Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Materials
2.2. Inoculation Technique
2.3. PTR-ToF-MS Data Acquisition
2.4. SPME of Garlic Cloves
2.5. Statistical Analysis
3. Results
3.1. Artificial Inoculations
3.2. VOC Analyses
3.2.1. PTR-ToF-MS in Pure Cultures and Infected Garlic Bulbs
3.2.2. SPME-GC/MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toledano Medina, M.A.; Merinas-Amo, T.; Fernández-Bedmar, Z.; Font, R.; del Río-Celestino, M.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Alonso-Moraga, A.; Moreno-Rojas, R. Physicochemical characterization and biological activities of black and white garlic: In vivo and in vitro assays. Foods 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Parreño, R.; Rodríguez-Alcocer, E.; Martínez-Guardiola, C.; Carrasco, L.; Castillo, P.; Arbona, V.; Jover-Gil, S.; Candela, H. Turning garlic into a modern crop: State of the art and perspectives. Plants 2023, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Dugan, F.M.; Hellier, B.C.; Lupien, S.L. First report of Fusarium proliferatum causing rot of garlic bulbs in North America. Plant Pathol. 2023, 52, 426. [Google Scholar] [CrossRef]
- Stankovic, S.; Levic, J.; Petrovic, T.; Logrieco, A.; Moretti, A. Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur. J. Plant Pathol. 2017, 118, 165–172. [Google Scholar] [CrossRef]
- Palmero, D.; De Cara, M.; Nosir, W.; Gàlvez, L.; Cruz, A.; Woodward, S.; Gonzàlez-Jaèn, M.T.; Tello, J.C. Fusarium proliferatum isolated from garlic in Spain: Identification, toxigenic potential and pathogenicity on related Allium species. Phytopathol. Mediterr. 2012, 51, 207–218. [Google Scholar]
- Tonti, S.; Dal Prà, M.; Nipoti, P.; Prodi, A.; Alberti, I. First report of Fusarium proliferatum causing rot of stored garlic bulbs (Allium sativum L.) in Italy. J. Phytopathol. 2012, 160, 761–763. [Google Scholar] [CrossRef]
- Moharam, M.M.A.; Farrag, E.S.H.; Mazhar, D.A.M. Pathogenic fungi in garlic seed cloves and first report of Fusarium proliferatum causing cloves rot of stored bulbs in upper Egypt. Arch. Phytopathol. 2013, 46, 2096–2103. [Google Scholar] [CrossRef]
- Salvalaggio, A.E.; Ridao, A.d.C. First report of Fusarium proliferatum causing rot on garlic and onion in Argentina. Plant Dis. 2013, 97, 556. [Google Scholar] [CrossRef]
- Infantino, A.; Aureli, G.; Costa, C.; Taiti, C.; Antonucci, F.; Menesatti, P.; Pallottino, F.; De Felice, S.; D’Egidio, M.G.; Mancuso, S. Potential application of PTR-TOFMS for the detection of deoxynivalenol (DON) in durum wheat. Food Control 2015, 57, 96–104. [Google Scholar] [CrossRef]
- Mondani, L.; Chiusa, G.; Battilani, P. Fungi associated with garlic during the cropping season, with focus on Fusarium proliferatum and F. oxysporum. Plant Health Prog. 2021, 22, 137–146. [Google Scholar] [CrossRef]
- Gálvez, L.; Palmero, D. Fusarium Dry Rot of garlic bulbs caused by Fusarium proliferatum: A review. Horticulturae 2022, 8, 628. [Google Scholar] [CrossRef]
- Chrétien, P.L.S.; Laurent, I.; Bornard, C.; Troulet, M.; El Maâtaoui, C.L. Unraveling the infection process of garlic by Fusarium proliferatum, the causal agent of root rot. Phytopathol. Mediterr. 2020, 59, 285–293. [Google Scholar]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal volatile organic compounds: More than just a funky smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Abraham, W.-R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Kim, N.Y.; Park, M.H.; Jang, E.Y.; Lee, J.H. Volatile distribution in garlic (Allium sativum L.) by solid phase microextraction (SPME) with different processing conditions. Food Sci. Biotechnol. 2011, 20, 775–782. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Infantino, A.; Costa, C.; Aragona, M.; Reverberi, M.; Taiti, C.; Mancuso, S. Identification of different Fusarium spp through mVOCs profiling by means of Proton-Transfer-Reaction Time-of-Flight (PTR-ToF-MS) analysis. Plant Pathol. J. 2017, 99, 663–669. [Google Scholar]
- Elshahawy, I.E.; Saied, N.M.; Morsy, A.A. Fusarium proliferatum, the main cause of clove rot during storage, reduces clove germination and causes wilt of established garlic plants. Plant Pathol. J. 2017, 99, 85–93. [Google Scholar]
- De Santis, D.; Garzoli, S.; Vettraino, A.M. Effect of gaseous ozone treatment on the aroma and clove rot by Fusarium proliferatum during garlic postharvest storage. Heliyon 2021, 7, e06634. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Guidi Nissim, W.; Bibbiani, S.; Azzarello, E.; Masi, E.; Pandolfi, C.; Pallottino, F.; Menesatti, P.; Mancuso, S. Assessing VOC emission by different wood cores using the PTR-ToF-MS technology. Wood Sci. Technol. 2017, 51, 273–295. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.M.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Mark, T.D.; Gasperi, F. On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Davis, J.C. Statistics and Data Analysis in Geology; John Wiley & Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- Harper, D.A.T. (Ed.) Numerical Palaeobiology; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Sjöström, M.; Wold, S.; Söderström, B. PLS discrimination plots. In Pattern Recognition in Practice II; Gelsema, E.S., Kanals, L.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 461–470. [Google Scholar]
- Sabatier, R.; Vivien, M.; Amenta, P. Two approaches for discriminant partial least squares. In Between Data Science and Applied Data Analysis; Schader, M., Gaul, W., Vichi, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 100–108. [Google Scholar]
- Giagnoni, L.; Taiti, C.; León, P.; Costa, C.; Menesatti, P.; Espejo, R.; Gómez-Paccard, C.; Hontoria, C.; Vázquez, E.; Benito, M.; et al. Volatile organic compound emissions and biochemical properties of degraded Ultisols ameliorated by no tillage and liming. Pedosphere 2020, 30, 597–606. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Swierenga, H.; de Groot, P.J.; de Weijer, A.P.; Derksen, M.W.J.; Buydens, L.M.C. Improvement of PLS model transferability by robust wavelength selection. Chemometr. Intell. Lab. Syst. 1998, 41, 237–248. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile compounds of fresh and processed garlic (Review). Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef]
- Wang, A.; Haapalainen, M.; Latvala, S.; Edelenbos, M.; Johansen, A. Discriminant analysis of volatile organic compounds of Fusarium oxysporum f. sp. cepae and Fusarium proliferatum isolates from onions as indicators of fungal growth. Fungal Biol. 2018, 22, 1013–1022. [Google Scholar] [CrossRef]
- Martinez, A.; Bennett, J.W. Fungal Volatile Organic Compounds. In Encyclopedia of Mycology; Zaragoza, O., Ed.; Elsevier: Oxford, UK, 2021; Volume 1, pp. 239–245. [Google Scholar] [CrossRef]
- Russo, A.; Pollastri, S.; Ruocco, M.; Monti, M.M.; Loreto, F. Volatile organic compounds in the interaction between plants and beneficial microorganisms. J. Plant Interact. 2022, 17, 840–852. [Google Scholar] [CrossRef]
- Sridharan, A.P.; Sugitha, T.; Karthikeyan, G.; Sivakumar, U. Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microb. Res. 2020, 236, 126436. [Google Scholar] [CrossRef]
- Girotti, J.; Malbrán, I.; Lori, G.; Juárez, M. Use of solid phase microextraction coupled to capillary gas chromatography-mass spectrometry for screening Fusarium spp. based on their volatile sesquiterpenes. World Mycotoxin J. 2010, 3, 121–128. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Ghirardo, A.; Antritter, F.; Benz, J.P.; Schnitzler, J.-P.; Rosenkranz, M. Sniffing fungi—Phenotyping of volatile chemical diversity in Trichoderma species. New Phytol. 2020, 227, 244–259. [Google Scholar] [CrossRef]
- Akhoundi, M.; Chebbah, D.; Elissa, N.; Brun, S.; Jan, J.; Lacaze, I.; Izri, A. Volatile Organic Compounds: A promising tool for bed bugdetection. Int. J. Environ. Res. Public Health 2023, 20, 5214. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.R.; Polle, A.; Benz, J.P.; Pritsch, K.; Schnitzler, J.P.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2021, 4, 673. [Google Scholar] [CrossRef]
- Honeker, L.K.; Graves, K.R.; Tfaily, M.M.; Krechmer, J.E.; Meredith, L.K. The Volatilome: A vital piece of the complete soil metabolome. Front. Environ. Sci. 2021, 9, 649905. [Google Scholar] [CrossRef]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an electronic nose and volatilome analysis to differentiate sparkling wines obtained under different conditions of temperature, ageing time and yeast formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Fang, G.-Y.; Mu, X.-J.; Huang, B.-W.; Wu, G.-Z.; Jiang, Y.-J. Fungal biodiversity and interaction complexity were the important drivers of multifunctionality for flavor production in a spontaneously fermented vinegar. Innov. Food Sci. Emerg. Technol. 2023, 83, 103259. [Google Scholar] [CrossRef]
- Salthammer, T. Acetaldehyde in the indoor environment. Environ. Sci. Atmos. 2023, 3, 474. [Google Scholar] [CrossRef]
- Gutiérrez-Corona, J.F.; González-Hernández, G.A.; Padillla-Guerreo, I.E.; Olmedo-Monfil, V.; Martìnez-Tocha, A.L.; Patiño-Medina, J.A.; Meza-Carmen, V.; Torres-Guzmàn, J.C. Fungal alcohol dehydrogenases: Physiological function, molecular properties, regulation of their production, and biotechnological potential. Cells 2023, 12, 2239. [Google Scholar] [CrossRef]
- Marcinkowska, M.A.; Jelen, H.H. Role of Sulfur Compounds in Vegetable and Mushroom Aroma. Molecules 2022, 27, 6116. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Buyukkurt, O.; Kelebek, H.; Bordiga, M.; Keskin, M.; Selli, S. Changes in the aroma and key odorants from white garlic to black garlic using approaches of molecular sensory science: A review. Heliyon 2023, 9, e19056. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, R.A.; Evans, G.; Graz, M.; Marti, G.M.; Puri, C.; Rogers, H.J.; Müller, C.T. From laboratory to industrial storage—Translating volatile organic compounds into markers for assessing garlic storage quality. Postharvest Biol. Technol. 2022, 191, 111976. [Google Scholar] [CrossRef] [PubMed]
- Biancolillo, A.; Aloia, R.; Rossi, L.; D’Archivio, A.A. Organosulfur volatile profiles in Italian red garlic (Allium sativum L.) varieties investigated by HS-SPME/GC-MS and chemometrics. Food Control 2022, 131, 108477. [Google Scholar] [CrossRef]
- Vita, F.; Giuntoli, B.; Bertolini, E.; Taiti, C.; Marone, E.; D’Ambrosio, C.; Trovato, E.; Sciarrone, D.; Zoccali, M.; Balestrini, R.; et al. Tuberomics: A molecular profiling for the adaption of edible fungi (Tuber magnatum Pico) to different natural environments. BMC Genom. 2020, 21, 90. [Google Scholar] [CrossRef]
- Cellini, A.; Blasioli, S.; Biondi, E.; Bertaccini, A.; Braschi, I.; Spinelli, F. Potential applications and limitations of electronic nose devices for plant disease diagnosis. Sensors 2017, 17, 2596. [Google Scholar] [CrossRef]
- Ali, A.; Mansol, A.S.; Khan, A.A.; Muthoosamy, K.; Siddiqui, Y. Electronic nose as a tool for early detection of diseases and quality monitoring in fresh postharvest produce: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2408–2432. [Google Scholar] [CrossRef]
- Abbey, L.; Aked, J.; Joyce, D.C. Discrimination amongst Alliums using an electronic nose. Ann. Appl. Biol. 2001, 139, 337–342. [Google Scholar] [CrossRef]
- Baby, R.E.; Sance, M.M.; Bauzá, M.; Messina, V.M.; Gómez, A.R.; Burba, J.L.; Walsöe de Reca, N.E. Electronic nose study of powdered garlic. Sens. Transducers 2009, 107, 26–34. [Google Scholar]
- Trirongjitmoah, S.; Juengmunkong, Z.; Srikulnath, K.; Somboon, P. Classification of garlic cultivars using an electronic nose. Comput. Electron. Agric. 2015, 113, 148–153. [Google Scholar] [CrossRef]
- Makarichian, A.; Chayjan, R.A.; Ahmadi, E.; Zafari, D. Early detection and classification of fungal infection in garlic (A. sativum) using electronic nose. Comput. Electron. Agric. 2022, 192, 106575. [Google Scholar] [CrossRef]
- Arora, M.; Zambrzycki, S.C.; Levy, J.M.; Esper, A.; Frediani, J.K.; Quave, C.L.; Fernández, F.M.; Kamaleswaran, R. Machine Learning Approaches to Identify Discriminative Signatures of Volatile Organic Compounds (VOCs) from Bacteria and Fungi Using SPME-DART-MS. Metabolites 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Zhao, Z.; Feng, X.; Wang, Z.; Jiao, M. Advanced algorithms for low dimensional metal oxides-based electronic nose application: A review. Crystals 2023, 13, 615. [Google Scholar] [CrossRef]

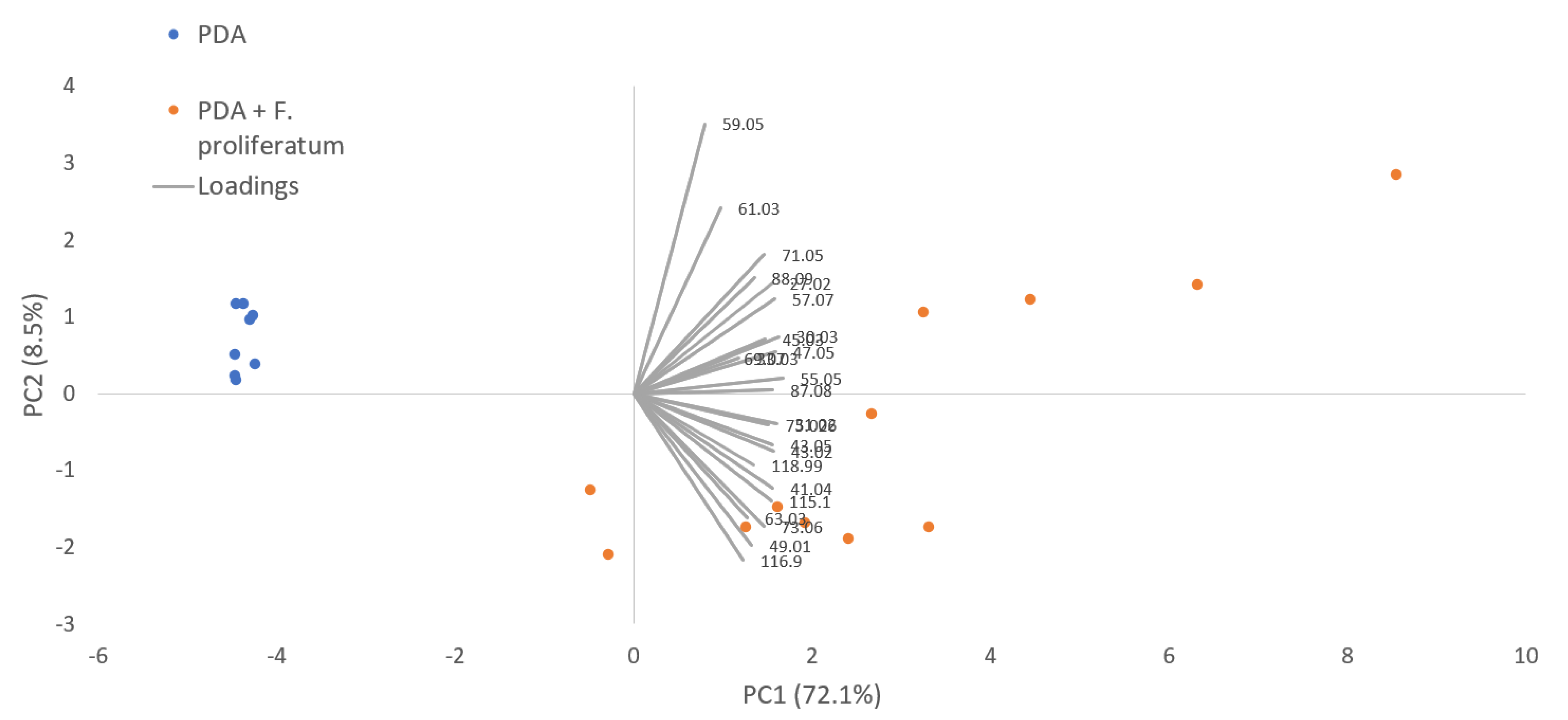
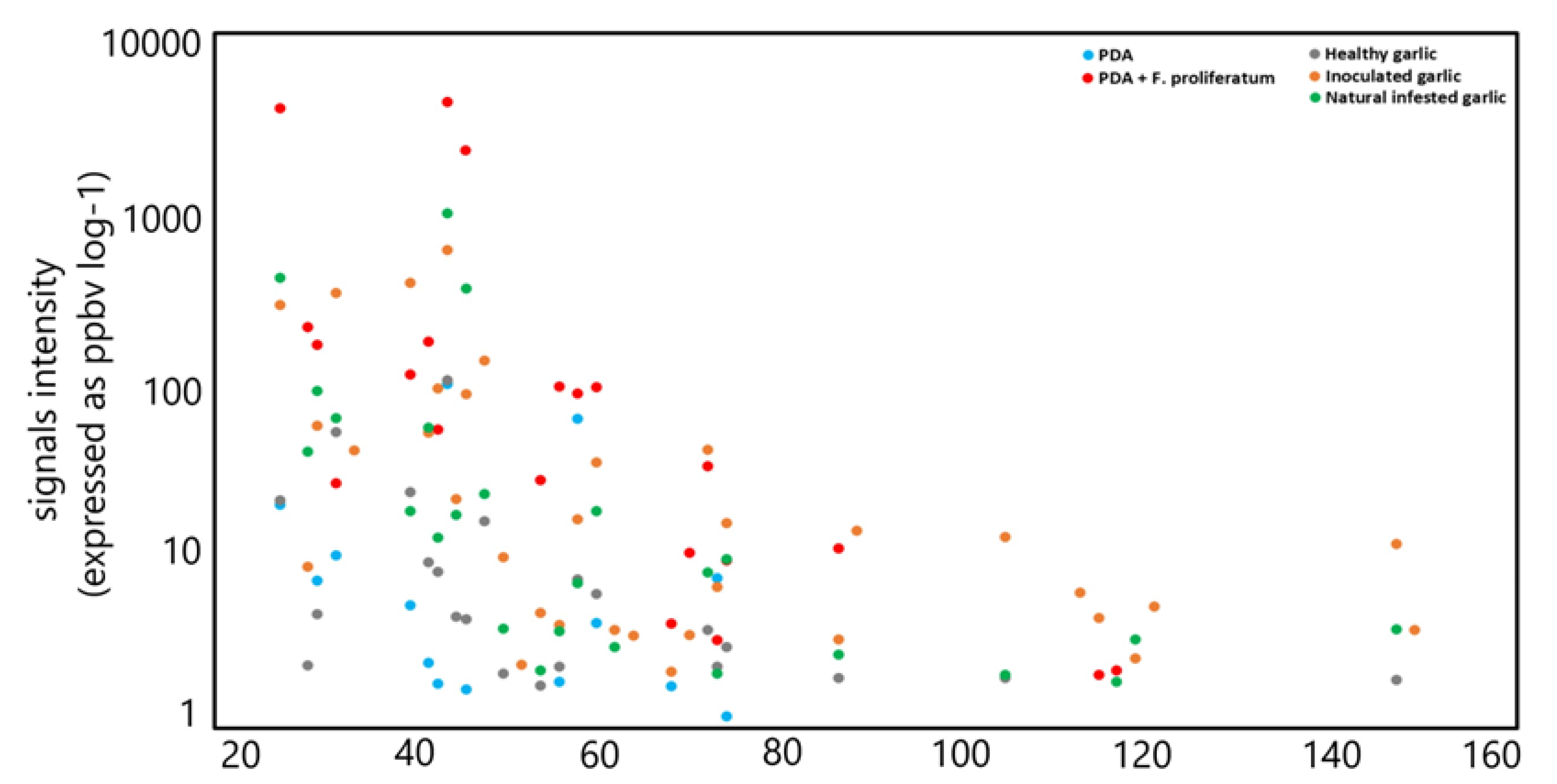
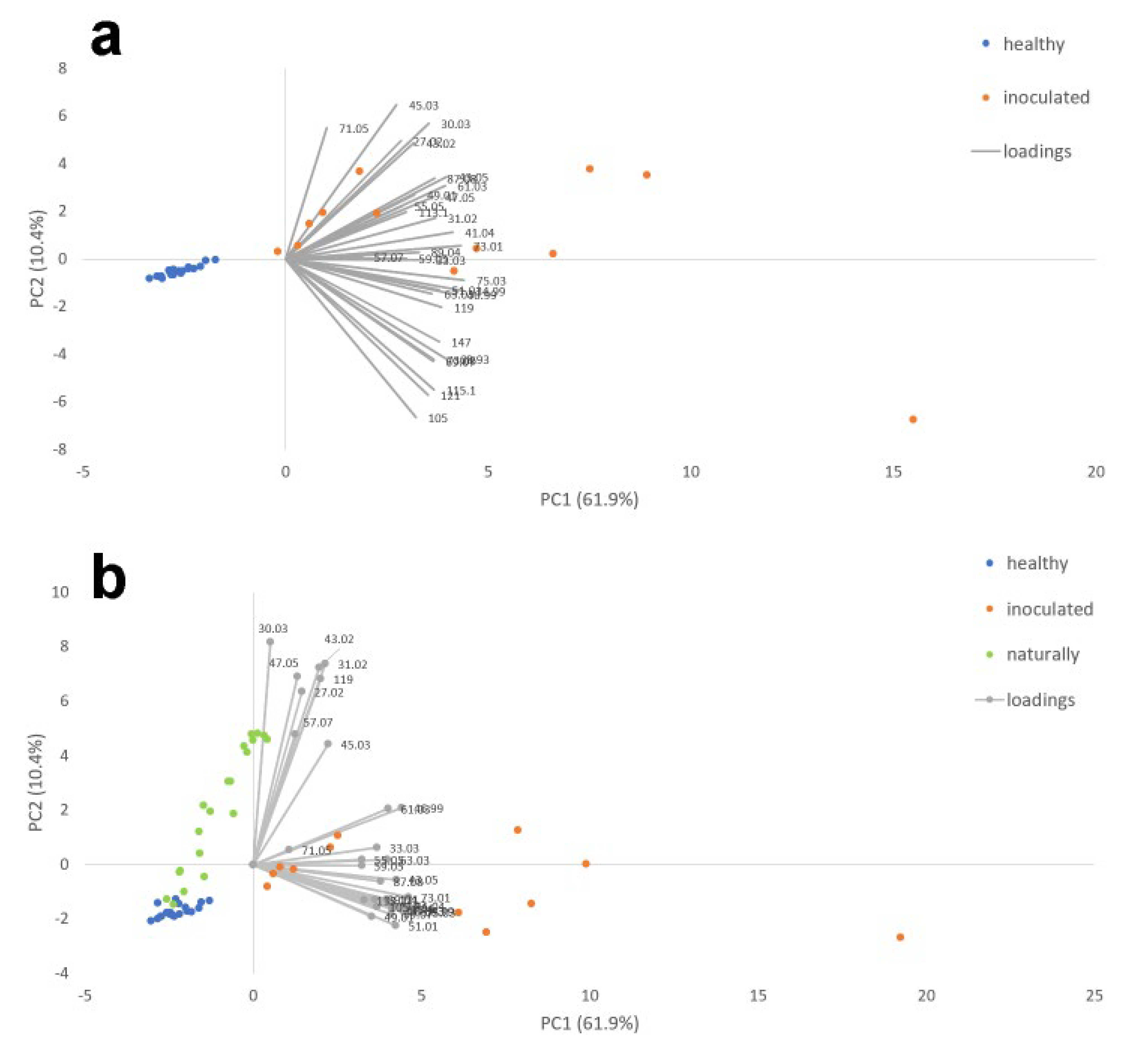
| m/z | Chemical Formula | Tentative Identification | PDA (n = 8) | PDA + F. proliferatum (n = 12) | Healthy Garlic (n = 20) | Inoculated Garlic (n = 12) | Naturally Infected Garlic (n = 20) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protonated Ion (H+) | Average | S.D. | Average | S.D. | Average | S.D. | Average | S.D. | Average | S.D. | ||
| 27.022 | C2H3+ | Acetylene and other fragments | 18.13 | 8.27 | 4770.97 | 2740.75 | 18.39 | 8.30 | 272.24 | 146.98 | 387.79 | 279.89 |
| 30.030 | C2H5+ | Ethylene (isotope) | 0.00 | 0.00 | 203.05 | 99.28 | 2.29 | 0.82 | 8.44 | 2.98 | 38.78 | 23.88 |
| 31.018 | CH3O+ | Formaldehyde | 6.02 | 2.81 | 160.56 | 50.80 | 4.52 | 2.57 | 54.70 | 37.71 | 86.48 | 43.79 |
| 33.033 | CH5O+ | Methanol | 9.86 | 4.40 | 25.47 | 12.16 | 50.16 | 35.12 | 318.23 | 156.54 | 60.34 | 39.58 |
| 34.994 | H3S+ | S compounds | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 39.33 | 17.68 | 0.00 | 0.00 |
| 41.038 | C2H5+ | Fragment (alcohol, ester) | 4.05 | 2.49 | 107.68 | 35.28 | 22.68 | 14.56 | 364.88 | 282.30 | 17.60 | 10.68 |
| 43.018 | C2H3O+ | Fragment (ester) | 1.37 | 0.62 | 166.88 | 66.98 | 8.93 | 5.32 | 49.96 | 17.77 | 53.40 | 30.46 |
| 43.054 | C3H7+ | Fragment (alcohol, ester, acetate) | 1.79 | 0.24 | 52.29 | 19.02 | 7.91 | 4.65 | 89.74 | 52.56 | 12.39 | 6.43 |
| 45.033 | C2H5O+ | Acetaldehyde | 94.11 | 39.86 | 5211.79 | 2738.41 | 100.32 | 75.71 | 563.74 | 338.81 | 918.04 | 472.29 |
| 46.990 | CH3S+ | S compounds (thioformaldehyde) | 0.00 | 0.00 | 0.00 | 0.00 | 4.35 | 2.49 | 20.74 | 5.12 | 16.78 | 6.68 |
| 47.049 | C2H7O+ | Ethanol | 1.66 | 1.08 | 2551.01 | 1034.38 | 4.22 | 2.31 | 83.21 | 58.72 | 337.71 | 206.18 |
| 49.011 | CH5S+ | S compounds (metanethiol) | 0.00 | 0.00 | 0.00 | 0.00 | 15.42 | 10.43 | 130.31 | 86.88 | 22.06 | 5.78 |
| 51.006 | CH5S+ (isotope) | S compounds (metanethiol-isotope) | 0.00 | 0.00 | 0.00 | 0.00 | 2.06 | 0.47 | 9.54 | 6.11 | 3.73 | 0.21 |
| 53.021 | CH9S+ | S compounds (metanethiol) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.30 | 0.64 | 0.00 | 0.00 |
| 55.057 | C4H11S+ | S compounds (butanethiol) | 0.00 | 0.00 | 26.69 | 10.24 | 1.75 | 0.42 | 4.56 | 1.20 | 2.14 | 0.49 |
| 57.070 | C4H9+ | Alcohol Fragment | 1.84 | 1.01 | 92.28 | 31.87 | 2.24 | 0.81 | 3.92 | 1.41 | 3.60 | 1.80 |
| 59.050 | C3H7O+ | Acetone/propanal | 60.16 | 23.12 | 84.19 | 51.79 | 7.17 | 2.94 | 15.83 | 5.92 | 6.78 | 3.82 |
| 61.027 | C2H5O2 | Acetic acid | 4.02 | 2.93 | 91.11 | 66.54 | 5.88 | 2.23 | 33.74 | 13.84 | 17.71 | 6.36 |
| 63.027 | C2H7S+ | S compounds (dimethylsulfide) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.65 | 1.47 | 2.92 | 1.03 |
| 63.998 | CH4OS+ | S compounds (sulphenic acid) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.38 | 1.37 | 0.00 | 0.00 |
| 69.074 | C2H13S+ | S compounds | 1.73 | 0.59 | 2.97 | 1.46 | 0.00 | 0.00 | 2.11 | 0.51 | 0.00 | 0.00 |
| 71.049 | C5H11+ | 2,3-Dihydrofuran | 0.00 | 0.00 | 10.18 | 6.33 | 0.00 | 0.00 | 3.41 | 1.56 | 2.22 | 0.76 |
| 73.010 | C3H5S+ | S compounds (thioacrolein) | 0.00 | 0.00 | 32.03 | 9.87 | 2.65 | 1.41 | 39.82 | 17.97 | 7.80 | 1.41 |
| 73.060 | C4H9O+ | Butanal | 7.27 | 2.42 | 2.20 | 0.76 | 2.25 | 0.69 | 6.48 | 3.76 | 2.06 | 0.55 |
| 75.026 | C3H7S+ | S compounds (1-Propene-1-thiol/allyl mercaptan) | 1.17 | 0.40 | 9.19 | 3.42 | 2.92 | 1.15 | 15.08 | 4.85 | 9.31 | 0.81 |
| 87.044 | C4H7S+ | S compounds (2,3-Dihydrothiophene) | 0.00 | 0.00 | 10.77 | 3.48 | 1.93 | 0.21 | 3.22 | 1.49 | 1.64 | 0.25 |
| 89.040 | C4H9S+ | S compounds (allyl methyl sulfide) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 13.64 | 8.02 | 0.00 | 0.00 |
| 104.982 | C3H5S2+ | S compounds (1,2-dithiole) | 0.00 | 0.00 | 0.00 | 0.00 | 1.93 | 0.50 | 12.47 | 7.49 | 3.01 | 0.63 |
| 113.045 | C3H13S2+ | S compounds | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.97 | 3.79 | 0.00 | 0.00 |
| 115.060 | C6H11S+ | S compounds (cyclohexanethiolate) | 0.00 | 0.00 | 2.02 | 0.28 | 0.00 | 0.00 | 4.27 | 2.43 | 0.00 | 0.00 |
| 116.982 | C4H5S2+ | S compounds | 0.00 | 0.00 | 2.14 | 0.59 | 0.00 | 0.00 | 0.00 | 0.00 | 2.83 | 0.46 |
| 118.994 | C7H3S+ | S compounds (heptahexaene-1-thione) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.52 | 0.95 | 3.21 | 1.16 |
| 121.014 | C4H9S2+ | S compounds (disulfide, methyl 2-propenyl) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 | 4.06 | 0.00 | 0.00 |
| 147.037 | C6H11S2+ | S compounds (diallyl sulfide) | 0.00 | 0.00 | 0.00 | 0.00 | 1.88 | 0.23 | 11.45 | 5.86 | 3.68 | 0.54 |
| 149.041 | C6H13S2+ | S compounds (disulfide, 1-methylethyl 2-propen-1-yl) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.65 | 1.25 | 0.00 | 0.00 |
| Total number of signals detected | 14 | 21 | 22 | 34 | 27 | |||||||
| Total VOC emission (average, ppbv) | 213.19 | 13,615.47 | 271.82 | 2201.53 | 2024.02 | |||||||
| Total S compound emission (average, ppbv) | 2.90 | 85.81 | 28.87 | 333.01 | 79.13 | |||||||
| Healthy vs. Artificially Infected Garlic Cloves | Healthy vs. Naturally Infected Garlic Cloves | Healthy vs. Artificially Infected vs. Naturally Infected Garlic Cloves | |
|---|---|---|---|
| N | 32 | 52 | 52 |
| n° units (X-block) | 32 | 32 | 32 |
| n° units (Y-block) | 2 | 2 | 3 |
| Preprocessing | Mean center | Autoscale | Median center |
| n° LV | 1 | 13 | 15 |
| % Cumulated variance X-block | 68.09 | 96.54 | 99.98 |
| % Cumulated variance Y-block | 37.46 | 43.05 | 86.39 |
| Mean specificity | 1 | 0.98 | 0.96 |
| Mean sensitivity | 1 | 0.98 | 0.96 |
| Random probability (%) | 50 | 50 | 33.3 |
| Mean class. err. (%) | 0 | 0.02 | 0.04 |
| Mean RMSEC | 0.550 | 0.410 | 0.274 |
| % Corr. class. model | 100 | 97.6 | 97.6 |
| % Corr. class. independent test | 100 | 100 | 100 |
| m/z | Tentative Identification | Chemical Formula | Healthy | Artificially Infected | Naturally Infected |
|---|---|---|---|---|---|
| 27.022 | Acetylene | C2H3+ | 0.930307 | 2.158485 | 2.181981 |
| 30.030 | Ethylene (isotope) | C2H5+ | 1.804159 | 0.814889 | 0.857015 |
| 33.033 | Methanol | CH5O+ | 0.616435 | 1.314073 | 1.329796 |
| 41.038 | Fragment Alchohol, Ester | C2H5+ | 1.107641 | 2.530064 | 2.590729 |
| 43.054 | Fragment Alchohol, ester, acetate | C3H7+ | 1.705502 | 0.645954 | 0.564028 |
| 45.033 | Acethaldehyde | C2H5O+ | 1.188507 | 2.728673 | 2.046816 |
| 47.049 | Ethanol | C2H7O+ | 1.351119 | 2.169554 | 2.971928 |
| 61.027 | Acetic acid | C2H5O2+ | 3.117157 | 0.745749 | 0.95885 |
| 71.050 | 2,3-Dihydrofuran | C5H11+ | 1.377662 | 0.934311 | 0.525653 |
| Component 1 | LRI 2 | LRI 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-propene-1-thiol | 590 | 595 | 0.34 ± 0.02 | 0.23 ± 0.01 | - | - | - | - | - | - | - | - |
| ethyl acetate | 596 | 599 | - | 7.89 ± 0.02 | 10.69 ± 0.02 | 2.80 ± 0.02 | 2.57 ± 0.02 | - | 7.85 ± 0.02 | 6.67 ± 0.02 | - | - |
| thiirane, methyl- | 653 | 650 | - | 17.04 ± 0.03 | 19.60 ± 0.03 | 6.70 ± 0.03 | - | - | 16.78 ± 0.04 | 17.44 ± 0.04 | - | - |
| allyl methyl sulfide | 685 | 678 | - | 1.96 ± 0.02 | 0.76 ± 0.01 | - | 1.82 ± 0.02 | 3.55 ± 0.02 | 5.86 ± 0.02 | - | - | - |
| diallyl sulfide | 876 | 871 | - | 1.51 ± 0.03 | - | 2.39 ± 0.02 | 3.10 ± 0.02 | 2.82 ± 0.01 | 1.76 ± 0.02 | 2.00 ± 0.02 | - | 6.67 ± 0.02 |
| allyl mercaptan | 896 | 891 | - | - | - | - | 6.35 ± 0.03 | - | - | - | - | - |
| diacetyl sulfide | 900 | * | - | - | - | - | - | 0.88 ± 0.01 | - | - | - | - |
| disulfide, methyl 2-propenyl | 927 | 922 | 5.90 ± 0.03 | 1.13 ± 0.03 | - | 1.94 ± 0.02 | 5.26 ± 0.02 | 4.93 ± 0.02 | 3.78 ± 0.02 | 1.92 ± 0.02 | - | - |
| diallyl disulfide | 1089 | 1085 | 91.39 ± 0.07 | 68.43 ± 0.11 | 66.92 ± 0.15 | 84.45 ± 0.11 | 79.78 ± 0.14 | 86.35 ± 0.18 | 61.70 ± 0.14 | 70.92 ± 0.17 | 87.93 ± 0.12 | 77.68 ± 0.15 |
| disulfide, 1-methylethyl 2-propen-1-yl | 1095 | 1099 | 1.26 ± 0.03 | 0.64 ± 0.02 | - | 0.55 ± 0.02 | 0.25 ± 0.02 | 1.08 ± 0.02 | 2.03 ± 0.02 | - | 8.03 ± 0.03 | 15.65 ± 0.02 |
| allyl thiocyanate | 1210 | * | 0.67 ± 0.02 | 0.88 ± 0.02 | - | - | - | - | 0.24 ± 0.02 | 1.05 ± 0.02 | 4.04 ± 0.02 | - |
| tetrasulfide, di-2-propenyl | 1516 | 1510 | - | - | 2.03 ± 0.02 | 1.17 ± 0.02 | 0.85 ± 0.01 | 0.39 ± 0.02 | - | - | - | - |
| SUM | 99.56 | 99.71 | 100.0 | 100.0 | 99.98 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infantino, A.; Taiti, C.; Grottoli, A.; Mancuso, S.; Costa, C.; Garzoli, S. Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry. Separations 2023, 10, 556. https://doi.org/10.3390/separations10110556
Infantino A, Taiti C, Grottoli A, Mancuso S, Costa C, Garzoli S. Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry. Separations. 2023; 10(11):556. https://doi.org/10.3390/separations10110556
Chicago/Turabian StyleInfantino, Alessandro, Cosimo Taiti, Alessandro Grottoli, Stefano Mancuso, Corrado Costa, and Stefania Garzoli. 2023. "Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry" Separations 10, no. 11: 556. https://doi.org/10.3390/separations10110556
APA StyleInfantino, A., Taiti, C., Grottoli, A., Mancuso, S., Costa, C., & Garzoli, S. (2023). Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry. Separations, 10(11), 556. https://doi.org/10.3390/separations10110556









