Enhancing Trace Metal Extraction from Wastewater: Magnetic Activated Carbon as a High-Performance Sorbent for Inductively Coupled Plasma Optical Emission Spectrometry Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Samples and Reagents
2.3. Synthesis of the Magnetic Sorbent
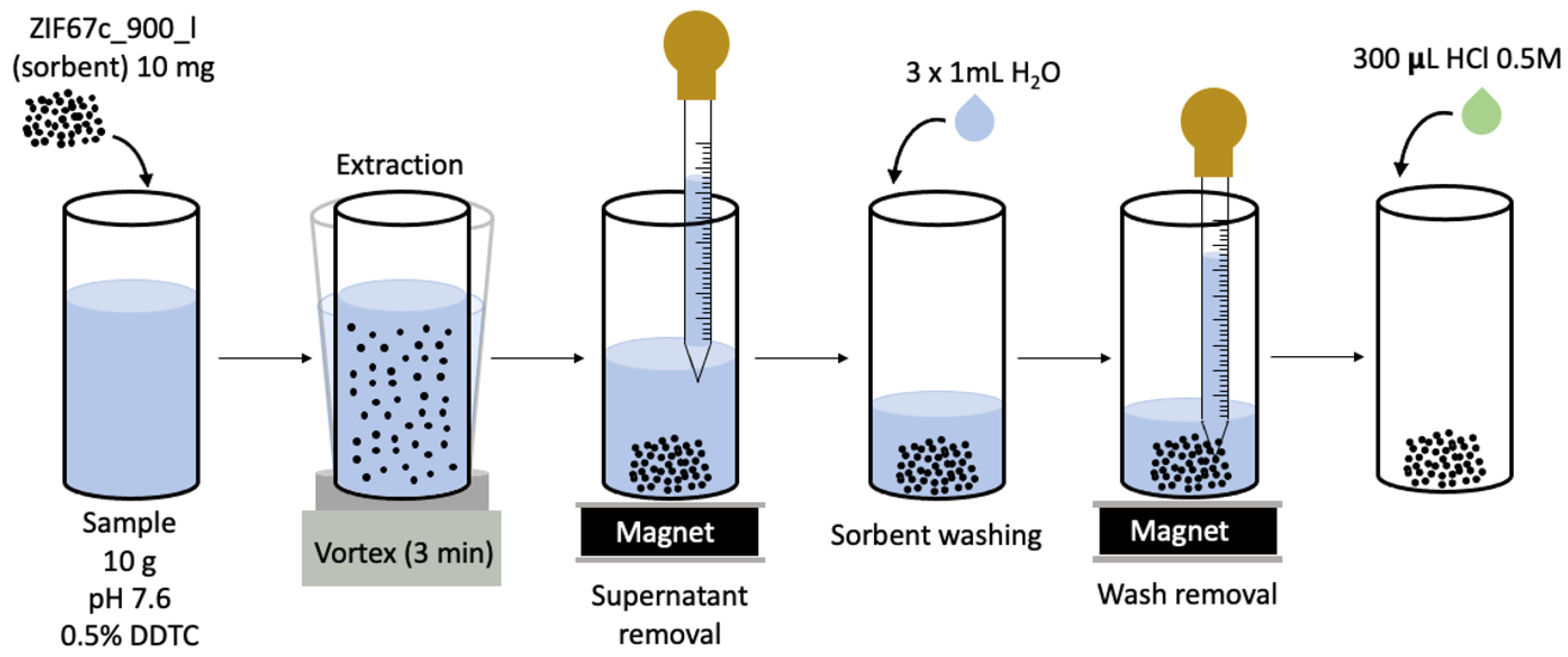
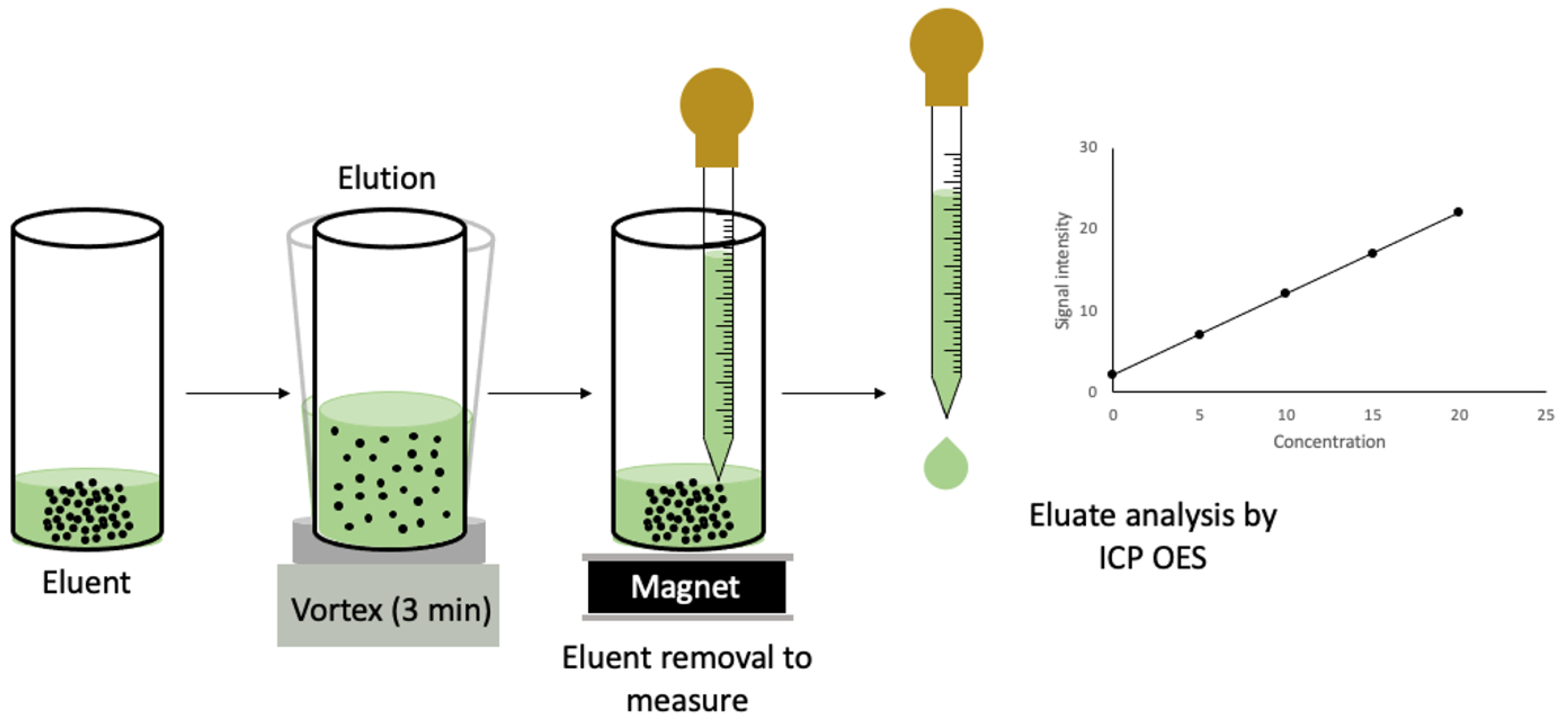
2.4. MDSPE Procedure
3. Result and Discussion
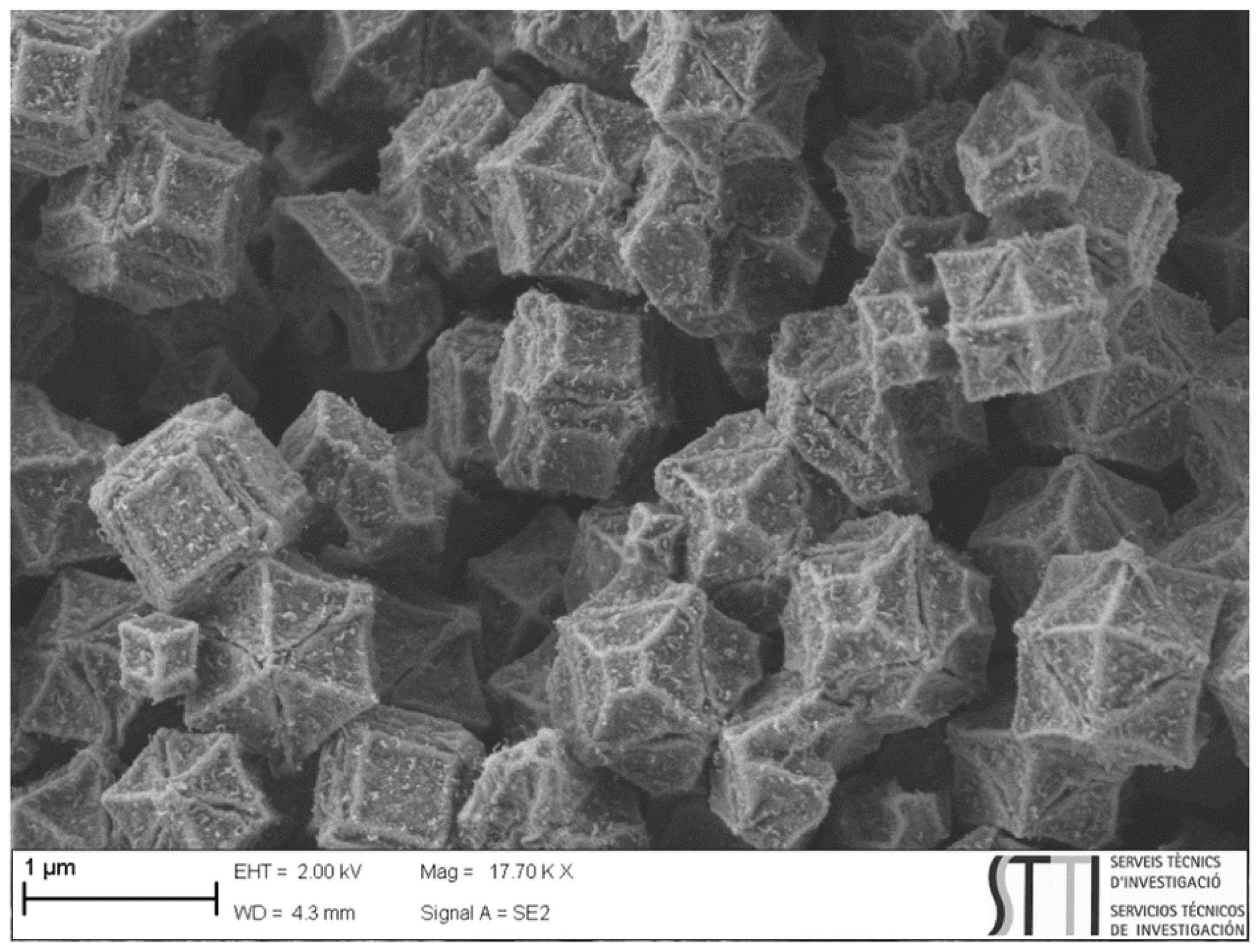
3.1. Characterization of ZIF67C_900_l
3.2. MDSPE Multivariate Optimization
3.2.1. Screening Study
3.2.2. Central Composite Design (CCD)
3.2.3. Desirability Function
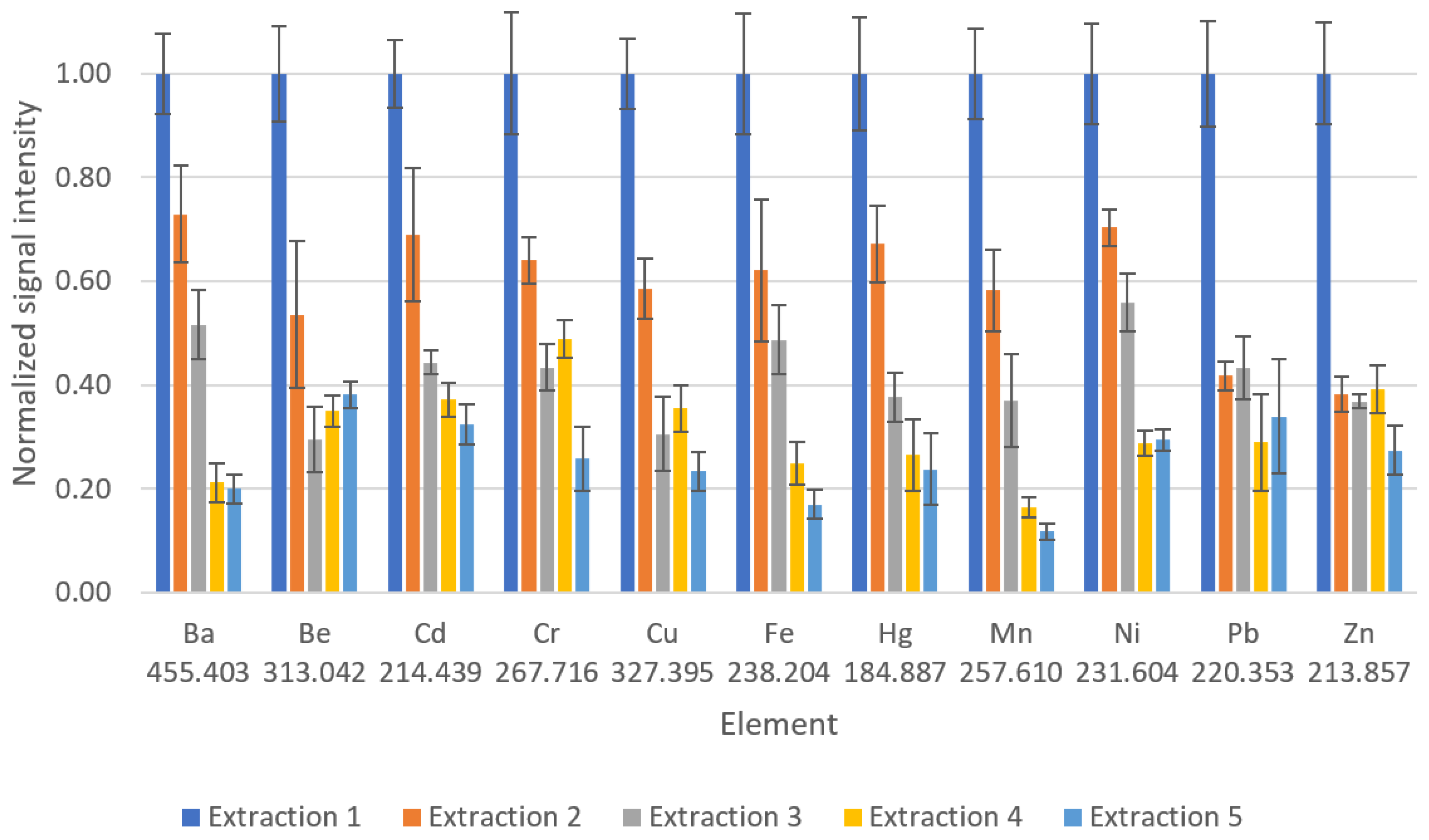
3.3. Sorbent Reutilization Study
3.4. Method Validation
3.5. Method Applicability
3.6. Comparison with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dogru, M.; Gul-Guven, R.; Erdogan, S. The Use of Bacillus Subtilis Immobilized on Amberlite XAD-4 as a New Biosorbent in Trace Metal Determination. J. Hazard. Mater. 2007, 149, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of Toxic Metals from Aqueous Solutions by Bacteria Strains Isolated from Metal-Polluted Soils. Process Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Bermejo-Barrera, P.; Martínez Alfonso, N.; Díaz López, C.; Bermejo Barrera, A. Use of Amberlite XAD-2 Loaded with 1-(2-Pyridylazo)-2-Naphthol as a Preconcentration System for River Water Prior to Determination of Cu2+, Cd2+ and Pb2+ by Flame Atomic Absorption Spectroscopy. Microchim. Acta 2003, 142, 101–108. [Google Scholar] [CrossRef]
- Sardans, J.; Montes, F.; Peñuelas, J. Determination of As, Cd, Cu, Hg and Pb in Biological Samples by Modern Electrothermal Atomic Absorption Spectrometry. Spectrochim. Acta B 2010, 65, 97–112. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for Heavy Metals Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A.N. Magnetic Materials as Sorbents for Metal/Metalloid Preconcentration and/or Separation. A Review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef]
- Harvey, P.J.; Handley, H.K.; Taylor, M.P. Identification of the Sources of Metal (Lead) Contamination in Drinking Waters in North-Eastern Tasmania Using Lead Isotopic Compositions. Environ. Sci. Pollut. Res. 2015, 22, 12276–12288. [Google Scholar] [CrossRef]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human Health Risk Assessment of Heavy Metals in Urban Stormwater. Sci. Total Environ. 2016, 557–558, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, C.; Xu, W. Magnetic Dendritic Materials for Highly Efficient Adsorption of Dyes and Drugs. ACS Appl. Mater. Interfaces 2010, 2, 1483–1491. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Saber-Tehrani, M.; Khoshsafar, H. Preparation and Characterization of Magnetic Nanocomposite of Schiff Base/Silica/Magnetite as a Preconcentration Phase for the Trace Determination of Heavy Metal Ions in Water, Food and Biological Samples Using Atomic Absorption Spectrometry. Talanta 2012, 97, 87–95. [Google Scholar] [CrossRef]
- Suleiman, J.S.; Hu, B.; Huang, C.; Zhang, N. Determination of Cd, Co, Ni and Pb in Biological Samples by Microcolumn Packed with Black Stone (Pierre Noire) Online Coupled with ICP-OES. J. Hazard. Mater. 2008, 157, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Yamini, Y.; Faraji, M.; Shariati, S.; Hassani, R.; Ghambarian, M. On-Line Metals Preconcentration and Simultaneous Determination Using Cloud Point Extraction and Inductively Coupled Plasma Optical Emission Spectrometry in Water Samples. Anal. Chim. Acta 2008, 612, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.C.; Calloway, C.P.; Jones, B.T. Direct Determination of Cadmium in Urine by Tungsten-Coil Inductively Coupled Plasma Atomic Emission Spectrometry Using Palladium as a Permanent Modifier. Talanta 2007, 71, 1144–1149. [Google Scholar] [CrossRef]
- Huang, C.; Hu, B. Silica-Coated Magnetic Nanoparticles Modified with γ-Mercaptopropyltrimethoxysilane for Fast and Selective Solid Phase Extraction of Trace Amounts of Cd, Cu, Hg, and Pb in Environmental and Biological Samples Prior to Their Determination by Inductively Co. Spectrochim. Acta B 2008, 63, 437–444. [Google Scholar] [CrossRef]
- Zheng, F.; Hu, B. Thermo-Responsive Polymer Coated Fiber-in-Tube Capillary Microextraction and Its Application to on-Line Determination of Co, Ni and Cd by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Talanta 2011, 85, 1166–1173. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, B.; Peng, H.; He, M.; Hu, B. Aminopropyltriethoxysilane-Silica Hybrid Monolithic Capillary Microextraction Combined with Inductively Coupled Plasma Mass Spectrometry for the Determination of Trace Elements in Biological Samples. J. Sep. Sci. 2011, 34, 2247–2254. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Taher, M.A.; Behzadi, M.; Moghaddam, F.H. Preparation a Novel Magnetic Natural Nano Zeolite for Preconcentration of Cadmium and Its Determination by ETAAS. Environ. Nanotechnol. Monit. Manag. 2017, 8, 261–267. [Google Scholar] [CrossRef]
- Jorhem, L.; Åstrand, C.; Sundström, B.; Baxter, M.; Stokes, P.; Lewis, J.; Grawe, K.P. Elements in Rice from the Swedish Market: 1. Cadmium, Lead and Arsenic (Total and Inorganic). Food Addit. Contam.-Part A 2008, 25, 284–292. [Google Scholar] [CrossRef]
- Montoro-Leal, P.; García-Mesa, J.C.; Siles Cordero, M.T.; López Guerrero, M.M.; Vereda Alonso, E. Magnetic Dispersive Solid Phase Extraction for Simultaneous Enrichment of Cadmium and Lead in Environmental Water Samples. Microchem. J. 2020, 155, 104796. [Google Scholar] [CrossRef]
- Xie, F.; Lin, X.; Wu, X.; Xie, Z. Solid Phase Extraction of Lead (II), Copper (II), Cadmium (II) and Nickel (II) Using Gallic Acid-Modified Silica Gel Prior to Determination by Flame Atomic Absorption Spectrometry. Talanta 2008, 74, 836–843. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. Column Solid-Phase Extraction of Nickel and Silver in Environmental Samples Prior to Their Flame Atomic Absorption Spectrometric Determinations. J. Hazard. Mater. 2009, 164, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Tavallali, H.; Shokrollahi, A.; Zahedi, M.; Montazerozohori, M.; Soylak, M. Flame Atomic Absorption Spectrometric Determination of Zinc, Nickel, Iron and Lead in Different Matrixes after Solid Phase Extraction on Sodium Dodecyl Sulfate (SDS)-Coated Alumina as Their Bis (2-Hydroxyacetophenone)-1, 3-Propanediimine Chelates. J. Hazard. Mater. 2009, 166, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Bryce, D.W.; Izquierdo, A.; Luque De Castro, M.D. Continuous Microwave Assisted Pervaporation/Atomic Fluorescence Detection: An Approach for Speciation in Solid Samples. Anal. Chim. Acta 1996, 324, 69–75. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Z.; Chu, X.; Yang, X. Evaluation of a New Electrolytic Cold Vapor Generation System for Mercury Determination by AFS. Talanta 2010, 80, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; He, M.; Peng, H.; Hu, B. Dithizone Modified Magnetic Nanoparticles for Fast and Selective Solid Phase Extraction of Trace Elements in Environmental and Biological Samples Prior to Their Determination by ICP-OES. Talanta 2012, 88, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Zeini Jahromi, E.; Bidari, A.; Assadi, Y.; Milani Hosseini, M.R.; Jamali, M.R. Dispersive Liquid-Liquid Microextraction Combined with Graphite Furnace Atomic Absorption Spectrometry. Ultra Trace Determination of Cadmium in Water Samples. Anal. Chim. Acta 2007, 585, 305–311. [Google Scholar] [CrossRef]
- Shabani, A.M.H.; Dadfarnia, S.; Nasirizadeh, N.; Shishehbore, M.R. Preconcentration of Copper with Dithizone-Naphthalene for Subsequent Determination by Atomic Absorption Spectrometry. J. Anal. Chem. 2007, 62, 46–50. [Google Scholar] [CrossRef]
- Miró, M.; Hansen, E.H. Recent Advances and Future Prospects of Mesofluidic Lab-on-a-Valve Platforms in Analytical Sciences—A Critical Review. Anal. Chim. Acta 2012, 750, 3–15. [Google Scholar] [CrossRef]
- Baghdadi, M.; Shemirani, F. Cold-Induced Aggregation Microextraction: A Novel Sample Preparation Technique Based on Ionic Liquids. Anal. Chim. Acta 2008, 613, 56–63. [Google Scholar] [CrossRef]
- Soylak, M.; Yilmaz, E. Ionic Liquid Dispersive Liquid-Liquid Microextraction of Lead as Pyrrolidinedithiocarbamate Chelate Prior to Its Flame Atomic Absorption Spectrometric Determination. Desalination 2011, 275, 297–301. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Miniaturized Preconcentration Methods Based on Liquid-Liquid Extraction and Their Application in Inorganic Ultratrace Analysis and Speciation: A Review. Spectrochim. Acta B 2009, 64, 1–15. [Google Scholar] [CrossRef]
- Soylak, M.; Aydin, A. Determination of Some Heavy Metals in Food and Environmental Samples by Flame Atomic Absorption Spectrometry after Coprecipitation. Food Chem. Toxicol. 2011, 49, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Kars, A.; Narin, I. Coprecipitation of Ni2+, Cd2+ and Pb2+ for Preconcentration in Environmental Samples Prior to Flame Atomic Absorption Spectrometric Determinations. J. Hazard. Mater. 2008, 159, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Saberyan, K.; Zolfonoun, E.; Shamsipur, M.; Salavati-Niasari, M. Separation and Preconcentration of Trace Gallium and Indium by Amberlite XAD-7 Resin Impregnated with a New Hexadentates Naphthol-Derivative Schiff Base. Sep. Sci. Technol. 2009, 44, 1851–1868. [Google Scholar] [CrossRef]
- Uludag, Y.; Özbelge, H.Ö.; Yilmaz, L. Removal of Mercury from Aqueous Solutions via Polymer-Enhanced Ultrafiltration. J. Membr. Sci. 1997, 129, 93–99. [Google Scholar] [CrossRef]
- Bruno, P.; Caselli, M.; De Gennaro, G.; Ielpo, P.; Ladisa, T.; Placentino, C.M. Ion Chromatography Determination of Heavy Metals in Airborne Particulate with Preconcentration and Large Volume Direct Injection. Chromatographia 2006, 64, 537–542. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Sánchez Rojas, F.; Ojeda, C.B.; Pavón, J.M.C. On-Line Preconcentration of Rhodium on an Anion-Exchange Resin Loaded with 1,5-Bis(2-Pyridyl)-3-Sulphophenyl Methylene Thiocarbonohydrazide and Its Determination in Environmental Samples. Talanta 2004, 64, 230–236. [Google Scholar] [CrossRef]
- Shariati, S.; Yamini, Y.; Zanjani, M.K. Simultaneous Preconcentration and Determination of U(VI), Th(IV), Zr(IV) and Hf(IV) Ions in Aqueous Samples Using Micelle-Mediated Extraction Coupled to Inductively Coupled Plasma-Optical Emission Spectrometry. J. Hazard. Mater. 2008, 156, 583–590. [Google Scholar] [CrossRef]
- Meeravali, N.N.; Jiang, S.J. Microwave Assisted Mixed-Micelle Cloud Point Extraction of Au and Tl from Environmental Samples without Using a Chelating Agent Prior to ICP-MS Determination. J. Anal. At. Spectrom. 2008, 23, 1365–1371. [Google Scholar] [CrossRef]
- Tuzen, M.; Karaman, I.; Citak, D.; Soylak, M. Mercury(II) and Methyl Mercury Determinations in Water and Fish Samples by Using Solid Phase Extraction and Cold Vapour Atomic Absorption Spectrometry Combination. Food Chem. Toxicol. 2009, 47, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Uluozlu, O.D.; Karaman, I.; Soylak, M. Mercury(II) and Methyl Mercury Speciation on Streptococcus Pyogenes Loaded Dowex Optipore SD-2. J. Hazard. Mater. 2009, 169, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Qin, Y.; Ye, C.; Peng, P.; Wu, C. Preparation of the Diphenylcarbazone-Functionalized Silica Gel and Its Application to on-Line Selective Solid-Phase Extraction and Determination of Mercury by Flow-Injection Spectrophotometry. J. Hazard. Mater. 2008, 150, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yamini, Y.; Rezaee, M. Extraction of Trace Amounts of Mercury with Sodium Dodecyle Sulphate-Coated Magnetite Nanoparticles and Its Determination by Flow Injection Inductively Coupled Plasma-Optical Emission Spectrometry. Talanta 2010, 81, 831–836. [Google Scholar] [CrossRef]
- Poole, C.F. New Trends in Solid-Phase Extraction. TrAC-Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, H.; Yang, L.; Fei, P.; Liu, H. Development Trend and Prospect of Solid Phase Extraction Technology. Chin. J. Chem. Eng. 2022, 42, 245–255. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A.N. Automated Magnetic Sorbent Extraction Based on Octadecylsilane Functionalized Maghemite Magnetic Particles in a Sequential Injection System Coupled with Electrothermal Atomic Absorption Spectrometry for Metal Determination. Talanta 2013, 110, 229–235. [Google Scholar] [CrossRef]
- Rao, T.P.; Metilda, P.; Gladis, J.M. Preconcentration Techniques for Uranium(VI) and Thorium(IV) Prior to Analytical Determination-an Overview. Talanta 2006, 68, 1047–1064. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, Z.; Qiu, K.; Peng, W. Separation of Ilmenite from Vanadium Titanomagnetite by Combining Magnetic Separation and Flotation Processes. Separations 2023, 10, 95. [Google Scholar] [CrossRef]
- Córdova Udaeta, M.; Dodbiba, G.; Ponou, J.; Sone, K.; Fujita, T. Recovery of Phosphorus from Sewage Sludge Ash (SSA) by Heat Treatment Followed by High Gradient Magnetic Separation and Flotation. Adv. Powder Technol. 2017, 28, 755–762. [Google Scholar] [CrossRef]
- Jiménez-Soto, J.M.; Cárdenas, S.; Valcárcel, M. Evaluation of Single-Walled Carbon Nanohorns as Sorbent in Dispersive Micro Solid-Phase Extraction. Anal. Chim. Acta 2012, 714, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Galán-Cano, F.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Direct Coupling of Dispersive Micro-Solid Phase Extraction and Thermal Desorption for Sensitive Gas Chromatographic Analysis. Anal. Methods 2011, 3, 991–995. [Google Scholar] [CrossRef]
- Skorek, R.; Zawisza, B.; Marguí, E.; Queralt, I.; Sitko, R. Dispersive Micro Solid-Phase Extraction Using Multiwalled Carbon Nanotubes for Simultaneous Determination of Trace Metal Ions by Energy-Dispersive x-Ray Fluorescence Spectrometry. Appl. Spectrosc. 2013, 67, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L.; Nomngongo, P.N. Ultrasonic Assisted Dispersive-Solid Phase Extraction for Preconcentration of Trace Metals in Wastewater Samples. J. Environ. Chem. Eng. 2022, 10, 108187. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC-Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Duan, C.; Shen, Z.; Wu, D.; Guan, Y. Recent Developments in Solid-Phase Microextraction for on-Site Sampling and Sample Preparation. TrAC-Trends Anal. Chem. 2011, 30, 1568–1574. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J. Dispersive Solid-Phase Extraction. In Analytical Separation Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1525–1570. [Google Scholar] [CrossRef]
- Ruiz, F.J.; Ripoll, L.; Hidalgo, M.; Canals, A. Dispersive Micro Solid-Phase Extraction (DµSPE) with Graphene Oxide as Adsorbent for Sensitive Elemental Analysis of Aqueous Samples by Laser Induced Breakdown Spectroscopy (LIBS). Talanta 2019, 191, 162–170. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Evolution of Solid-Phase Microextraction Technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I. Magnetic Adsorbents Based on Micro- and Nano-Structured Materials. RSC Adv. 2015, 5, 6695–6719. [Google Scholar] [CrossRef]
- Tian, J.; Xu, J.; Zhu, F.; Lu, T.; Su, C.; Ouyang, G. Application of Nanomaterials in Sample Preparation. J. Chromatogr. A 2013, 1300, 2–16. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized Solid-Phase Extraction Techniques. TrAC-Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Wierucka, M.; Biziuk, M. Application of Magnetic Nanoparticles for Magnetic Solid-Phase Extraction in Preparing Biological, Environmental and Food Samples. TrAC-Trends Anal. Chem. 2014, 59, 50–58. [Google Scholar] [CrossRef]
- Safarikova, M.; Kibrikova, I.; Ptackova, L.; Hubka, T.; Komarek, K.; Safarik, I. Magnetic Solid Phase Extraction of Non-Ionic Surfactants from Water. J. Magn. Magn. Mater. 2005, 293, 377–381. [Google Scholar] [CrossRef]
- Aliyari, E.; Alvand, M.; Shemirani, F. Modified Surface-Active Ionic Liquid-Coated Magnetic Graphene Oxide as a New Magnetic Solid Phase Extraction Sorbent for Preconcentration of Trace Nickel. RSC Adv. 2016, 6, 64193–64202. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Goudarzi, A.; Soylak, M.; Mehdizadeh Langroodi, S. Magnetic Nanoparticle Based Dispersive Micro-Solid-Phase Extraction for the Determination of Malachite Green in Water Samples: Optimized Experimental Design. New J. Chem. 2015, 39, 9813–9823. [Google Scholar] [CrossRef]
- Costa dos Reis, L.; Vidal, L.; Canals, A. Determination of Siloxanes in Water Samples Employing Graphene Oxide/Fe3O4 Nanocomposite as Sorbent for Magnetic Solid-Phase Extraction Prior to GC–MS. J. Sep. Sci. 2018, 41, 4177–4184. [Google Scholar] [CrossRef]
- Aguilar-Arteaga, K.; Rodriguez, J.A.; Barrado, E. Magnetic Solids in Analytical Chemistry: A Review. Anal. Chim. Acta 2010, 674, 157–165. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wei, X.; Xu, P.; Xu, W.; Ni, R.; Meng, J. Magnetic Solid-Phase Extraction for the Removal of Mercury from Water with Ternary Hydrosulphonyl-Based Deep Eutectic Solvent Modified Magnetic Graphene Oxide. Talanta 2018, 188, 454–462. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Toghroli, M.; Kamankesh, M. Zeolite/Fe3O4 as a New Sorbent in Magnetic Solid-Phase Extraction Followed by Gas Chromatography for Determining Phthalates in Aqueous Samples. J. Sep. Sci. 2015, 38, 3750–3757. [Google Scholar] [CrossRef]
- Tolmacheva, V.V.; Apyari, V.V.; Kochuk, E.V.; Dmitrienko, S.G. Magnetic Adsorbents Based on Iron Oxide Nanoparticles for the Extraction and Preconcentration of Organic Compounds. J. Anal. Chem. 2016, 71, 321–338. [Google Scholar] [CrossRef]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic Nanoparticle Based Solid-Phase Extraction of Heavy Metal Ions: A Review on Recent Advances. Microchim. Acta 2018, 185, 160. [Google Scholar] [CrossRef] [PubMed]
- Baile, P.; Vidal, L.; Canals, A. A Modified Zeolite/Iron Oxide Composite as a Sorbent for Magnetic Dispersive Solid-Phase Extraction for the Preconcentration of Nonsteroidal Anti-Inflammatory Drugs in Water and Urine Samples. J. Chromatogr. A 2019, 1603, 33–43. [Google Scholar] [CrossRef]
- Baile, P.; Fernández, E.; Vidal, L.; Canals, A. Zeolites and Zeolite-Based Materials in Extraction and Microextraction Techniques. Analyst 2019, 144, 366–387. [Google Scholar] [CrossRef] [PubMed]
- Baile, P.; Vidal, L.; Canals, A. Magnetic Dispersive Solid-Phase Extraction Using ZSM-5 Zeolite/Fe2O3 Composite Coupled with Screen-Printed Electrodes Based Electrochemical Detector for Determination of Cadmium in Urine Samples. Talanta 2020, 220, 121394. [Google Scholar] [CrossRef]
- Villalgordo-Hernández, D.; Grau-Atienza, A.; García-Marín, A.A.; Ramos-Fernández, E.V.; Narciso, J. Manufacture of Carbon Materials with High Nitrogen Content. Materials 2022, 15, 2415. [Google Scholar] [CrossRef]
- Zabiegaj, D.; Caccia, M.; Casco, M.E.; Ravera, F.; Narciso, J. Synthesis of Carbon Monoliths with a Tailored Hierarchical Pore Structure for Selective CO2 Capture. J. CO2 Util. 2018, 26, 36–44. [Google Scholar] [CrossRef]
- Narciso, J.; Ramos-Fernandez, E.V.; Delgado-Marín, J.J.; Affolter, C.W.; Olsbye, U.; Redekop, E.A. New Route for the Synthesis of Co-MOF from Metal Substrates. Microporous Mesoporous Mater. 2021, 324, 111310. [Google Scholar] [CrossRef]
- Ramos-Fernandez, E.V.; Redondo-Murcia, A.; Grau-Atienza, A.; Sepúlveda-Escribano, A.; Narciso, J. Clean Production of Zeolitic Imidazolate Framework 8 Using Zamak Residues as Metal Precursor and Substrate. J. Clean. Prod. 2020, 260, 121081. [Google Scholar] [CrossRef]
- Delgado-Marín, J.J.; Martín-García, I.; Villalgordo-Hernández, D.; Alonso, F.; Ramos-Fernández, E.V.; Narciso, J. Valorization of CO2 through the Synthesis of Cyclic Carbonates Catalyzed by ZIFs. Molecules 2022, 27, 7791. [Google Scholar] [CrossRef]
- Caccia, M.; Giuranno, D.; Molina-Jorda, J.M.; Moral, M.; Nowak, R.; Ricci, E.; Sobczak, N.; Narciso, J.; Fernández Sanz, J. Graphene Translucency and Interfacial Interactions in the Gold/Graphene/SiC System. J. Phys. Chem. Lett. 2018, 9, 3850–3855. [Google Scholar] [CrossRef]
- Costa, D.A.S.; Mambrini, R.V.; Fernandez-Outon, L.E.; Macedo, W.A.A.; Moura, F.C.C. Magnetic Adsorbent Based on Cobalt Core Nanoparticles Coated with Carbon Filaments and Nanotubes Produced by Chemical Vapor Deposition with Ethanol. Chem. Eng. J. 2013, 229, 35–41. [Google Scholar] [CrossRef]
- Baile, P.; Vidal, L.; Aguirre, M.Á.; Canals, A. A Modified ZSM-5 Zeolite/Fe2O3 Composite as a Sorbent for Magnetic Dispersive Solid-Phase Microextraction of Cadmium, Mercury and Lead from Urine Samples Prior to Inductively Coupled Plasma Optical Emission Spectrometry. J. Anal. At. Spectrom. 2018, 33, 856–866. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; LGC: Teddington Ltd.: Teddington, UK, 2014; ISBN 9789187461590. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC-Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]

| Direct ICP OES | ||||||||
| Emission Line (nm) | Working Range (µg L−1) | R2 | Sensitivity a (cps µg−1 L) | Repeatability (RSD%) | LOD (µg L−1) | LOQ (µg L−1) | EF | |
| Ba 455.403 | 250–1000 | 0.998 | 534 ± 17 | - | 2.6 | 8.8 | - | |
| Be 313.042 | 250–1000 | 0.998 | 877 ± 28 | - | 0.39 | 1.3 | - | |
| Cd 214.439 | 250–1000 | 0.998 | 8.3 ± 0.2 | - | 1.5 | 5.0 | - | |
| Cr 267.716 | 250–1000 | 0.9994 | 11.37 ± 0.19 | - | 1.3 | 4.4 | - | |
| Cu 327.395 | 250–1000 | 0.998 | 11.0 ± 0.4 | - | 1.2 | 3.9 | - | |
| Fe 238.204 | 250–1000 | 0.998 | 9.1 ± 0.2 | - | 9.8 | 33 | - | |
| Hg 184.887 | 250–1000 | 0.9991 | 2.86 ± 0.06 | - | 5.1 | 17 | - | |
| Mn 257.610 | 250–1000 | 0.996 | 34.3 ± 1.6 | - | 0.82 | 2.7 | - | |
| Ni 231.604 | 250–1000 | 0.98 | 1.24 ± 0.12 | - | 9.9 | 33 | - | |
| Pb 220.353 | 250–1000 | 0.98 | 0.39 ± 0.04 | - | 21 | 70 | - | |
| Zn 213.857 | 250–1000 | 0.992 | 25.0 ± 1.6 | - | 0.81 | 2.7 | - | |
| MDSPE-ICP OES d | ||||||||
| Emission line (nm) | Working range (µg L−1) | R2 | Sensitivity a (cps µg−1 L) | Repeatability (RSD%) b | LOD (µg L−1) | LOQ (µg L−1) | EF c | |
| 25 (µg L−1) | 300 (µg L−1) | |||||||
| Ba 455.403 | 0.5–500 | 0.9991 | 7103 ± 93 | 5 | 5 | 0.085 | 0.29 | 13 |
| Be 313.042 | 0.5–500 | 0.997 | 5638 ± 131 | 7 | 6 | 0.073 | 0.24 | 6.4 |
| Cd 214.439 | 0.5–500 | 0.993 | 84 ± 3 | 6 | 4 | 0.12 | 0.41 | 10 |
| Cr 267.716 | 0.5–500 | 0.998 | 50.2 ± 1.0 | 7 | 6 | 0.14 | 0.45 | 4.4 |
| Cu 327.395 | 0.5–500 | 0.998 | 85.9 ± 1.5 | 6 | 6 | 0.10 | 0.34 | 7.8 |
| Fe 238.204 | 5–500 | 0.997 | 64.6 ± 1.8 | 8 | 5 | 1.3 | 4.3 | 7.1 |
| Hg 184.887 | 0.5–500 | 0.997 | 33.8 ± 0.8 | 7 | 6 | 0.13 | 0.43 | 12 |
| Mn 257.610 | 0.5–500 | 0.997 | 109 ± 3 | 6 | 6 | 0.11 | 0.37 | 3.2 |
| Ni 231.604 | 5–500 | 0.998 | 8.23 ± 0.16 | 7 | 7 | 1.1 | 3.8 | 6.6 |
| Pb 220.353 | 5–500 | 0.9996 | 3.03 ± 0.03 | 8 | 7 | 1.1 | 3.8 | 7.9 |
| Zn 213.857 | 1–500 | 0.998 | 92.2 ± 1.6 | 8 | 6 | 0.22 | 0.75 | 3.7 |
| Analyte | Cd | Cr | Cu | Fe | Hg | Mn | Ni | Pb |
|---|---|---|---|---|---|---|---|---|
| Certified value (µg L−1) b | 5.09 ± 0.20 | 20.9 ± 1.3 | 101 ± 7 | 445 ± 27 | 1.84 ± 0.11 | 95 ± 4 | 50.3 ± 1.4 | 49.7 ± 1.7 |
| Found value (µg L−1) c | 4.8 ± 0.4 | 21.6 ± 1.3 | 94 ± 8 | 424 ± 33 | 1.66 ± 0.14 | 90 ± 2 | 55 ± 3 | 50 ± 4 |
| Recovery (%) | 95 ± 8 | 103 ± 6 | 93 ± 8 | 95 ± 7 | 90 ± 8 | 95 ± 2 | 109 ± 5 | 100 ± 7 |
| Analyte | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Added Value (µg L−1) | Ba | Be | Cd | Cr | Cu | Fe | Hg | Mn | Ni | Pb | Zn | |
| Found value (µg L−1) b | - | <LOQ | <LOQ | <LOQ | 308.4 ± 0.5 | 53.6 ± 1.0 | 62.6 ± 1.3 | 1.27 ± 0.05 | 2.41 ± 0.02 | <LOQ | <LOQ | 108.4 ± 1.4 |
| 5.05 | 5.4 ± 0.2 (106 ± 4) | 5.2 ± 0.2 (101 ± 6) | 5.02 ± 0.16 (99 ± 3) | 313.8 ± 0.4 (108 ± 8) | 58.4 ± 0.3 (95 ± 6) | 67.6 ± 0.2 (98 ± 4) | 6.1 ± 0.2 (94 ± 3) | 7.2 ± 0.2 (94 ± 5) | 5.2 ± 0.2 (103 ± 4) | 5.2 ± 0.2 (103 ± 4) | 113.4 ± 0.3 (98 ± 6) | |
| 25.4 | 26.1 ± 1.1 (102 ± 5) | 24.0 ± 0.9 (94 ± 3) | 24.0 ± 0.8 (94 ± 4) | 334.2 ± 1.4 (101 ± 5) | 79.6 ± 1.9 (102 ± 6) | 88.8 ± 0.7 (103 ± 3) | 27.0 ± 1.4 (100 ± 7) | 26.5 ± 0.8 (95 ± 3) | 27.2 ± 0.7 (107 ± 2) | 27.2 ± 1.3 (107 ± 4) | 133.4 ± 0.8 (98 ± 4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán-Martín, S.J.; Villalgordo-Hernández, D.; Aguirre, M.Á.; Ramos-Fernández, E.V.; Narciso, J.; Canals, A. Enhancing Trace Metal Extraction from Wastewater: Magnetic Activated Carbon as a High-Performance Sorbent for Inductively Coupled Plasma Optical Emission Spectrometry Analysis. Separations 2023, 10, 563. https://doi.org/10.3390/separations10110563
Abellán-Martín SJ, Villalgordo-Hernández D, Aguirre MÁ, Ramos-Fernández EV, Narciso J, Canals A. Enhancing Trace Metal Extraction from Wastewater: Magnetic Activated Carbon as a High-Performance Sorbent for Inductively Coupled Plasma Optical Emission Spectrometry Analysis. Separations. 2023; 10(11):563. https://doi.org/10.3390/separations10110563
Chicago/Turabian StyleAbellán-Martín, Sergio J., David Villalgordo-Hernández, Miguel Ángel Aguirre, Enrique V. Ramos-Fernández, Javier Narciso, and Antonio Canals. 2023. "Enhancing Trace Metal Extraction from Wastewater: Magnetic Activated Carbon as a High-Performance Sorbent for Inductively Coupled Plasma Optical Emission Spectrometry Analysis" Separations 10, no. 11: 563. https://doi.org/10.3390/separations10110563
APA StyleAbellán-Martín, S. J., Villalgordo-Hernández, D., Aguirre, M. Á., Ramos-Fernández, E. V., Narciso, J., & Canals, A. (2023). Enhancing Trace Metal Extraction from Wastewater: Magnetic Activated Carbon as a High-Performance Sorbent for Inductively Coupled Plasma Optical Emission Spectrometry Analysis. Separations, 10(11), 563. https://doi.org/10.3390/separations10110563








