Spectroscopic Tracking of the Characteristics of Microplastic-Derived Dissolved Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microplastics
2.2. Leaching Experiments
2.3. Fluorescence Quenching Experiments
2.4. Analytical Methods
3. Results and Discussion
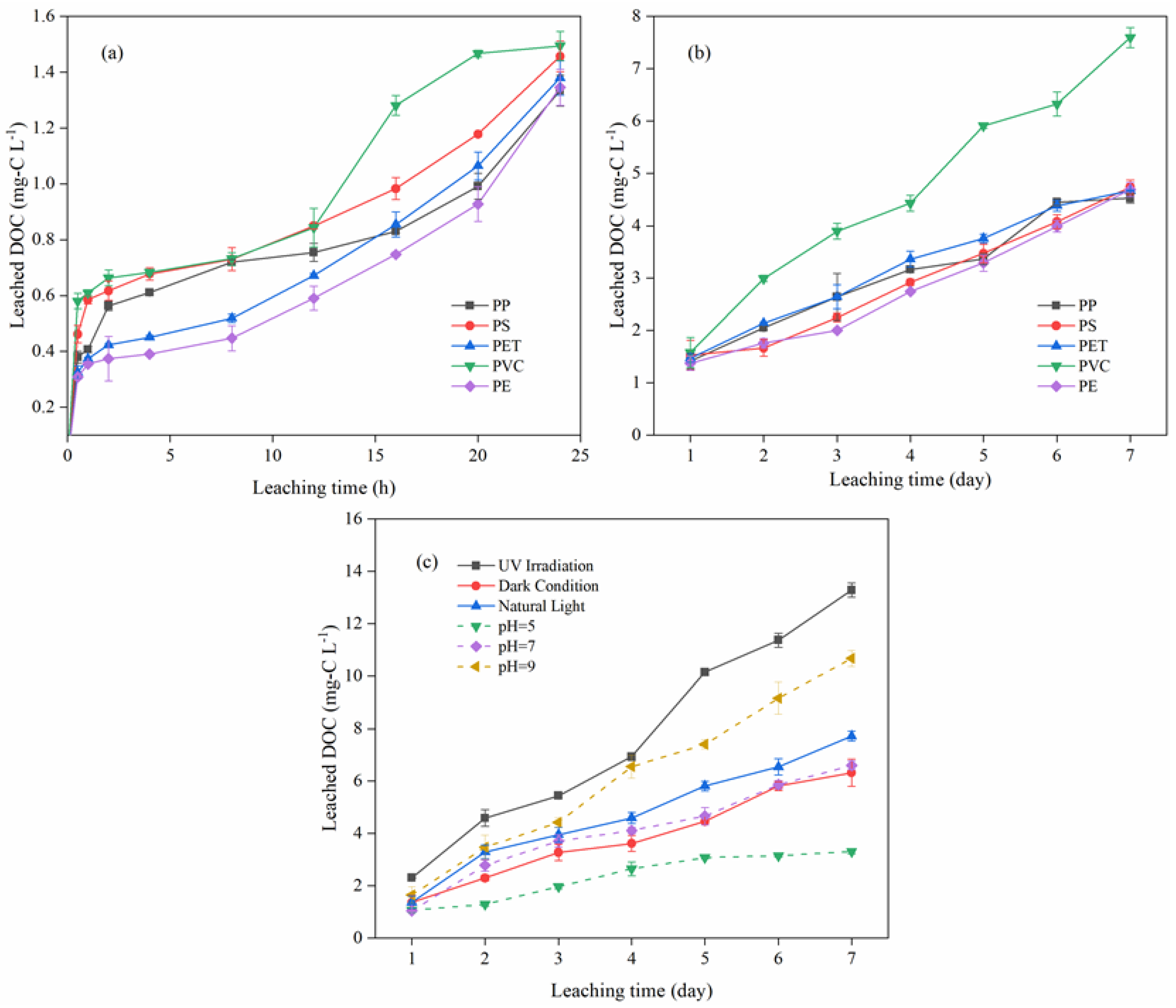
3.1. DOC Leaching from the Microplastics
3.1.1. Different Types of Microplastics
3.1.2. Factors concerning DOC Leaching of Microplastic
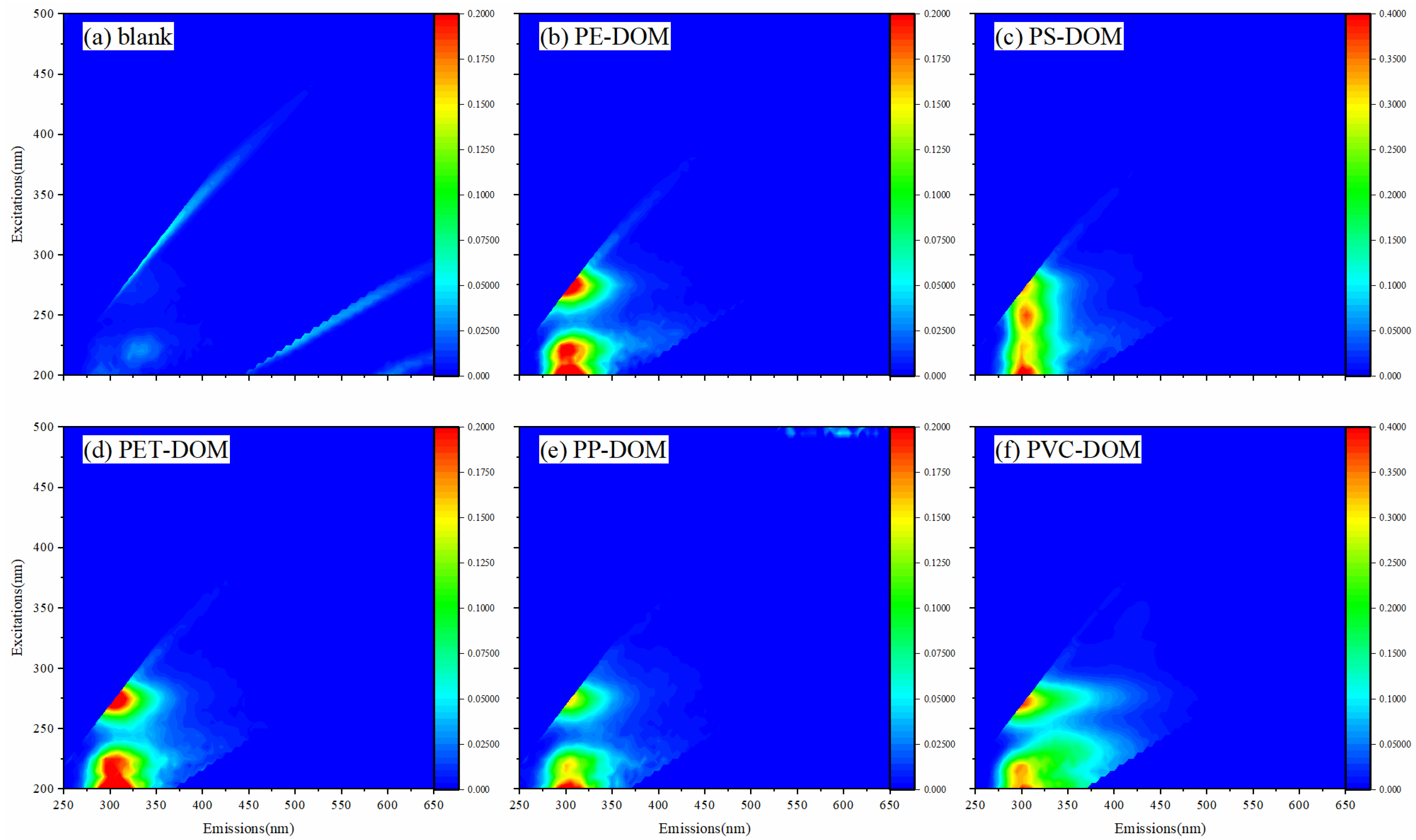
3.2. Fluorescence EEMs
3.2.1. Different Types of Microplastics
3.2.2. PVC-DOM under Different Leaching Conditions
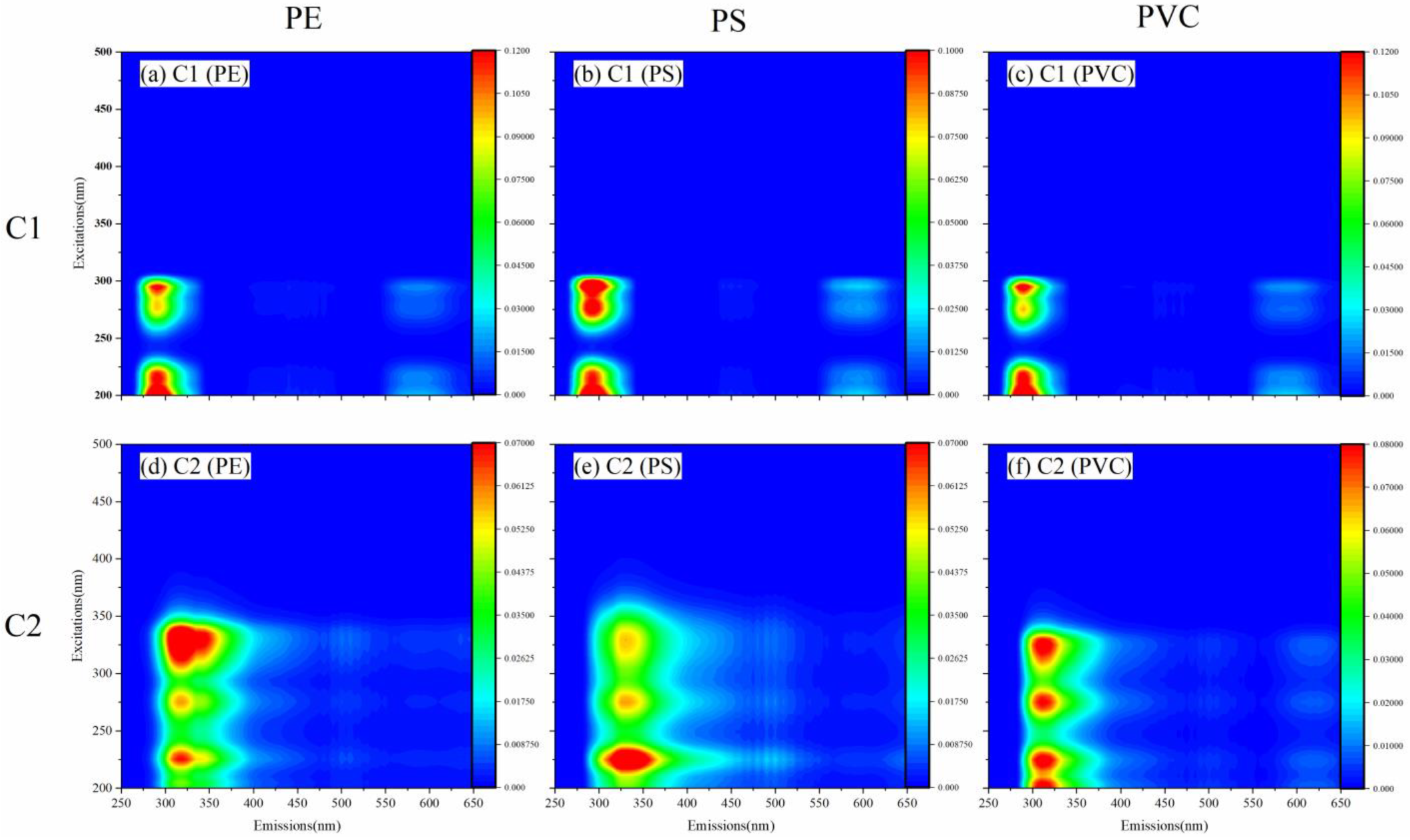
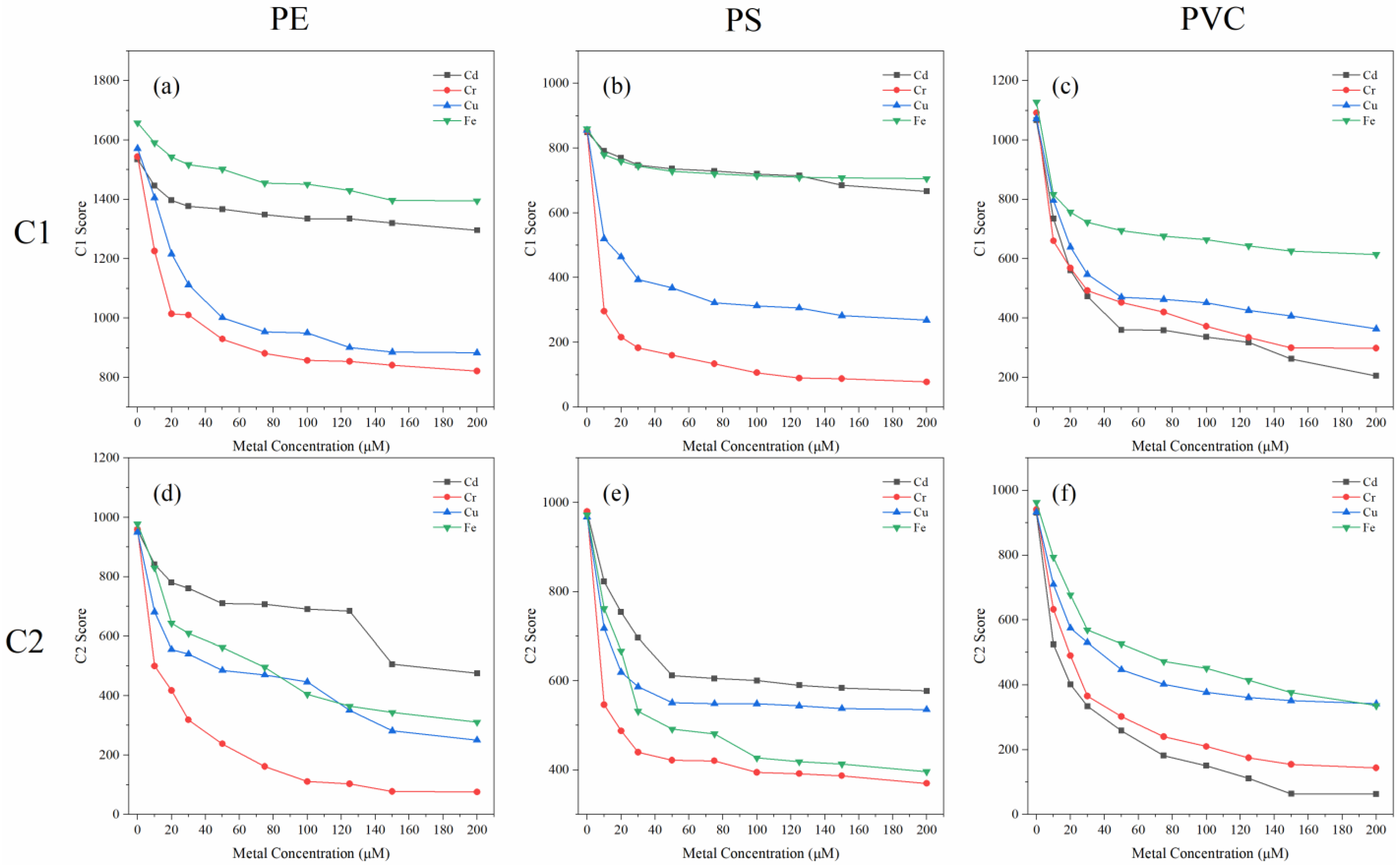
3.3. Metal-Binding Characteristics of Different Fluorescent Components
3.3.1. Fluorescent Components Identified by PARAFAC Modeling
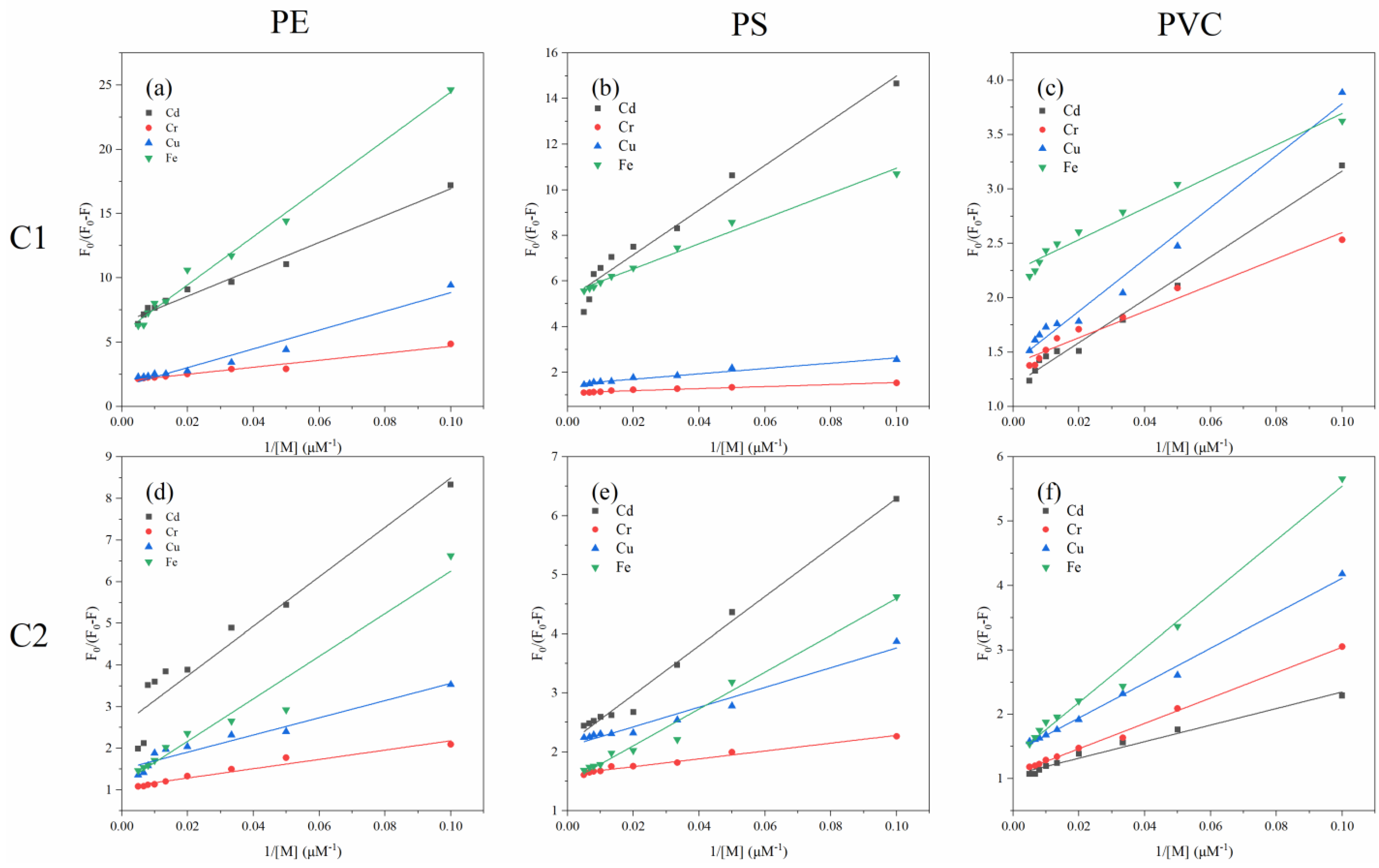
3.3.2. Fluorescence Quenching Behavior of Different Fluorescent Components
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garcial, J.; Robertson, M. The future of plastics recycling chemical advances are increasing the proportion of polymer waste that can be recycled. Sci. Adv. 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of Nonrecyclable Plastic Waste over a Carbon Nitride/Nickel Phosphide Catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210. [Google Scholar] [CrossRef]
- Tian, W.; Song, P.; Zhang, H.; Duan, X.; Wei, Y.; Wang, H.; Wang, S. Microplastic materials in the environment: Problem and strategical solutions. Prog. Mater. Sci 2023, 132, 105303. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Man, K.Y.; Wong, M.H. Direct and indirect effects of microplastics on bivalves, with a focus on edible species: A mini-review. Title Crit. Rev. Environ. Sci. Technol. 2019, 50, 2109–2143. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.-J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth. Planet. Sci. 2021, 8, 1–26. [Google Scholar] [CrossRef]
- Priya, K.L.; Renjith, K.R.; Joseph, C.J.; Indu, M.S.; Srinivas, R.; Haddout, S. Fate, transport and degradation pathway of microplastics in aquatic environment—A critical review. Reg. Stud. Mar. Sci. 2022, 56, 102647. [Google Scholar] [CrossRef]
- Wei, X.F.; Capezza, A.J.; Cui, Y.; Li, L.; Hakonen, A.; Liu, B.; Hedenqvist, M.S. Millions of microplastics released from a biodegradable polymer during biodegradation/enzymatic hydrolysis. Water Res. 2022, 211, 118068. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Sun, Y.; Jiang, W.; Zhang, C.; Dai, Y.; Liu, Z.; Wang, T.; Guo, X.; Jia, H. Inorganic anions influenced the photoaging kinetics and mechanism of polystyrene microplastic under the simulated sunlight: Role of reactive radical species. Water Res. 2022, 216, 118294. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Akhtar, N.; Naqash, N.; Rahayu, F.; Djajadi, D.; Chopra, C.; Singh, R.; Mulla, S.I.; Sher, F.; Americo-Pinheiro, J.H.P. Discovering untapped microbial communities through metagenomics for microplastic remediation: Recent advances, challenges, and way forward. Environ. Sci. Pollut. Res. Int. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, A.; Oelschlagel, K.; Schmitt-Jansen, M.; Rummel, C.D.; Kuhnel, D. From the sea to the laboratory: Characterization of microplastic as prerequisite for the assessment of ecotoxicological impact. Integr. Environ. Assess. Manag. 2017, 13, 500–504. [Google Scholar] [CrossRef]
- Sait, S.T.L.; Sorensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef] [PubMed]
- Mortula, M.M.; Atabay, S.; Fattah, K.P.; Madbuly, A. Leachability of microplastic from different plastic materials. J. Environ. Manag. 2021, 294, 112995. [Google Scholar] [CrossRef]
- Lee, Y.K.; Murphy, K.R.; Hur, J. Fluorescence Signatures of Dissolved Organic Matter Leached from Microplastics: Polymers and Additives. Environ. Sci. Technol. 2020, 54, 11905–11914. [Google Scholar] [CrossRef]
- M’Rabet, C.; Kefi-Daly Yahia, O.; Couet, D.; Gueroun, S.K.M.; Pringault, O. Consequences of a contaminant mixture of bisphenol A (BPA) and di-(2-ethylhexyl) phthalate (DEHP), two plastic-derived chemicals, on the diversity of coastal phytoplankton. Mar. Pollut. Bull. 2019, 138, 385–396. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Sun, F.; Li, Y.; Wang, X.; Chi, Z.; Yu, G. Using new hetero-spectral two-dimensional correlation analyses and synchrotron-radiation-based spectromicroscopy to characterize binding of Cu to soil dissolved organic matter. Environ. Pollut. 2017, 223, 457–465. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation− emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.Q. Advances in the characterization and monitoring of natural organic matter using spectroscopic approaches. Water. Res. 2021, 190, 116759. [Google Scholar] [CrossRef] [PubMed]
- Amorello, D.; Barreca, S.; Gambacurta, S.; Gulotta, M.G.; Orecchio, S.; Pace, A. An analytical method for monitoring micro-traces of landfill leachate in groundwater using fluorescence excitation–emission matrix spectroscopy. Anal. Methods 2016, 8, 3475–3480. [Google Scholar] [CrossRef]
- Majedi, S.M.; Kelly, B.C.; Lee, H.K. Combined effects of water temperature and chemistry on the environmental fate and behavior of nanosized zinc oxide. Sci. Total. Environ. 2014, 496, 585–593. [Google Scholar] [CrossRef]
- Suhrhoff, T.J.; Scholz-Bottcher, B.M. Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—A lab experiment. Mar. Pollut. Bull. 2016, 102, 84–94. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hur, J. Adsorption of microplastic-derived organic matter onto minerals. Water. Res. 2020, 187, 116426. [Google Scholar] [CrossRef]
- Hays, M.D.; Ryan, D.K.; Pennell, S. A modified multisite Stern− Volmer equation for the determination of conditional stability constants and ligand concentrations of soil fulvic acid with metal ions. Anal. Chem. 2004, 76, 848–854. [Google Scholar] [CrossRef]
- Da Silva, J.C.E.; Machado, A.A.; Oliveira, C.J.; Pinto, M.S. Fluorescence quenching of anthropogenic fulvic acids by Cu (II), Fe (III) and UO22+. Talanta 1998, 45, 1155–1165. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Lee, Y.K.; Romera-Castillo, C.; Hong, S.; Hur, J. Characteristics of microplastic polymer-derived dissolved organic matter and its potential as a disinfection byproduct precursor. Water. Res. 2020, 175, 115678. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, J.; Tang, R.; Yuan, S.; Min, Y.; Xu, Q.; Shi, P. Roles of microplastic-derived dissolved organic matter on the photodegradation of organic micropollutants. J. Hazard. Mater. 2022, 440, 129784. [Google Scholar] [CrossRef]
- Achar, J.C.; Na, J.; Im, H.; Jung, J. Role of extracellular polymeric substances in leaching and bioconcentration of benzophenone-3 from microplastic fragments. J. Hazard. Mater. 2021, 416, 125832. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, S.; Bittar, T.B.; Stubbins, A.; Li, D. Photochemical dissolution of buoyant microplastics to dissolved organic carbon: Rates and microbial impacts. J. Hazard. Mater. 2020, 383, 121065. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Shim, W.J. The fragmentation of nano- and microplastic particles from thermoplastics accelerated by simulated-sunlight-mediated photooxidation. Environ. Pollut. 2022, 311, 119847. [Google Scholar] [CrossRef]
- Carstea, E.M.; Popa, C.L.; Baker, A.; Bridgeman, J. In situ fluorescence measurements of dissolved organic matter: A review. Sci. Total. Environ. 2020, 699, 134361. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence spectroscopy for wastewater monitoring: A review. Water. Res. 2016, 95, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, H.; Gao, P.; Li, B.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Effects and mechanisms of aged polystyrene microplastics on the photodegradation of sulfamethoxazole in water under simulated sunlight. J. Hazard. Mater. 2022, 433, 128813. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hong, S.; Hur, J. Copper-binding properties of microplastic-derived dissolved organic matter revealed by fluorescence spectroscopy and two-dimensional correlation spectroscopy. Water. Res. 2021, 190, 116775. [Google Scholar] [CrossRef] [PubMed]
- Croué, J.-P.; Benedetti, M.; Violleau, D.; Leenheer, J. Characterization and copper binding of humic and nonhumic organic matter isolated from the South Platte River: Evidence for the presence of nitrogenous binding site. Environ. Sci. Technol. 2003, 37, 328–336. [Google Scholar] [CrossRef] [PubMed]

| Type | Components | Cd | Cr | Cu | Fe |
|---|---|---|---|---|---|
| PE | C1 | 15.62% | 46.81% | 43.85% | 15.88% |
| C2 | 50.23% | 92.14% | 73.62% | 68.22% | |
| PS | C1 | 21.55% | 90.94% | 68.65% | 17.95% |
| C2 | 40.96% | 62.27% | 44.60% | 59.23% | |
| PVC | C1 | 80.79% | 72.67% | 66.05% | 45.52% |
| C2 | 93.24% | 84.67% | 63.36% | 65.10% |
| Type | C1 | C2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| f (a) | log K (b) | β(c) | r (d) | f | log K | β | r | ||
| PE | Cd | 15.42 | 4.13 | 104.53 | 0.98 | 39.13 | 3.76 | 59.38 | 0.92 |
| Cr | 51.1 | 4.27 | 27.26 | 0.96 | 94.17 | 4.55 | 11.2 | 0.96 | |
| Cu | 64.3 | 3.06 | 73.06 | 0.96 | 66.96 | 4.28 | 20.68 | 0.93 | |
| Fe | 17.54 | 3.41 | 187.64 | 0.99 | 87.21 | 3.11 | 51.08 | 0.96 | |
| PS | Cd | 19.27 | 3.97 | 98.04 | 0.97 | 46.87 | 3.94 | 41.63 | 0.99 |
| Cr | 90.61 | 5.51 | 4.47 | 0.97 | 61.93 | 5.49 | 6.65 | 0.98 | |
| Cu | 68.33 | 4.83 | 11.67 | 0.97 | 47.89 | 4.83 | 16.74 | 0.97 | |
| Fe | 19.06 | 4.55 | 55.2 | 0.99 | 67.68 | 3.86 | 31.22 | 0.98 | |
| PVC | Cd | 83.93 | 4.1 | 19.74 | 0.99 | 94.38 | 4.41 | 12.88 | 0.98 |
| Cr | 87.8 | 4.55 | 12.09 | 0.97 | 93.56 | 3.99 | 19.75 | 1 | |
| Cu | 71.53 | 4.07 | 23.86 | 0.98 | 71.41 | 3.94 | 27.12 | 0.99 | |
| Fe | 44.59 | 5.03 | 14.53 | 0.97 | 74.49 | 3.46 | 42.05 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Xiang, H.; Tong, Y.; Zhou, K.; Peng, C.; Chen, W. Spectroscopic Tracking of the Characteristics of Microplastic-Derived Dissolved Organic Matter. Separations 2023, 10, 101. https://doi.org/10.3390/separations10020101
Yuan M, Xiang H, Tong Y, Zhou K, Peng C, Chen W. Spectroscopic Tracking of the Characteristics of Microplastic-Derived Dissolved Organic Matter. Separations. 2023; 10(2):101. https://doi.org/10.3390/separations10020101
Chicago/Turabian StyleYuan, Mengna, Huiqing Xiang, Yang Tong, Kanggen Zhou, Changhong Peng, and Wei Chen. 2023. "Spectroscopic Tracking of the Characteristics of Microplastic-Derived Dissolved Organic Matter" Separations 10, no. 2: 101. https://doi.org/10.3390/separations10020101
APA StyleYuan, M., Xiang, H., Tong, Y., Zhou, K., Peng, C., & Chen, W. (2023). Spectroscopic Tracking of the Characteristics of Microplastic-Derived Dissolved Organic Matter. Separations, 10(2), 101. https://doi.org/10.3390/separations10020101






