each factor and the interaction effects among factors. The final objective was to optimize the response y, and therefore, the best estimation for the correlation between independent factors and the response surface must be found. The regression coefficients were obtained by the least-squares regression method. The resulting mathematical model obtained using partial least-squares regression gave the following regression equation:
y = 12,880.3 + 500.1 X1 − 11.2 X2 + 1833.5 X3 − 6.0 X12 + 2.0 X22 − 247.3 X32 − 1.06 X1X2 + 36.8 X1X3 − 9.3 X2X3
ANOVA was used to determine the significance of each effect. The mean squares against an estimate of the experimental error were compared. Table 3 shows the absolute values of the estimated effects divided by their standard. Variables or combinations of variables had a significant effect on the response if p < 0.05. The results obtained in fitting the second-order polynomial model to the data are shown in Table 3. The resulting value for the square coefficient of regression (R2) was 0.9359.

Table 3.
The p-values of the estimated effects for the extraction variables in the regression model and for lack of fit.
The results shown in Table 3 indicate that only the concentration of methanol and its quadratic term had a significant effect on the area of the caffeic acid (p < 0.05). The p-values of the other single factors, quadratic terms, and interactions were higher than 0.05, which indicates that they were not significant regarding the response.
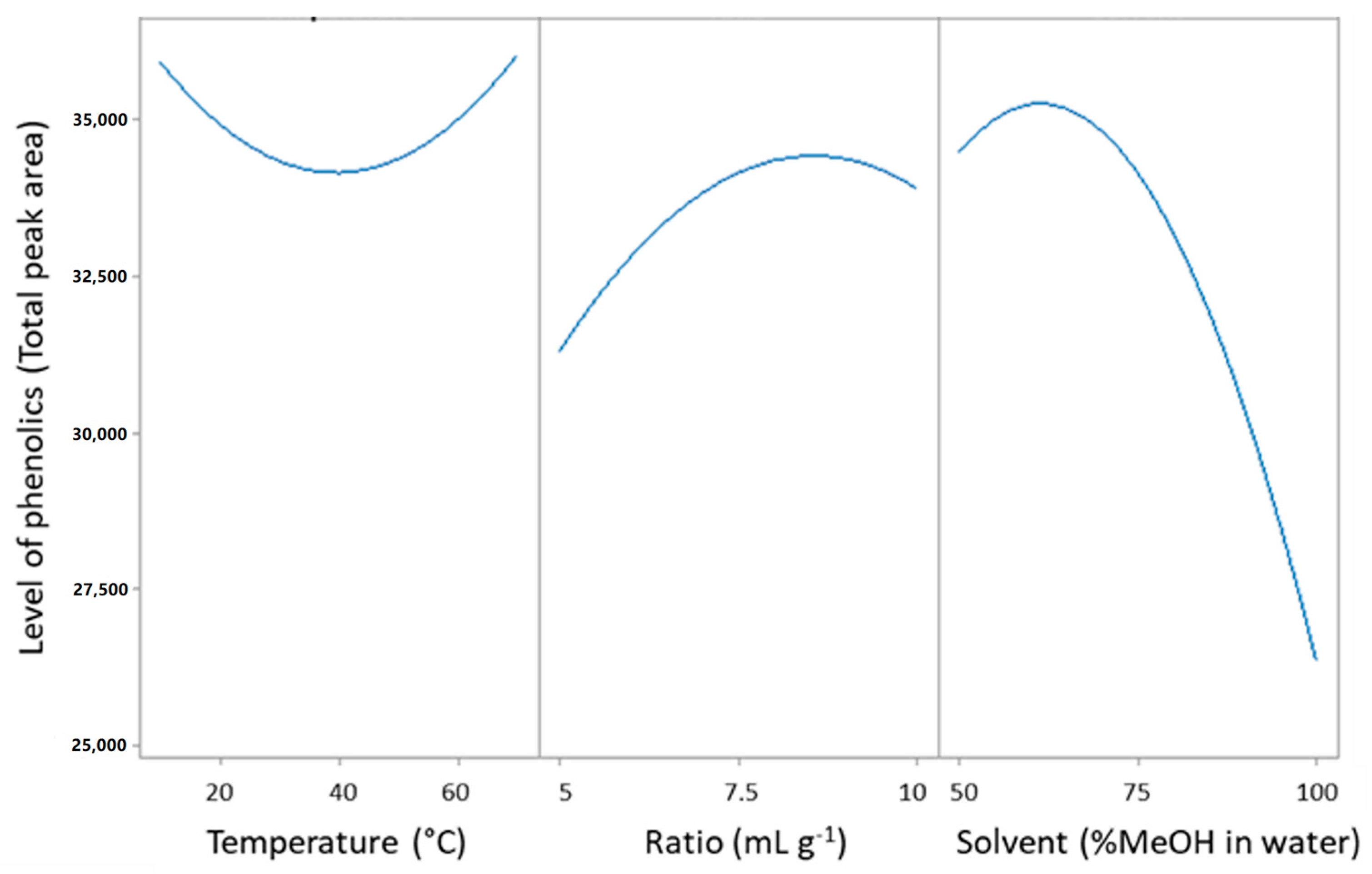
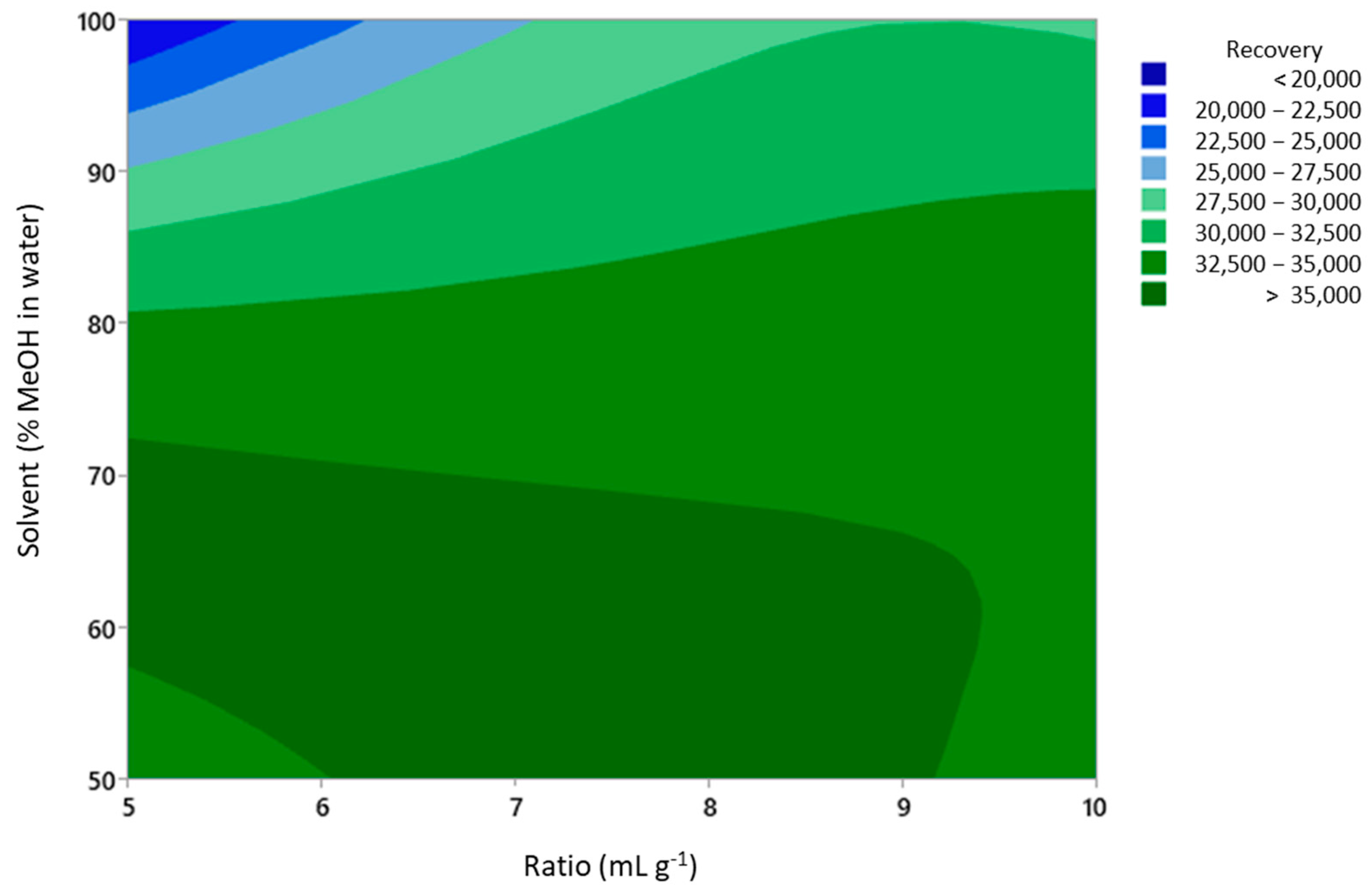
It was observed that the methanol concentration was the most critical factor that influenced the UAE performance. It can clearly be seen from Figure 1 (main effects plot) and Figure 2 (response surface contour plot) that the highest recovery was obtained in the region where the methanol concentration ranged from 50 to 70%. A methanol concentration above 70% led to a decrease in the recovery of phenolic compounds.
Figure 1.
Main effect plots for the effect of extraction variables on the recovery of the phenolic compounds.
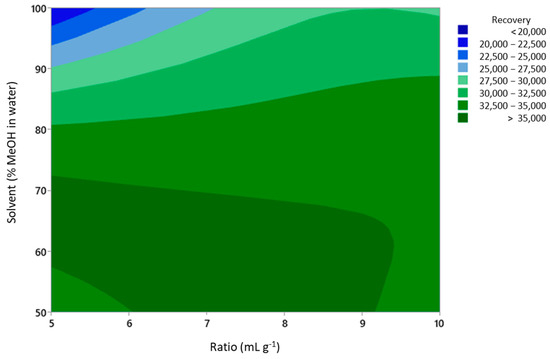
Figure 2.
Contour plot of phenolic compound recovery for solvent and ratio extraction variables.
3.4. Optimization of the Response and Verification of the Model
The resulting mathematical model provided the best extraction conditions to reach the highest recovery of phenolic compounds from coriander samples. The values for the extraction variables were 1.5 g of the coriander sample in 10 mL of solvent (ratio = 6.67), using 55% of methanol in water and a temperature of 70 °C. The contour plot for the solvent and ratio variables is included in Figure 2.
3.5. Extraction Kinetics
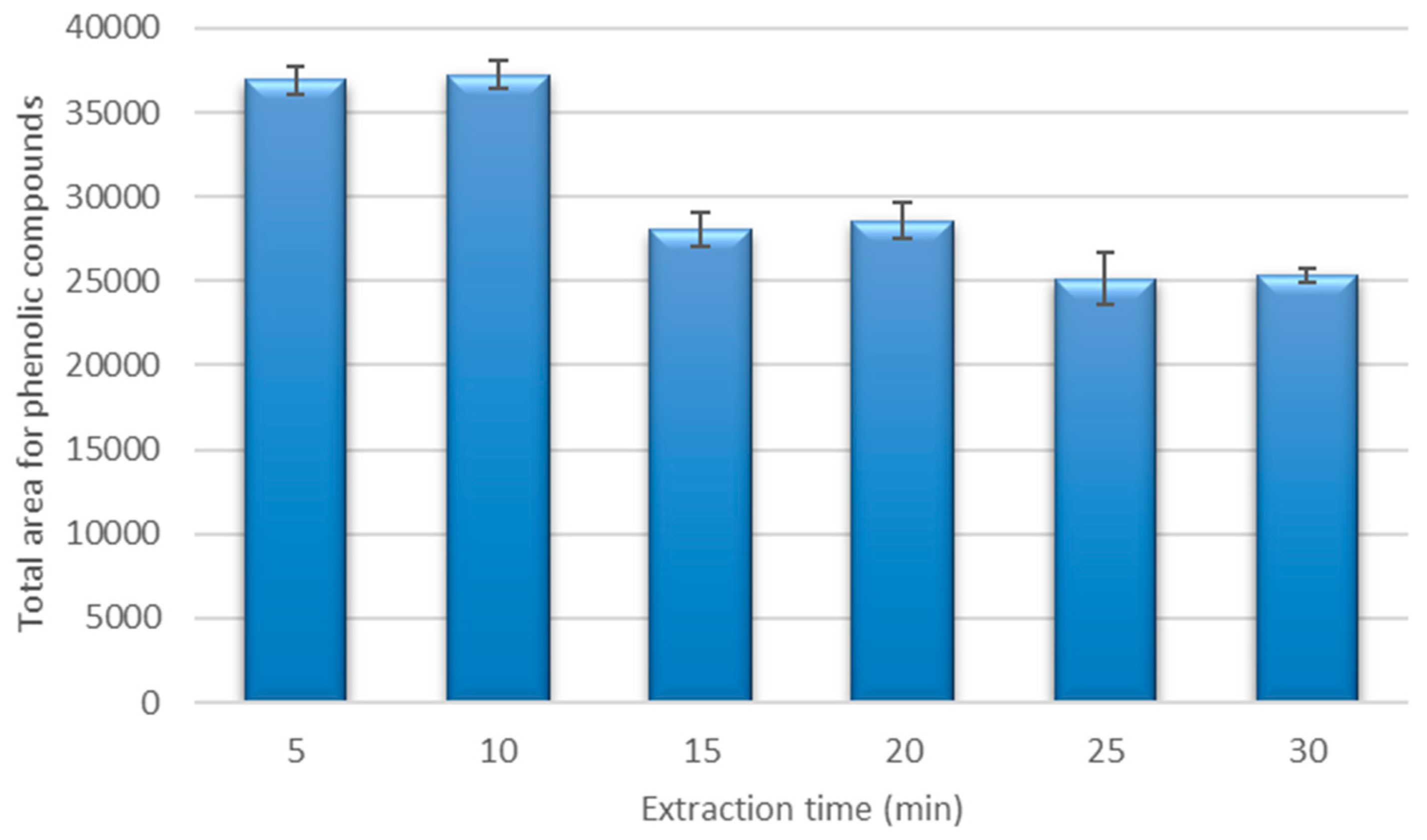
The extraction method should provide a full recovery of phenolic compounds from the coriander samples, and therefore, the extraction time that provides a sufficient recovery should be established. The recoveries from the same sample under the best extraction conditions at different extraction times (from 5 to 30 min) were studied. The results of these extractions are represented in Figure 3.
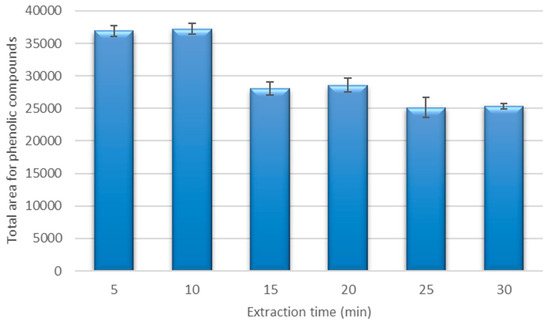
Figure 3.
Recovery for the phenolic compounds under the optimized extraction conditions using different extraction times.
3.6. Repeatability and Intermediate Precision of the Method
The repeatability and intermediate precision were used to determine the precision of the method. They were evaluated under the best extraction conditions, which were a methanol concentration of 55%, a temperature of 70 °C, a time of 10 min, and 1.5 g of the coriander sample in 10 mL of solvent. The repeatability was determined by carrying out nine extractions under the optimal conditions on the same day. The intermediate precision was calculated by carrying out extractions on three different days (four extractions per day). The relative standard deviation (RSD) values for the developed method were 2.4% for repeatability and 5.4% for intermediate precision. The RSD values were below the acceptable limits (±10%).
3.7. Application of the Developed Method to Real Samples
The applicability of the developed method was assessed by applying it to some coriander samples. Three different fresh coriander samples available in Spanish markets were extracted in triplicate under the best working conditions. All of them were produced in the same area in South Spain. The results are shown in Table 4.

Table 4.
Levels for phenolic compounds (mg kg−1) in fresh coriander.
4. Discussion
Caffeic acid, isoquercitrin, quercetin-3-O-glucuronide, and rutin were the phenolic compounds identified in the studied samples, with a predominance of caffeic and quercetin-3-O-glucuronide. According to Melo et al. [40], caffeic acid and flavonoids have been found in several coriander samples. A similar composition has been previously described for the vegetative parts of coriander by Hadjmohammadi et al. [41]. Specifically, several flavonoids, including quercetin derivatives, have been described in extracts from coriander [17]; in addition, quercetin derivatives have been studied from coriander samples under different preharvest treatments by EL-Zaeddi et al. [42].
The experimental design for the optimization of the extraction conditions provided a model that was reported as non-significant in the lack-of-fit test (p-value = 0.308). Moreover, the quadratic coefficient of regression (R2) showed a value close to one (0.9359); this implies a good correlation between the observed and predicted values and suggests that the model fit the experimental values for the responses. The predicted and real values for the total area of phenolic compounds are shown in Table 3. The standard error of the predicted values showed that that average of the residuals was 3.8% and the standard deviation of the residuals was 3.59. Therefore, the model is useful for estimating the response for the purposes of optimization.
4.1. Effect of Experimental Parameters on the Recovery of the Phenolic Compounds
The use of an adequate solvent system was the crucial step in obtaining a high recovery of phenolic compounds from ultrasound-assisted extraction, and this effect has been previously described [43]. Methanol has been used with very good results for the extraction of phenolic compounds [43,44,45,46].
It can clearly be seen from Figure 1 that the highest recovery was obtained in the region where the methanol concentration ranged from 50 to 70%. A methanol level above 70% led to a decrease in the recovery. This result indicates that a solvent/water mixture is more efficient than a mono-solvent system in the extraction of phenolic compounds, as has been previously reported [47].
Water added to an organic solvent usually promotes the swelling of plant material, therefore facilitating the penetration of the solvent into the solid matrix and improving the recovery of the phenolic compounds [48]. In addition, three of the studied phenolic compounds were glycosides, which have a higher solubility in water than in methanol [49]. Therefore, adding water to the solvent system is convenient, but the final level of water in the solvent has to be controlled to minimize the co-extraction of non-phenolic compounds.
The extraction temperature was another important extraction variable [32]; however, in the results shown in Table 1, it was found to be non-significant regarding the total area of phenolic compounds in Table 3, suggesting that the temperature had a negligible effect on the recovery of caffeic acid, with a p-value of 0.934. Similar findings have been reported in previous studies on the extraction of phenolic compounds from apples [49]. However, other studies carried out on other plant material have highlighted the remarkable effect of temperature during ultrasound-assisted extraction [31,48]. This difference could be due to the complexity of the plant material studied and to the phenolic compound considered [31,50,51].
The influence of the liquid solvent-to-solid sample ratio was studied by carrying out the extraction of different quantities of coriander samples in the same volume of solvent (10 mL). It can be seen from the results in Table 1 that the liquid-to-solid ratio did not significantly influence the recovery of the phenolic compounds.
4.2. Optimization of the Response and Verification of the Model
Under optimal extraction conditions, the developed model predicted an area of 37,790 (µv×sec). In order to verify the reliability of the model, new coriander samples were extracted in triplicate using the conditions identified for the highest recovery. An average of 37,599.7 ± 2376.61 (µv×sec) was obtained. It must be noted that this finding is in full agreement with the predicted value. Therefore, the mathematical model is capable of correctly predicting values under the best extraction conditions.
4.3. Extraction Kinetic
A higher recovery was obtained when the extractions were carried out using times between 5 and 10 min. The best recovery was obtained at 10 min, and this was marginally higher than the recovery at 5 min (difference was non-significant, <5%). The mass transfer of phenolic compounds from a solid matrix to a solvent is related to the extraction time, because the mass transfer increases as time increases until a maximum is achieved. A further increase in the extraction time led to a decrease in the recovery, and this may have been due to the thermal degradation of phenolic compounds, or it may have been due to the extraction temperature and the effect of oxygen dissolved in the solvent during the application of ultrasounds. This kind of effect has been reported in previous work with phenolic compounds [6,48]. Consequently, the best extraction time was fixed at 10 min.
4.4. Repeatability and Intermediate Precision of the Method
The values of the relative standard deviation for the repeatability and for the intermediate precision were 2.41% and 5.39%, respectively. The RSD values were below the acceptable limits (±10%, according to the AOAC International Manual [52]), and they were similar to previously reported extraction methods for solid samples using ultrasound-assisted extraction [28]. It must be noted that these values cover the error both during the extraction step and during the chromatographic analysis, with the latter one usually found to be around 0.5–1% [53].
4.5. Application of the Developed Method to Real Samples
Caffeic acid and quercetin-3-O-glucuronide were the dominant phenolic compounds in all samples (Table 4). Small differences were found for the three coriander samples. After the extraction, the solid material was extracted again using the same procedure. The levels were below 5% of the levels in the first extract; therefore, it can be concluded that the first extraction produced a quantitative recovery of the major phenolic compounds in the aerial parts of coriander.
5. Conclusions
To summarize, four phenolic compounds were identified in the coriander samples: caffeic acid, isoquercitrin, quercetin-3-O-glucuronide, and rutin. The effect of the extraction conditions on the recovery of these compounds in coriander samples was studied using the response surface methodology. The results show that the methanol concentration was the pivotal factor that affected the recovery. The optimal extraction conditions were found to be: 10 min for the extraction time, 70 °C for the incubation temperature, 50% for the methanol concentration, and 1.5 g of the coriander sample in 10 mL of solvent. The developed method was employed for the extraction of real coriander samples, and it is suggested that this method can potentially be applied in the pharmaceutical and food industries for quality control analyses due to its high recovery, repeatability, and intermediate precision.
Author Contributions
Conceptualization, L.M. and M.P.; methodology, L.M. and C.A.C.; software, M.P.; validation, L.M. and C.A.C.; formal analysis, L.M. and R.D.; investigation, L.M. and C.A.C.; resources, M.P. and R.D.; data curation, L.M. and W.S.; writing—original draft preparation, L.M.; writing—review and editing, M.P., W.S., M.H. and R.D.; visualization, M.H. and C.A.C.; supervision, M.P., M.H. and R.D.; project administration, M.P., M.H. and R.D.; funding acquisition, M.P., M.H. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Cádiz, grant number Plan Propio—UCA 2022-2023 REPINF-011.
Data Availability Statement
All data used are included in the manuscript.
Acknowledgments
L.M. is grateful to the Ministry of Higher Education and Scientific Research of Algeria for financial assistance for this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Thangavelu, S.; Balasubramanian, B.; Palanisamy, S.; Shanmugam, V.; Natchiappan, S.; Kalibulla, S.I.; Rathinasamy, B.; Arumugam, V.A. Characterization and phytoconstituents of Petroselinum crispum (Mill) and Coriandrum sativum (Linn) and their impacts on inflammation—An in vitro analysis against human adenocarcinoma cells with molecular docking. S. Afr. J. Bot. 2022, 146, 776–788. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef]
- Harborne, J.B. Role of secondary metabolites in chemical defence mechanisms in plants. In Ciba Foundation Symposium 154-Bioactive Compounds from Plants: Bioactive Compounds from Plants: Ciba Foundation Symposium; John Wiley & Sons, Ltd.: Chichester, UK, 2007; Volume 154, pp. 126–139. [Google Scholar]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, P.; Bisht, S. Coriandrum sativum: A daily use spice with great medicinal effect. Pharmacogn. J. 2011, 3, 84–88. [Google Scholar] [CrossRef]
- Sahib, N.G.; Anwar, F.; Gilani, A.H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals-a review. Phyther. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef]
- Yashni, G.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Abirama Shanmugan, V.; Abu Bakar, J. Characterization of Coriandrum sativum leaves as a sustainable green biosorbent. Mater. Today Proc. 2021, 47, 1345–1349. [Google Scholar] [CrossRef]
- Wei, J.N.; Liu, Z.H.; Zhao, Y.P.; Zhao, L.L.; Xue, T.K.; Lan, Q.K. Phytochemical and bioactive profile of Coriandrum sativum L. Food Chem. 2019, 286, 260–267. [Google Scholar] [CrossRef]
- Inan, M.; Kirici, S.; Sultan Giray, E.; Turk, M.; Taghikhani, H. Determination of suitable coriander (Coriandrum sativum L.) cultivars for eastern mediterranean region. Turkish J. Field Crops 2014, 19, 1–6. [Google Scholar] [CrossRef]
- Nadeem, M.; Anjum, F.M.; Khan, M.I.; Tehseen, S.; El-Ghorab, A.; Sultan, J.I. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.): A review. Br. Food J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- Divya, P.; Puthusseri, B.; Neelwarne, B. The effect of plant regulators on the concentration of carotenoids and phenolic compounds in foliage of coriander. LWT 2014, 56, 101–110. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 2018, 105, 305–323. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem. 2012, 132, 841–848. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Teixeira Filho, J.; Wagner, R.; Teixeira Godoy, H. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019, 82, 103244. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J. Ultrasound-assisted extraction of β-glucans from barley. LWT 2013, 50, 57–63. [Google Scholar] [CrossRef]
- Odabaş, H.İ.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crops Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Barbero, G.F.; PiñEiro, Z.; Liazid, A.; Barroso, C.G.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Extraction of Natural Products: Principles and Fundamental Aspects; The Royal Society of Chemistry: London, UK, 2013; ISBN 9781849736060. [Google Scholar]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of amino acids from grapes. Ultrason. Sonochem. 2015, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Fan, X.H.; Li, T.; Zhang, Z.Q.; Liu, Y.K.; Li, X.P. Optimisation of ultrasound extraction for flavonoids from semen astragali complanati and its identification by HPLC-DAD-MS/MS. Int. J. Food Sci. Technol. 2013, 48, 1970–1976. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Guerrero, R.F.; Fernández-Marin, M.I.; Cantos-Villar, E.; Palma, M. Ultrasound-assisted extraction of stilbenoids from grape stems. J. Agric. Food Chem. 2013, 61, 12549–12556. [Google Scholar] [CrossRef] [PubMed]
- Aadil, R.M.; Zeng, X.A.; Wang, M.S.; Liu, Z.W.; Han, Z.; Zhang, Z.H.; Hong, J.; Jabbar, S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1144–1150. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M. Optimisation of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019, 288, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of phenolic compounds from rice (Oryza sativa) grains. Food Chem. 2016, 192, 452–459. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar] [CrossRef]
- Ivanovic, M.; Razboršek, M.I.; Košir, I.J.; Kolar, M. Response surface methodology: An optimal design applied for maximum ultrasound-assisted extraction efficiency of phenolic acids from Coriandrum sativum L. J. Appl. Bot. Food Qual. 2019, 92, 378–387. [Google Scholar] [CrossRef]
- Sriti, J.; Aidi Wannes, W.; Talou, T.; Ben Jemia, M.; Elyes Kchouk, M.; Marzouk, B. Antioxidant properties and polyphenol contents of different parts of coriander (Coriandrum sativum L.) fruit. Riv. Ital. Delle Sostanze Grasse 2012, 89, 253–262. [Google Scholar]
- Zeković, Z.; Vladić, J.; Vidović, S.; Adamović, D.; Pavlić, B. Optimization of microwave-assisted extraction (MAE) of coriander phenolic antioxidants-response surface methodology approach. J. Sci. Food Agric. 2016, 96, 4613–4622. [Google Scholar] [CrossRef]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canli, O.; Vidović, S. Chemical characterization of polyphenols and volatile fraction of coriander (Coriandrum sativum L.) extracts obtained by subcritical water extraction. Ind. Crops Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Zeković, Z.; Pavlić, B.; Cvetanović, A.; Đurović, S. Supercritical fluid extraction of coriander seeds: Process optimization, chemical profile and antioxidant activity of lipid extracts. Ind. Crops Prod. 2016, 94, 353–362. [Google Scholar] [CrossRef]
- Zeković, Z.; Bušić, A.; Komes, D.; Vladić, J.; Adamović, D.; Pavlić, B. Coriander seeds processing: Sequential extraction of non-polar and polar fractions using supercritical carbon dioxide extraction and ultrasound-assisted extraction. Food Bioprod. Process. 2015, 95, 218–227. [Google Scholar] [CrossRef]
- De Almeida Melo, E.; Mancini Filho, J.; Barbosa Guerra, N. Characterization of antioxidant compounds in aqueous coriander extract (Coriandrum sativum L.). LWT 2005, 38, 15–19. [Google Scholar] [CrossRef]
- Hadjmohammadi, M.; Sharifi, V. Investigation of optimum extraction conditions for determination of quercetin and kaempferol in coriander (Conundrum sativum L.) by using experimental design and HPLC. J. Food Drug Anal. 2009, 17, 7. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Nowicka, P.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef]
- Bouafia, M.; Colak, N.; Ayaz, F.A.; Benarfa, A.; Harrat, M.; Gourine, N.; Yousfi, M. The optimization of ultrasonic-assisted extraction of Centaurea sp. antioxidative phenolic compounds using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100330. [Google Scholar] [CrossRef]
- Palma, M.; Taylor, L.T. Supercritical Fluid Extraction of 5-Hydroxymethyl-2-furaldehyde from Raisins. J. Agric. Food Chem. 2001, 49, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Shakeri, A.; Sohrabi, M.R.; Khalajzadeh, S.; Ghasemi, E. Optimization of ultrasonic assisted extraction of fatty acids from Aesculus hippocastanum fruit by response surface methodology. Food Chem. 2019, 271, 762–766. [Google Scholar] [CrossRef]
- Dziri, S.; Hassen, I.; Fatnassi, S.; Mrabet, Y.; Casabianca, H.; Hanchi, B.; Hosni, K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Foods 2012, 4, 423–432. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Goldsmith, C.D.; Dang, T.T.; Nguyen, V.T.; Bhuyan, D.J.; Sadeqzadeh, E.; Scarlett, C.J.; Bowyer, M.C. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant capacity from Euphorbia tirucalli using response surface methodology. Antioxidants 2014, 3, 604–617. [Google Scholar] [CrossRef]
- Teofilović, B.; Grujić-Letić, N.; Goločorbin-Kon, S.; Stojanović, S.; Vastag, G.; Gadžurić, S. Experimental and chemometric study of antioxidant capacity of basil (Ocimum basilicum) extracts. Ind. Crops Prod. 2017, 100, 176–182. [Google Scholar] [CrossRef]
- AOAC International. Appendix F: Guidelines for Standard Method Performance Requirements. AOAC Official Methods of Analysis. 2016. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=2613575 (accessed on 10 January 2023).
- Stipcovich, T.; Barbero, G.F.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Fast analysis of capsaicinoids in Naga Jolokia extracts (Capsicum chinense) by high-performance liquid chromatography using fused core columns. Food Chem. 2018, 239, 217–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).