Mineralogical Characteristics of Baima Vanadium Titanomagnetite during Magnetic Separation Upgrading
Abstract
:1. Introduction
2. Sample and Analytical Methods
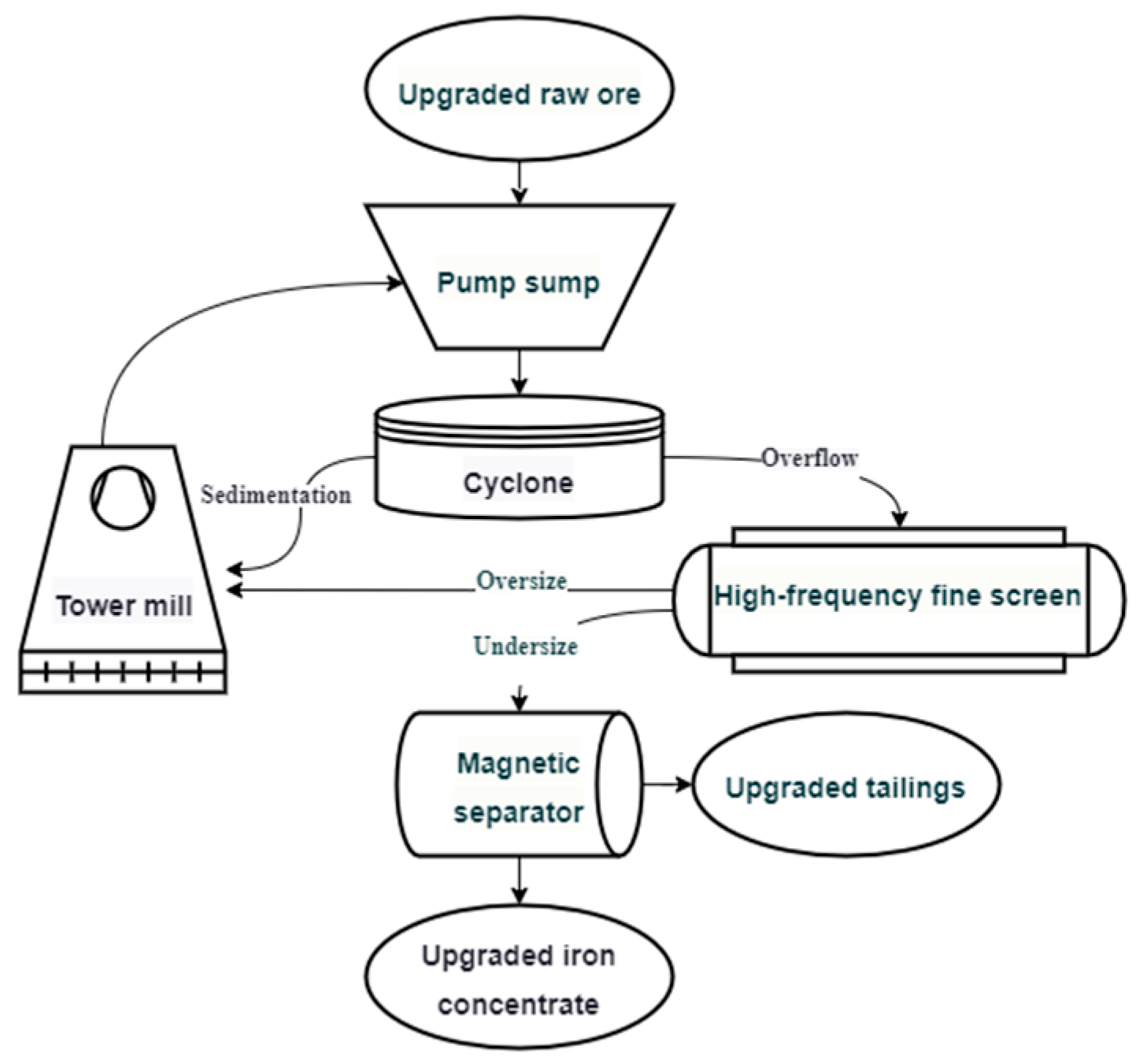
2.1. Sample
2.2. Sample Preparation for AMICS Analysis
2.3. Analytical Methods
3. Results
3.1. Chemical Composition
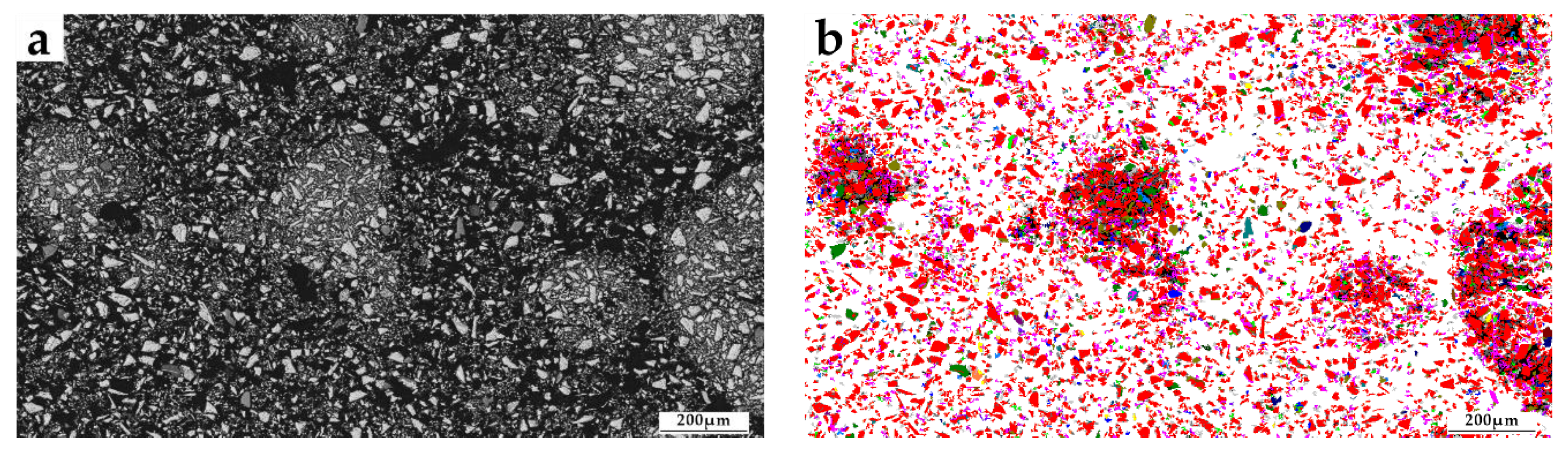
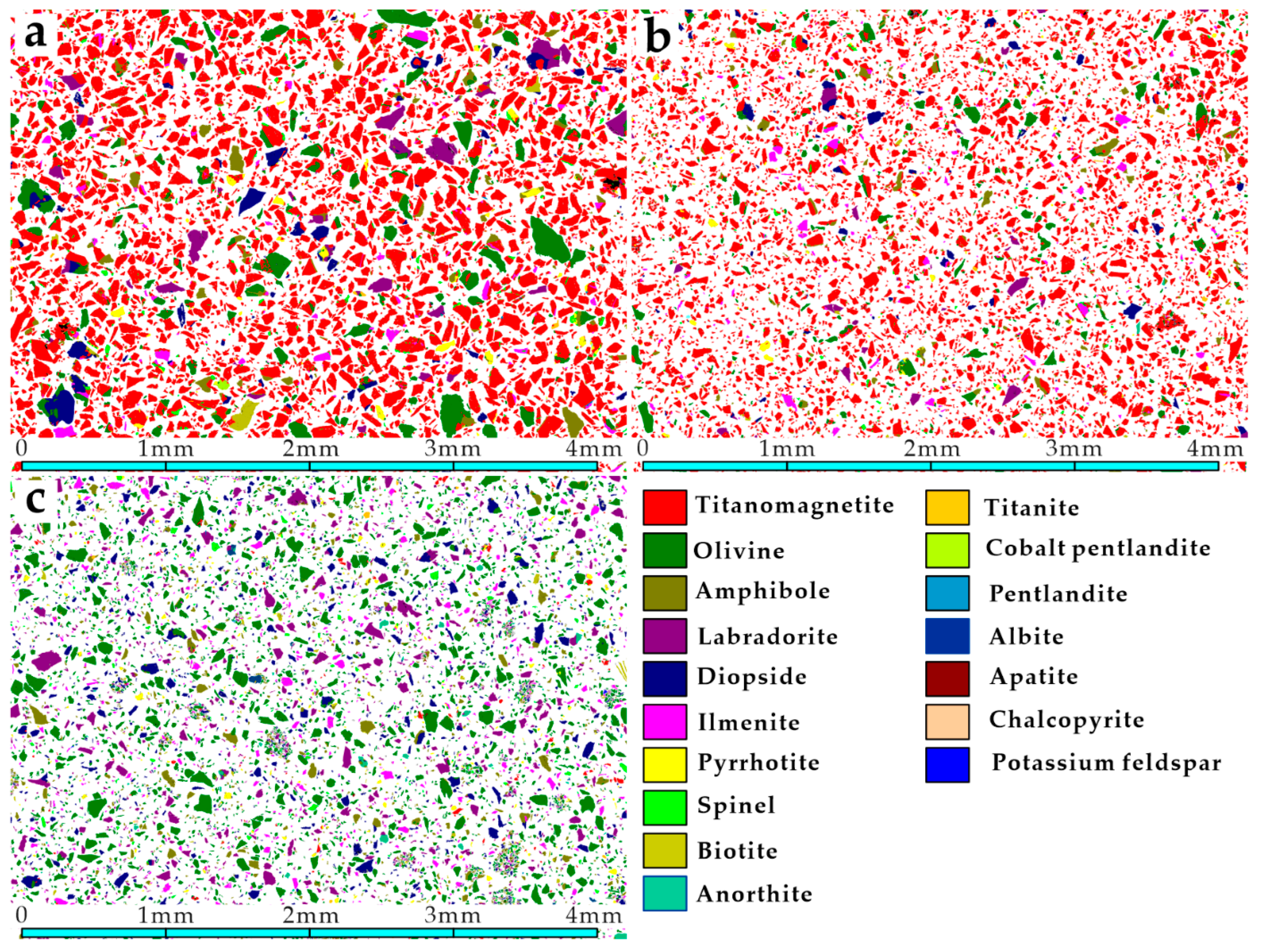
3.2. Mineral Composition
3.3. Iron and Titanium Element Distribution
3.3.1. Iron Element Distribution
3.3.2. Titanium Element Distribution
3.4. Titanomagnetite Mineral-Processing Properties
3.4.1. Chemical Composition of Titanomagnetite
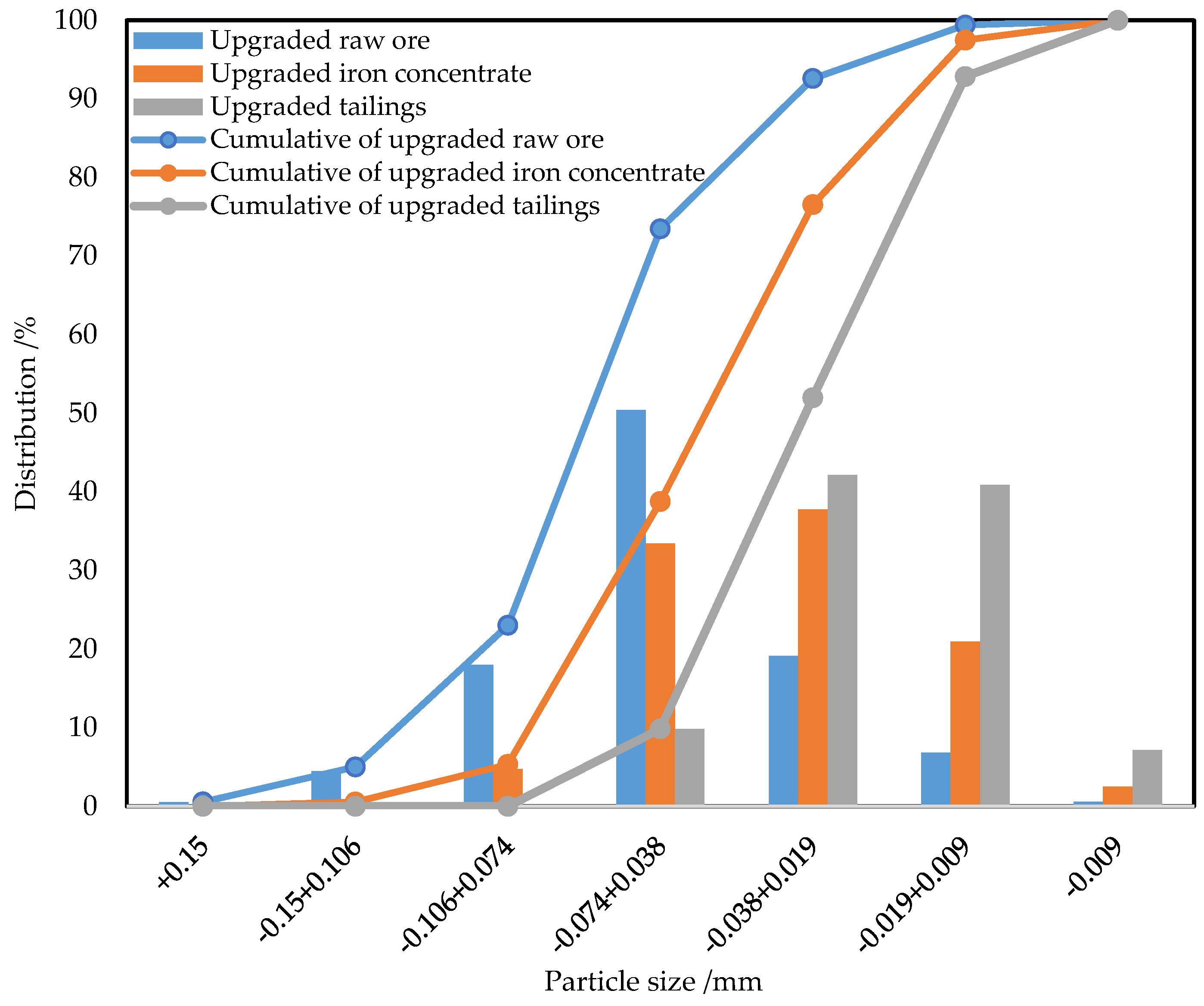
3.4.2. Particle Size of Titanomagnetite
3.4.3. Liberation Degree of Titanomagnetite
3.4.4. Intergrowth Characteristics of Titanium Magnetite and Other Minerals
4. Discussion
5. Conclusions
- (1)
- This study indicates that adding an appropriate amount of acetone before preparing resin-embedded fine-grained powder samples can effectively disperse the powder and prevent agglomeration, leading to improved data analysis precision using the AMICS.
- (2)
- After the upgrading process, the iron grade increased from 55.68% to 57.89%, and the titanomagnetite content increased from 77.41% to 82.10%. The improvement effect is evident.
- (3)
- Titanomagnetite contains titanium, magnesium, aluminum, chromium, manganese, and other impurity elements, which seriously affects the grade of iron in the iron concentrate. The grade of iron in titanomagnetite is calculated to be 65.08% based on the EPMA data analysis.
- (4)
- The main minerals that affect the grade of the iron concentrate are olivine, amphibole, diopside, and labradorite. Most of these gangue minerals are symbiotic with titanomagnetite, indicating that there is room for continued improvement.
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Fu, X.; Chu, M.; Liu, Z.; Tang, J. Life Cycle Assessment of the Comprehensive Utilisation of Vanadium Titano-Magnetite. J. Clean. Prod. 2015, 101, 122–128. [Google Scholar] [CrossRef]
- Luo, J.H.; Qiu, K.H.; Qiu, Y.C.; Zhang, P.C. Studies of Mineralogical Characteristics on Vanadium Titanium Magnetite in Hongge Area, Panzhihua, Sichuan, China. Adv. Mater. Res. 2013, 813, 292–297. [Google Scholar] [CrossRef]
- Liu, L.; Du, T.; Tan, W.; Zhang, X.; Yang, F. A Novel Process for Comprehensive Utilization of Vanadium Slag. Int. J. Miner. Metall. Mater. 2016, 23, 156–160. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, Z.; Gao, G.; Qiu, K.; Peng, W. Process Mineralogy of Vanadium Titanomagnetite Ore in Panzhihua, China. Separations 2023, 10, 147. [Google Scholar] [CrossRef]
- Guo, X.; Dai, S.; Wang, Q. Influence of Different Comminution Flowsheets on the Separation of Vanadium Titano-Magnetite. Miner. Eng. 2020, 149, 106268. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The Extraction of Vanadium from Titanomagnetites and Other Sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Pang, K.-N.; Zhou, M.-F.; Qi, L.; Shellnutt, G.; Wang, C.Y.; Zhao, D. Flood Basalt-Related Fe–Ti Oxide Deposits in the Emeishan Large Igneous Province, SW China. Lithos 2010, 119, 123–136. [Google Scholar] [CrossRef]
- Liu, P.-P.; Zhou, M.-F.; Ren, Z.; Wang, C.Y.; Wang, K. Immiscible Fe- and Si-Rich Silicate Melts in Plagioclase from the Baima Mafic Intrusion (SW China): Implications for the Origin of Bi-Modal Igneous Suites in Large Igneous Provinces. J. Asian Earth Sci. 2016, 127, 211–230. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.-H.; Zhang, S.-X.; Zhou, M.-K. Research on the process of improving iron and reducing impurities by magnetic separation of Panzhihua iron concentrate. Multipurp. Util. Miner. Resour. 2018, 2, 69–73. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y. Mineralogical Characteristics and Genetic Significance of Vanadium-Titanium Magnetite in Panxi Area. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2017. (In Chinese). [Google Scholar]
- Zhong, X.; Shi, Z.; Gao, J. Discussion on the process mineralogy of Baima vanadium titanium magnetite in Panxi area. Metall. Anal. 2021, 41, 29–35. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, W.; Bian, X. Investigations on Mineralogical Characteristics of Rare Earth Minerals in Bayan Obo Tailings during the Roasting Process. J. Rare Earths 2017, 35, 300–308. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Sun, Y.; Gao, P.; Li, Y.; Gong, G. Growth Behavior and Size Characterization of Metallic Iron Particles in Coal-Based Reduction of Oolitic Hematite–Coal Composite Briquettes. Minerals 2018, 8, 177. [Google Scholar] [CrossRef]
- Leißner, T.; Bachmann, K.; Gutzmer, J.; Peuker, U.A. MLA-Based Partition Curves for Magnetic Separation. Miner. Eng. 2016, 94, 94–103. [Google Scholar] [CrossRef]
- Pszonka, J.; Schulz, B.; Sala, D. Application of Mineral Liberation Analysis (MLA) for Investigations of Grain Size Distribution in Submarine Density Flow Deposits. Mar. Pet. Geol. 2021, 129, 105109. [Google Scholar] [CrossRef]
- Schulz, B.; Merker, G.; Gutzmer, J. Automated SEM Mineral Liberation Analysis (MLA) with Generically Labelled EDX Spectra in the Mineral Processing of Rare Earth Element Ores. Minerals 2019, 9, 527. [Google Scholar] [CrossRef]
- Sripal, E.; Grant, D.; James, L. Application of SEM Imaging and MLA Mapping Method as a Tool for Wettability Restoration in Reservoir Core Samples for SCAL Experiments. Minerals 2021, 11, 285. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, K.-H.; Zhang, P.-C.; Li, J.-F.; Zhang, W.-T.; Chen, X.-F. Process Mineralogy of Dalucao Rare Earth Ore and Design of Beneficiation Process Based on AMICS. Rare Met. 2020, 39, 959–966. [Google Scholar] [CrossRef]
- She, H.-D.; Fan, H.-R.; Yang, K.-F.; Li, X.-C.; Yang, Z.-F.; Wang, Q.-W.; Zhang, L.-F.; Wang, Z.-J. Complex, Multi-Stage Mineralization Processes in the Giant Bayan Obo REE-Nb-Fe Deposit, China. Ore Geol. Rev. 2021, 139, 104461. [Google Scholar] [CrossRef]
- Xu, W.; Shi, B.; Tian, Y.; Chen, Y.; Li, S.; Cheng, Q.; Mei, G. Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite from Guizhou, China. Minerals 2021, 11, 1249. [Google Scholar] [CrossRef]
- ISO/TS 2597-4:2019; Iron Ores Determination of Total Iron Content Part 4: Potentiometric Titration Method. Available online: https://www.iso.org/standard/50478.html (accessed on 11 October 2023).
- Zelenova, I.M. Iron-Ore Concentrates in Iron-Powder Production. Steel Transl. 2009, 39, 827–830. [Google Scholar] [CrossRef]
- Bonadiman, C.; Nazzareni, S.; Coltorti, M.; Comodi, P.; Giuli, G.; Faccini, B. Crystal Chemistry of Amphiboles: Implications for Oxygen Fugacity and Water Activity in Lithospheric Mantle beneath Victoria Land, Antarctica. Contrib. Mineral. Petrol. 2014, 167, 984. [Google Scholar] [CrossRef]
- Harrison, R.J.; Putnis, A. Magnetic Properties of the Magnetite-Spinel Solid Solution: Curie Temperatures, Magnetic Susceptibilities, and Cation Ordering. Am. Mineral. 1996, 81, 375–384. [Google Scholar] [CrossRef]
- Harrison, R.J.; Putnis, A. The Magnetic Properties and Crystal Chemistry of Oxide Spinel Solid Solutions. Surv. Geophys. 1998, 19, 461–520. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Clout, J.M.F. Importance of Fine Ore Chemical Composition and High Temperature Phase Relations: Applications to Iron Ore Sintering and Pelletising. Miner. Process. Extr. Metall. 2003, 112, 44–51. [Google Scholar] [CrossRef]
- Dhana Raju, R. Placer Magnetite-Sand and By-Product Iron, Generated during the Beneficiation of Mineral Sand Ilmenite to High-Titanium Products, as Potential Alternatives to the High-Grade Fe-Ore for Steel Making. J. Geol. Soc. India 2022, 98, 165–168. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, C.; Ding, W.; Peng, Y.; Chen, T.; Zou, K. Extraction of Phosphorous from a Phosphorous-Containing Vanadium Titano-Magnetite Tailings by Direct Flotation. Processes 2020, 8, 874. [Google Scholar] [CrossRef]
- Kumar, A.; Sahu, R.; Tripathy, S.K. Energy-Efficient Advanced Ultrafine Grinding of Particles Using Stirred Mills—A Review. Energies 2023, 16, 5277. [Google Scholar] [CrossRef]
- He, H.; Cao, J.; Duan, N. Novel Bead-Milling Mechanically Pulverized Bulk Mineral Particles to Ultrafine Scale: Energy Storage and Cleaner Promotion of Mineral Extraction. J. Clean. Prod. 2018, 198, 46–53. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Boston, MA, USA, 2015; ISBN 978-0-08-097054-7. [Google Scholar]
- Bamber, A.S. Integrated Mining, Pre-Concentration and Waste Disposal Systems for the Increased Sustainability of Hard Rock Metal Mining. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2008. [Google Scholar]
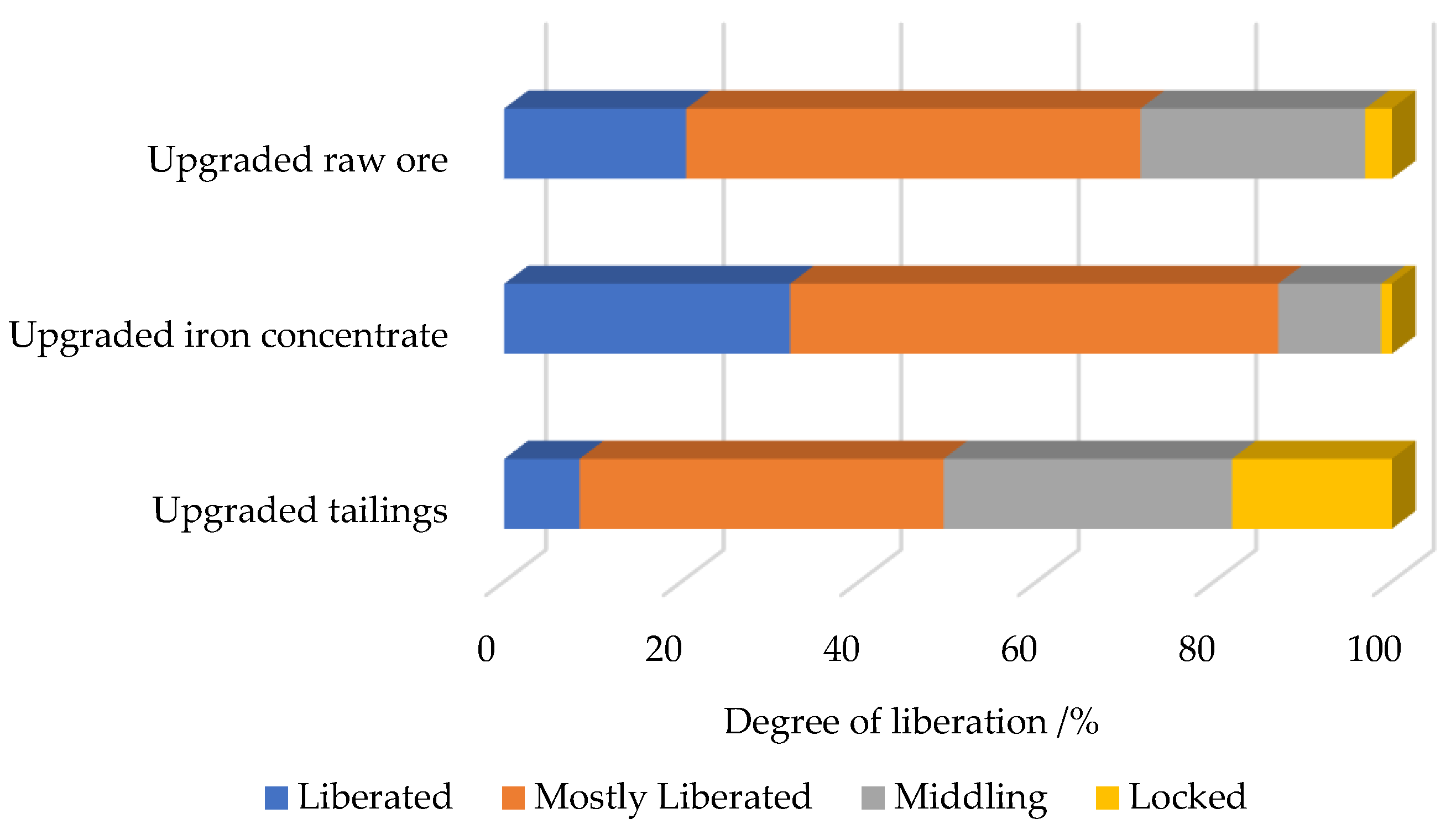
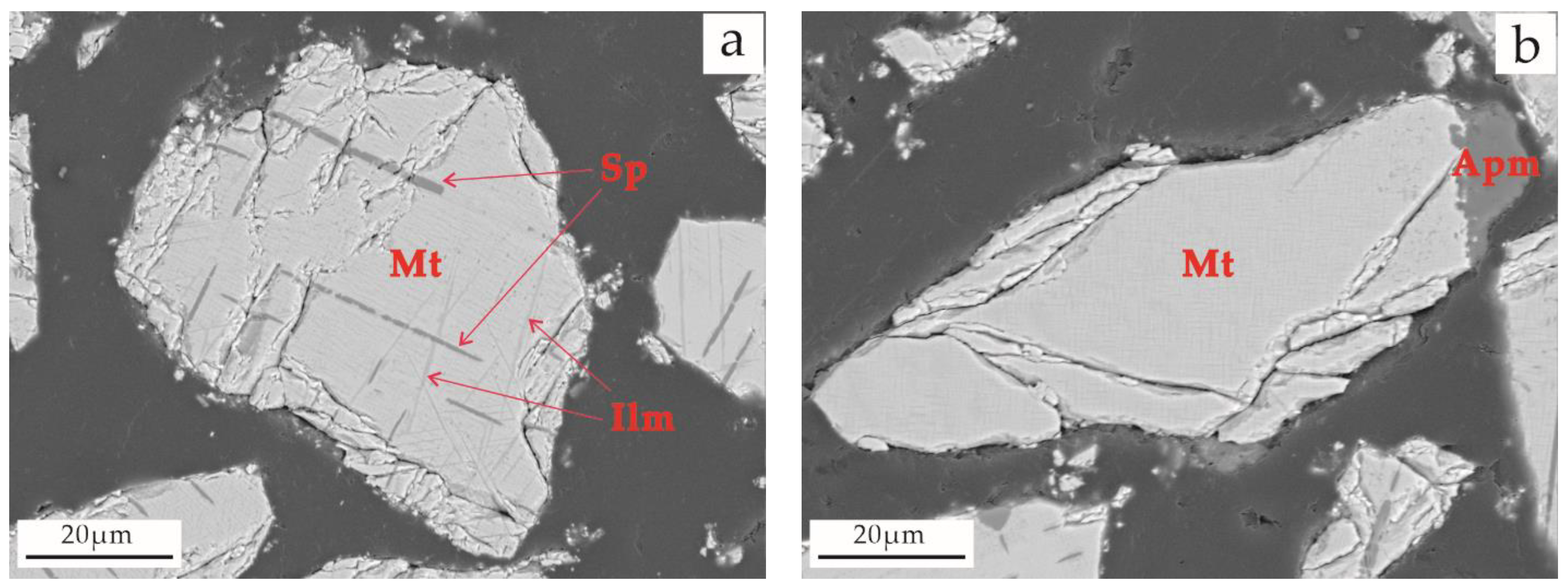
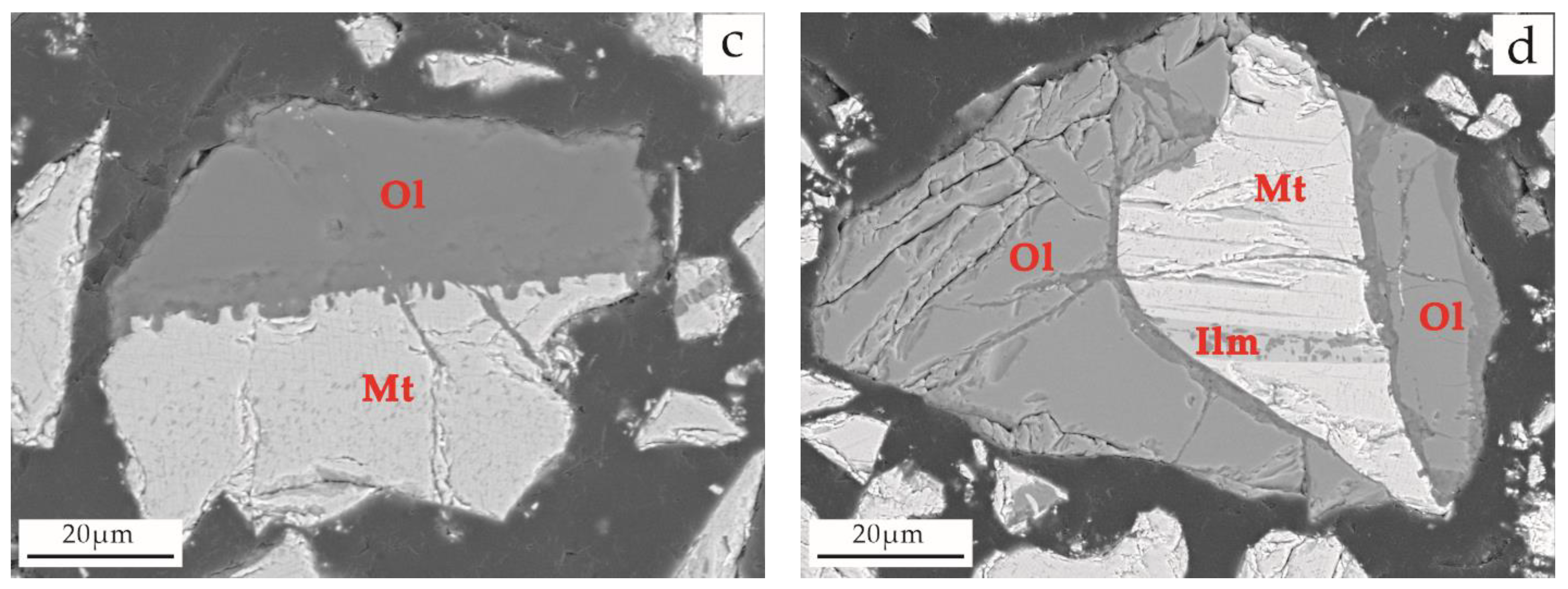

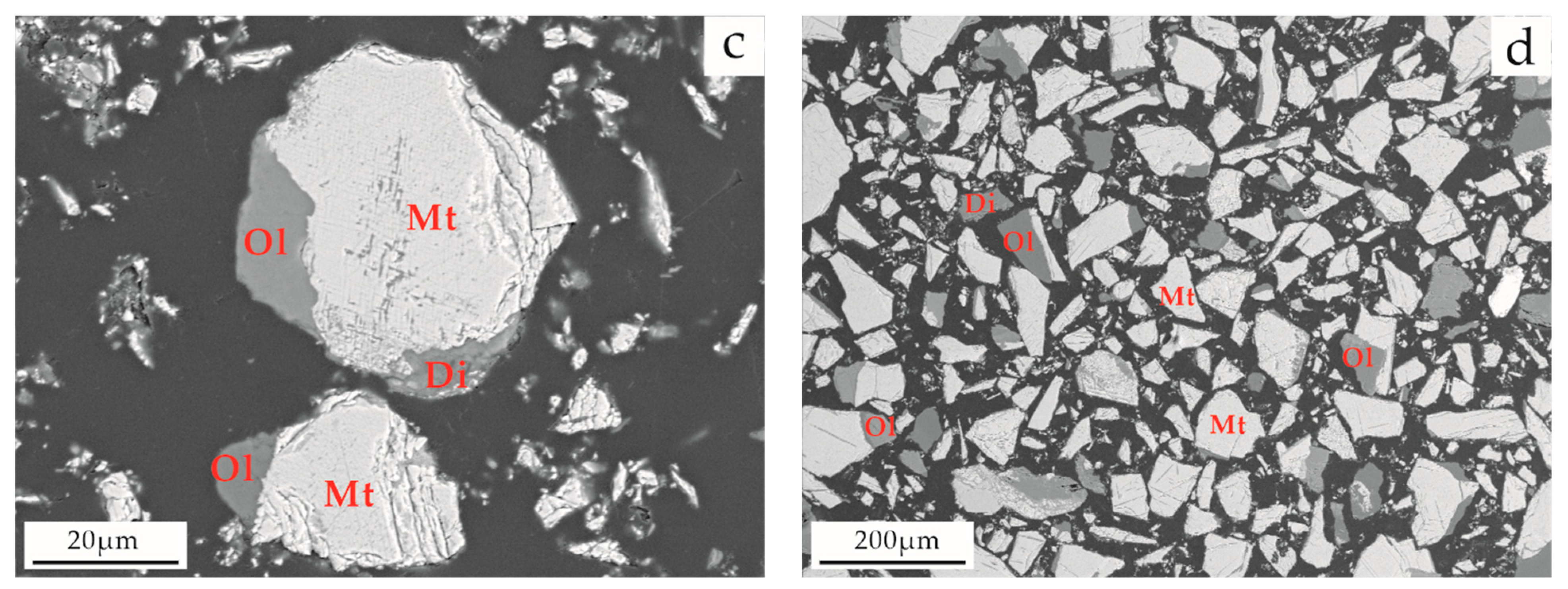
| Sample Type | Main Chemical Composition (wt. %) | ||||||
|---|---|---|---|---|---|---|---|
| TFe | TiO2 | V2O5 | SiO2 | CaO | MgO | Al2O3 | |
| Upgraded raw ore | 55.68 | 10.11 | 0.70 | 4.00 | 0.35 | 3.95 | 3.66 |
| Upgraded iron concentrate | 57.89 | 10.30 | 0.74 | 2.85 | 0.22 | 3.17 | 3.34 |
| Upgraded tailings | 15.62 | 6.59 | <0.1 | 32.06 | 3.98 | 19.93 | 9.46 |
| Mineral Name | Mineral Composition (wt. %) | ||
|---|---|---|---|
| Upgraded Raw Ore | Upgraded Iron Concentrate | Upgraded Tailings | |
| Titanomagnetite | 77.41 | 82.10 | 1.36 |
| Olivine | 8.02 | 5.86 | 44.51 |
| Amphibole | 3.37 | 3.06 | 10.41 |
| Labradorite | 3.22 | 2.04 | 18.57 |
| Diopside | 3.08 | 1.85 | 13.63 |
| Ilmenite | 2.09 | 2.53 | 6.12 |
| Pyrrhotite | 1.07 | 0.92 | 1.72 |
| Spinel | 0.94 | 0.97 | 1.33 |
| Biotite | 0.31 | 0.15 | 0.79 |
| Anorthite | 0.17 | 0.08 | 0.80 |
| Titanite | 0.16 | 0.30 | 0.16 |
| Cobalt pentlandite | 0.04 | 0.02 | 0.04 |
| Pentlandite | 0.03 | 0.02 | 0.04 |
| Albite | 0.03 | 0.06 | 0.30 |
| Apatite | 0.02 | 0.01 | 0.11 |
| Chalcopyrite | 0.02 | 0.02 | 0.03 |
| Potassium feldspar | 0.02 | 0.01 | 0.08 |
| Mineral Name | Iron Element Distribution (%) | ||
|---|---|---|---|
| Upgraded Raw Ore | Upgraded Iron Concentrate | Upgraded Tailings | |
| Titanomagnetite | 91.05 | 93.14 | 5.52 |
| Olivine | 4.16 | 2.51 | 52.18 |
| Ilmenite | 1.38 | 1.45 | 13.94 |
| Pyrrhotite | 1.29 | 1.08 | 6.99 |
| Amphibole | 1.03 | 1.02 | 10.59 |
| Diopside | 0.57 | 0.32 | 8.13 |
| Spinel | 0.38 | 0.39 | 1.83 |
| Biotite | 0.06 | 0.03 | 0.61 |
| Pentlandite | 0.04 | 0.03 | 0.09 |
| Cobalt pentlandite | 0.02 | 0.01 | 0.06 |
| Chalcopyrite | 0.02 | 0.02 | 0.06 |
| Mineral Name | Titanium Element Distribution (%) | ||
|---|---|---|---|
| Upgraded Raw Ore | Upgraded Iron Concentrate | Upgraded Tailings | |
| Titanomagnetite | 88.85 | 87.86 | 3.95 |
| Ilmenite | 10.21 | 10.95 | 87.56 |
| Diopside | 0.69 | 0.25 | 6.58 |
| Titanite | 0.25 | 0.94 | 1.91 |
| Chemical Compositions | FeO | Fe2O3 | TiO2 | MgO | Al2O3 | V2O5 | MnO | Total |
|---|---|---|---|---|---|---|---|---|
| 100 spots average | 30.50 | 61.50 | 5.30 | 0.42 | 1.82 | 0.15 | 0.19 | 99.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Hu, H.; Li, S.; Gao, J.; Shi, Z.; Zhu, F. Mineralogical Characteristics of Baima Vanadium Titanomagnetite during Magnetic Separation Upgrading. Separations 2023, 10, 574. https://doi.org/10.3390/separations10110574
Zhong X, Hu H, Li S, Gao J, Shi Z, Zhu F. Mineralogical Characteristics of Baima Vanadium Titanomagnetite during Magnetic Separation Upgrading. Separations. 2023; 10(11):574. https://doi.org/10.3390/separations10110574
Chicago/Turabian StyleZhong, Xiang, Haoran Hu, Shuo Li, Jian Gao, Zhixin Shi, and Fuxing Zhu. 2023. "Mineralogical Characteristics of Baima Vanadium Titanomagnetite during Magnetic Separation Upgrading" Separations 10, no. 11: 574. https://doi.org/10.3390/separations10110574
APA StyleZhong, X., Hu, H., Li, S., Gao, J., Shi, Z., & Zhu, F. (2023). Mineralogical Characteristics of Baima Vanadium Titanomagnetite during Magnetic Separation Upgrading. Separations, 10(11), 574. https://doi.org/10.3390/separations10110574






