Abstract
The synthesis of 14 hydrazone compounds derived from pyridoxal, pyridine-4-carbaldehyde, and quinoline-2-carbaldehyde using two methods, conventional method in deep eutectic solvents (DESs) and effective combination of ultrasound and DESs, is presented in this paper. In addition, the possibility of using 12 choline chloride (ChCl)-based DESs as an alternative to organic solvents was investigated. The results show that the application of ultrasound not only improves the reaction yield but also shortens the reaction time. The prepared compounds synthesized at room temperature were analyzed via NMR spectroscopy and MS spectrometry. The studies confirmed that the DESs ChCl:malonic, oxalic, levulinic, and trans-cinnamic acid can be excellent alternatives to classical organic solvents. By the combined use of DESs and the ultrasonic method, compound 11 was obtained in a nearly quantitative yield of 98% in DES ChCl:oxalic acid. The advantages of using DESs as reaction media are that they are biodegradable, nontoxic, recyclable, and can be easily prepared with inexpensive starting materials. The results of recycling DESs show that they can be used up to the fourth recycling cycle without significantly changing the reaction yield.
1. Introduction
Aldehydes and ketones are susceptible to nucleophilic addition reactions. Among the numerous products formed by nucleophilic addition, hydrazones attract special attention. Hydrazones contain the active azomethine functional group (–NH–N=CH–). They are very important intermediates in the field of heterocyclic chemistry and usually show broad biological activity [1]. Recently, scientific papers have reported numerous pharmacological activities of hydrazone derivatives, such as antispasmodic [2,3], anti-inflammatory [4], antihypertensive [5], anticancer [6,7,8,9,10,11], antimicrobial [12,13], antimalarial [14,15,16], anti-HIV [17], antitubercular [18,19,20], antifungal [21,22], antioxidant [23], cardioprotective [24], and antidepressant [25,26].
The most commonly used organic solvents in the conventional synthesis of pyridine-derived hydrazones are acetonitrile [27,28], ethanol [29,30,31,32], propan-1-ol [33], a mixture of ethanol and glacial acetic acid [34], tetrahydrofuran, butan-1-ol, and glacial acetic acid [35]. The classical organic solvents used in the synthesis of quinoline hydrazones are mainly ethanol [36] and methanol [37]. All of the above organic solvents belong to the group of volatile organic compounds, which have direct and indirect effects on humans and the environment. As chemists have become more environmentally conscious, they have changed their synthetic approaches by replacing traditional organic solvents with more environmentally friendly alternatives. Deep eutectic solvents (DESs) were first mentioned by Abbott et al. in 2003 [38], and numerous scientific reports have been published to date [39,40,41,42]. They can be easily prepared by combining two components, a hydrogen bond acceptor (HBA), mainly choline chloride (ChCl), and a hydrogen bond donor (HBD).
They have received extraordinary attention because of their advantages over the classical volatile organic solvents. They have low vapor pressure, low melting point, good electrical conductivity, chemical and thermal stability, and are non-flammable. Some of them can be obtained from renewable sources. Most of them are nontoxic and biodegradable, and the raw materials for their preparation are inexpensive. Therefore, large quantities of DESs can be easily produced on an industrial scale with minimal or no waste [43].
Although the number of publications on deep eutectic solvents is increasing, there are very few studies that address the evaluation of their toxicity and biodegradability [44,45,46,47]. Toxicity is important to control the solvents used in important sectors such as food and drug production, medicine, and cosmetics. Toxicity should also be tested after recycling because reaction conditions such as elevated temperature, microwave radiation, and ultrasound can lead to the formation of harmful byproducts. DESs are presented as green and safe solvents because they are synthesized from mostly nontoxic and non-carcinogenic starting materials (with the exception of some such as thiourea). There is also little research on the thermal stability of DESs [48]. Previous research has shown that DESs degrade at high temperatures by weakening and breaking hydrogen bonds to form HBA and HBD. Hydrogen bonds play a key role in thermal stability [49]. The exploration and use of DESs on an industrial scale will certainly increase their concentration in the environment, so monitoring their biological effects will play an important role. Therefore, their viability should be discussed with caution.
DESs are known to be safe and environmentally friendly solvents that offer enormous potential as reaction media for various chemical reactions and are substitutes for volatile organic solvents. In the last decade, deep eutectic solvents have been used as an alternative medium for numerous organic syntheses in the field of organometallic chemistry [50] and radical thiol-ene reactions [51], where they have been shown to be a good compatible medium for protein processing. They have been used for the synthesis of active pharmaceutical ingredients [52], in biocatalysis [53,54], organocatalysis [55,56], and electrochemistry [57].
In order to choose the most suitable medium for organic reactions, it is essential to understand the physicochemical properties of the solvent. For this reason, scientists try to design solvents with suitable properties by adjusting the chemical nature and the molar ratio between their components.
The synthesis of hydrazones using DESs has been reported in the literature [58], but the types of DESs are limited, so it would certainly be useful to investigate a range of other eutectic solvents. In this study, we investigated whether eutectic solvents can be a good alternative to volatile organic solvents and whether the type of HBD affects the efficiency of pyridinium and quinoline hydrazones formation.
Therefore, it is important to perform the synthesis of hydrazones according to the principles of green chemistry, i.e., to support long-term ecological balance, energy savings, lower toxicity of reagents, and reduced environmental and human health impacts.
For this reason, we selected 12 different choline chloride-based DESs, in which we investigated the possibility of pyridoxal, pyridinium, and quinoline hydrazones formation. For the synthesis of the hydrazones, we used two methods: a conventional and an ultrasound (US) method, which we performed under mild reaction conditions at room temperature. Since the presence of hydrophilic groups in choline chloride is responsible for the hygroscopic properties of DESs, it is important to determine the water content in the prepared DESs. Water impurities have been shown to affect the properties of eutectic solvents. It mainly affects their chemical structure [59], decreases kinematic viscosity, and increases electrical conductivity [60,61]. In this paper, the possibility of reusing DESs is also investigated, which is important for ecological as well as economic reasons.
2. Materials and Methods
The ultrasonic bath (US) was a device from BANDELIN GmbH & Co., Berlin, Germany, DT 510 H, with a frequency of 35 kHz, a power of 160 W, a temperature range of 20–80 °C, and a power of 400 W). Fluorescent silica gel plates F254 (Merck, Darmstadt, Germany) and the solvent system chloroform:methanol (6:1.5) were used for thin-layer chromatography under UV light and wavelengths 254 and 365 nm. Melting points were determined in the SMP3 electrothermal melting point apparatus (Mettler Toledo, Zagreb, Croatia). NMR spectra were recorded on a Bruker Avance 600 spectrometer at the Rudjer Bošković Institute using a 5 mm broadband probe head with z-gradient coils operating at 600.130 MHz for 1H and 150.903 MHz for 13C. All spectra were measured in DMSO-d6 at 25 °C. The signal shifts in the spectrum were determined according to the solvent signal at 2.50 ppm in the 1H spectrum and at 39.51 ppm in the 13C spectrum, respectively. Individual resonances were assigned based on their chemical shifts, multiplicities, integrals, and cross peaks in the recorded 2D NMR spectra: 1H-1H COSY (Correlation Spectroscopy), 1H-13C HMQC (Heteronuclear Multiple Quantum Coherence), and 1H-13C HMBC (Heteronuclear Multiple Bond Correlation).
Solvents and reagents were purchased from various manufacturers and used without further purification: pyridoxal and pyridine-4-carbaldehyde (97%) (Aldrich, St. Louis, MO, USA), quinoline-2-carbaldehyde (97%), 4-chlorophenylhydrazine hydrochloride, 4-fluorophenylhydrazine hydrochloride, N-methylurea, 4-methylphenylhydrazine hydrochloride, acetamide, oxalic acid and levulinic acid (Acros Organics, Antwerp, Belgium), phenylhydrazine hydrochloride and malic acid (Sigma-Aldrich), 2,4-dinitrophenylhydrazine (Merck), urea and citric acid (Gram mol, Zagreb, Croatia), thiourea and glycerol (Kemika, Zagreb, Croatia), malonic and trans-cinnamic acid (Fisher Chemical, Hampton, NH, USA), and lactic acid (Carlo Erba Reagents, Milano, Italy).
2.1. Synthesis of DESs and Detecting Water Content
DESs were prepared by mixing and heating two starting substances: ChCl and the various HBDs (the ratio is given in Table 1 and Table 2) on a magnetic stirrer for the appropriate time, depending on each HBD, at 80 °C. The cooled stable and homogeneous liquid was used for the synthesis of hydrazone without further purification. The water content of the DESs was determined using Karl Fischer titration according to HRN EN 13466- 1:2003 method (accredited according to HRN EN ISO/IEC 17025:2017) and is shown in Table 1.

Table 1.
Yields obtained for the prepared compounds (1–14) by the conventional method at room temperature in 12 different DESs.

Table 2.
Yields obtained for the prepared compounds (1–14) in the ultrasound method at room temperature in DESs.
2.2. Conventional Method
An equimolar mixture of pyridoxal, pyridine-4-carbaldehyde, and quinoline-2-carbaldehyde (1.12 mmol), and phenylhydrazine and substituted phenylhydrazine (2,4-dinitrophenylhydrazine, 4-fluorophenylhydrazine, 4-chlorophenylhydrazine, 4-methylphenylhydrazine) was transferred to DES (the molar ratios of starting reactant and ChCl were 1:10). The progress of the reaction was monitored via TLC. The reaction mixture was stirred at room temperature for 3 h. Cold water was then added to the mixture to precipitate the product. The reaction mixture was allowed to stand overnight at room temperature. After standing, colored crystals of the crude product were precipitated and separated using vacuum filtration. The crude products were washed with cold ethanol and cold diethyl ether, recrystallized from the ethanol, and dried in a desiccator.
2.3. Ultrasound Method
An equimolar mixture of pyridoxal, pyridine-4-carbaldehyde, and quino-line-2-carbaldehyde (1.12 mmol), and phenylhydrazine and substituted phenylhydrazine (2,4-dinitrophenylhydrazine, 4-fluorophenylhydrazine, 4-chlorophenylhydrazine, 4-methylphenylhydrazine) was dissolved in DES (the molar ratios of starting reactant and ChCl were 1:10). The reaction mixture was placed in an ultrasonic bath and sonicated (10–15 min, rt, 35 kHz) to give the product. To precipitate the product, cold water was added to the reaction mixture and left in the dark for 24 h. The crude products obtained were washed with cold ethanol and cold diethyl ether and recrystallized from the ethanol to give the chromatographically pure product.
The structure of all 14 products was characterized using NMR spectroscopy and mass spectrometry (MS).
2.4. Recycling and Reuse of DESs
DESs contained in the filtrate were recovered by evaporating the water and drying under reduced pressure to recycle and reuse DESs. The model reaction for recycling and reuse of DESs was the reaction of quinoline-2-carbaldehyde with 2,4-dinitrophenylhydrazine in DES ChCl:oxalic acid (1:1) according to the US method.
- 5-(hydroxymethyl)-2-methyl-4-((2-phenylhydrazineylidene)methyl)pyridin-3-ol (1)
Yellow solid; m.p. 210–213 °C; 1H NMR (DMSO-d6, 600 MHz): δ 11.96 (1H, br s, OH-5), 11.84 (1H, s, NH), 8.42 (1H, s, H-8), 8.17 (1H, s, H-2), 7.37 (2H, t, J = 7.91 Hz, H-13/15), 7.09 (2H, d, J = 7.91 Hz, H-12/16), 6.98 (1H, t, J = 6.59 Hz, H-14), 5.81 (1H, br s, OH-7), 4.79 (2H, s, CH2-7), and 2.62 (3H, s, CH3-6) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 150.5 (1C, C-5), 142.6 (1C, C-11), 140.6 (1C, C-6), 134.8 (1C, C-3 or C-4), 131.9 (1C, C-8), 130.3 (1C, C-3 or C-4), 129.6 (2C, C-13/15), 129.3 (1C, C-2), 121.6 (1C, C-14), 112.8 (2C, C-12/16), 58.8 (1C, CH2-7), and 14.4 (1C, CH3-6) ppm. MS: m/z 258 (M+, 100%).
- 4-((2-(2,4-dinitrophenyl)hydrazineylidene)methyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol (2)
Dark yellow solid; m.p. 210–213 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.06 (1H, s, OH-5), 9.19 (1H, s, H-8), 8.89 (1H, d, J = 2.67 Hz, H-15), 8.50 (1H, dd, J = 9.50, 2.67 Hz, H-13), 8.26 (1H, s, H-2), 7.87 (1H, d, J = 9.50 Hz, H-12), 4.88 (2H, s, CH2-7), and 2.65 (3H, s, CH3-14) ppm. The 13C NMR (DMSO-d6, 150 MHz,): δ 151.8 (1C, C-5), 143.8 (1C, C-8), 143.3 (1C, C-11), 142.9 (1C, C-6), 138.5 (1C, C-14), 137.4 (1C, C-3 or C-4), 131.1 (1C, C-16), 130.5 (1C, CH3-2), 130.1 (1C, C-13), 129.6 (1C, C-3 or C-4), 122.8 (1C, C-15), 116.7 (1C, C-12), 59.4 (1C, CH2-7), and 15.6 (1C, CH3-6) ppm. MS: m/z 349 (M+, 100%).
- 4-((2-(4-fluorophenyl)hydrazineylidene)methyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol (3)
Yellow solid; m.p. 285–290 °C; 1H NMR (DMSO-d6, 600 MHz): δ 11.82 (2H, br s, OH-5 and NH), 8.39 (1H, s, H-8), 8.17 (1H, s, H-2), 7.22 (2H, t, J = 8.66 Hz, H-13/15), 7.13–7.06 (2H, m, H-12/16), 5.79 (1H, br s, OH-7), 4.78 (2H, s, CH2-7), and 2.61 (3H, s, CH3-6) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 157.4 (1C, d, JC,F = 235 Hz, C-14), 150.4 (1C, C-5), 139.3 (1C, JC,F = 1.66 Hz, C-11), 140.6 (1C, C-6), 134.8 (1C, C-3 or C-4), 131.8 (1C, C-8), 130.5 (1C, C-3 or C-4), 129.5 (1C, C-2), 129.3 (1C, C-2), 116.3 (2C, d, JC,F = 24 Hz, C-13/15), 114.2 (2C, d, J = 8 Hz, C-12/16), 58.9 (1C, CH2-7), and 14.6 (1C, CH3-6) ppm. MS: m/z 276 (M+, 100%).
- 4-((2-(4-chlorophenyl)hydrazineylidene)methyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol (4)
Dark yellow solid; m.p. 292–295 °C; 1H NMR (DMSO-d6, 600 MHz): δ 11.87 (1H, br s, NH), 11.74 (1H, s, OH-5), 8.42 (1H, s, H-8), 8.19 (1H, s, H-2), 7.41 (2H, d, J = 8.79 Hz, H-13/15), 7.09 (2H, d, J = 8.79 Hz, H-12/16), 5.79 (1H, br s, OH), 4.79 (2H, s, CH2-7), and 2.61 (3H, s, CH3-6) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 150.5 (1C, C-5), 141.7 (1C, C-11), 140.8 (1C, C-6), 135.1 (1C, C-4), 137.7 (1C, C-8), 130.5 (2C, C-13/15), 129.5 (2C, C-2), 124.9 (1C, C-14), 114.3 (2C, C-12/16), 58.9 (1C, CH2-7), and 14.6 (1C, CH3-6) ppm. MS: m/z 292 (M+, 100%).
- 5-(hydroxymethyl)-2-methyl-4-((2-(p-tolyl)hydrazineylidene)methyl)pyridin-3-ol (5)
Brown solid; m.p. 250–268 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.00 (1H, s, OH-5), 11.73 (1H, s, NH), 8.36 (1H, s, H-8), 8.16 (1H, s, H-2), 7.18 (2H, d, J = 8.19 Hz, H-13/15), 6.99 (2H, d, J = 8.19 Hz, H-12/16), 5.79 (1H, br s, OH-7), 4.77 (2H, s, CH2-8), 2.60 (3H, s, CH3-6), and 2.27 (3H, s, CH3-14) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 150.4 (1C, C-5), 140.6 (1C, C-6), 140.2 (1C, C-11), 134.4 (1C, C-4), 131.3 (1C, C-8), 130.8 (2C, C-13/15), 130.1 (1C, C-2), 112.8 (2C, C-12/16), 58.7 (1C, CH2-7), 20.3 (1C, CH3-14), and 14.5 (1C, CH3-6) ppm. C-3 was not detected. MS: m/z 272 (M+, 100%).
- Pyridine-4-carbaldehyde-phenylhydrazone (6)
Dark yellow solid; m.p. 285–293 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.01 (1H, s, NH), 8.70 (2H, d, J = 6.83 Hz, H-2/6), 8.11 (2H, d, J = 6.83 HZ, H-3/5), 8.00 (1H, s, H-7), 7.35–7.29 (4H, m, H-11/15, and H-12/14), and 6.96 (1H, ddd, J = 7.27, 6.87, 1.74 Hz, H-13) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 151.8 (1C, C-4), 143.4 (1C, C-10), 141.0 (2C, C-2/6), 129.9 (1C, C-7), 129.3 (2C, C-12/14), 121.7 (1C, C-13), 121.2 (2C, C-3/5), and 113.6 (2C, C-11/15) ppm. MS: m/z 198 (M+, 100%).
- Pyridine-4-carbaldehyde-2,4-dinitrophenylhydrazone (7)
Orange solid; m.p. 205–212 °C; 1H NMR (DMSO-d6, 600 MHz): δ 11.82 (1H, s, NH), 8.87 (1H, d, J = 2.60 Hz, H-14), 8.69 (1H, s, H-7), 8.689 (2H, d, J = 6.03 Hz, H-2/6), 8.40 (1H, dd, J = 9.48, 2.46 Hz, H-12), 8.16 (1H, d, J = 9.48 Hz, H-11), and 7.74 (2H, d, J = 6.03 Hz, H-3/5) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 150.4 (1C or 2C, C-7 or C-2/6), 146.4 (1C or 2C, C-7 or C-2/6), 144.3 (1C, C-10), 141.0 (1C, C-4), 137.8 (1C, C-13), 130.3 (1C, C-15), 129.7 (1C, C-12), 122.8 (1C, C-14), 121.0 (2C, C-3/5), and 117.0 (1C, C-11) ppm. MS: m/z 286 (M+, 100%).
- Pyridine-4-carbaldehyde-4-fluorophenylhydrazone (8)
Orange solid; m.p. 270–285 °C; 1H NMR (600 MHz, DMSO-d6): δ 12.12 (1H, s, NH), 8.69 (2H, d, JH,F = 6.79 Hz, H-2/6), 8.10 (2H, d, J = 6.79 Hz, H-3/5), 7.98 (1H, s, H-7), 7.31 (2H, dd, J = 9.32 Hz, JH,F = 4.85 Hz, H-11/15), and 7.16 (2H, t, JH,H = JH,F = 9.03 Hz, H-12/14) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 157.5 (1C, JC,F = 240 Hz, C-13), 151.8 (1C, C-4), 140.9 (2C, C-2/6), 140.0 (1C, C-10), 129.8 (1C, C-7), 121.1 (2C, C-3/5), 115.9 (2C, d, JC,F = 22 Hz, C-12/14), and 114.9 (2C, d, JC,F = 8 Hz, C-11/15) ppm. MS: m/z 216 (M+, 100%).
- Pyridine-4-carbaldehyde-4-chlorophenylhydrazone (9)
Orange solid; m.p. 292–293 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.05 (1H, s, NH), 8.71 (2H, d, J = 6.99 HZ, H-2/6), 8.13 (2H, d, J = 6.55 HZ, H-3/5), 8.00 (1H, s, H-7), 7.36 (2H, d, J = 8.79 Hz, H-12/14), and 7.30 (2H, d, J = 8.64 Hz, H-11/15) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 151.5 (1C, C-4), 142.4 (1C, C-10), 141.3 (2C, C-2/6), 130.8 (1C, C-7), 129.2 (2C, C-12/14), 125.1 (1C, C-13), 121.4 (2C, C-3/5), and 115.0 (2C, C-11/15) ppm. MS: m/z 232 (M+, 100%).
- Quinoline-2-carbaldehyde-phenylhydrazone (10)
Red solid; m.p. 262–266 °C; 1H NMR (DMSO-d6, 600 MHz,): δ 12.37 (1H, s, NH), 8.77 (1H, d, J = 8.04 Hz, H-4), 8.42 (1H, d, J = 9.38 Hz, H-3), 8.37 (1H, s, H-11), 8.25 (1H, d, J = 7.43 Hz, H-8), 8.19 (1H, d, J = 8.04 Hz, H-5), 7.99 (1H, t, J = 6.70 Hz, H-7), 7.69 (1H, t, J = 8.04 Hz, H-6), 7.40–7.35 (4H, m, H-15/19, and H-16/18), and 7.02 (1H, t, J = 6.70 Hz, H-17) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 151.8 (1C, C-2), 143.0 (1C, C-14), 142.3 (1C, C-9), 141.8 (1C, C-4), 133.2 (1C, C-7), 129.4 (2C, C-16/18), 128.8 (1C, C-5), 128.0 (1C, C-6), 127.0 (1C, C-11), 126.7 (1C, C-10), 122.4 (1C, C-17), 121.0 (1C, C-8), 118.0 (1C, C-3), and 114.0 (2C, C-15/19) ppm. MS: m/z 248 (M+, 100%).
- Quinoline-2-carbaldehyde-2,4-dinitrophenylhydrazone (11)
Yellow solid; m.p. 250–257 °C; 1H NMR (DMSO-d6, 600 MHz,): δ 11.96 (1H, s, NH), 8.89 (1H, d, J = 2.66 Hz, H-18), 8.87 (1H, s, H-11), 8.46 (1H, d, J = 8.72 Hz, H-4), 8.43 (1H, dd, J = 9.52, 2.68 Hz, H-16), 8.275 (1H, d, J = 8.58 Hz, H-3), 8.267 (1H, d, J = 9.52 Hz, H-15), 8.06 (1H, d, J = 7.85 Hz, H-8), 8.03 (1H, d, J = 7.85 Hz, H-5), 7.81 (1H, ddd, J = 8.86, 6.54, 1.50 Hz, H-7), and 7.66 (1H, ddd, J = 8.59, 6.54, 1.09 Hz, H-6) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 153.4 (1C, C-2), 148.9 (1C, C-11), 147.4 (1C, C-9), 144.2 (1C, C-14), 137.8 (1C, C-17), 136.8 (1C, C-4), 130.5 (1C, C-19), 130.2 (1C, C-7), 129.8 (1C, C-16), 129.0 (1C, C-8), 128.2 (1C, C-5), 127.9 (1C, C-10), 127.5 (1C, C-6), 122.7 (1C, C-18), 117.8 (1C, C-15), and 117.4 (1C, C-3) ppm. MS: m/z 336 (M+, 100%)
- Quinoline-2-carbaldehyde-4-fluorophenylhydrazone (12)
Orange solid; m.p. 245–258 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.42 (1H, s, NH), 8.76 (1H, d, J = 7.81 Hz, H-4), 8.41 (1H, d, J = 8.79 Hz, H-3), 8.34 (1H, s, H-11), 8.24 (1H, d, J = 8.79 Hz, H-8), 8.19 (1H, d, J = 7.81 Hz, H-5), 7.99 (1H, t, J = 7.81 Hz, H-7), 7.77 (1H, t, J = 7.81 Hz, H-6), 7.40 (2H, JH,F = 4.84 Hz, H-15/19), and 7.21 (2H, JH,F = 8.75 Hz, H-16/18) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 157.9 (1C, d, JC,F = 238.5 Hz, C-17), 151.8 (1C, C-2), 147.5 (1C, C-9), 142.2 (1C, C-4), 139.7 (1C, C-14), 133.2 (1C, C-7), 128.7 (1C, C-5), 128.0 (1C, C-6), 127.0 (1C, C-11), 126.7 (1C, C-10), 121.1 (1C, C-8), 118.0 (1C, C-3), 116.1 (2C, t, JC,F = 22.8 Hz, C-16/18), and 115.4 (2C, d, JC,F = 5.9 Hz, C-15/19) ppm. MS: m/z 266 (M+, 100%).
- Quinoline-2-carbaldehyde-4-chlorophenylhydrazone (13)
Red solid; m.p. 205–207 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.32 (1H, s, NH), 8.75 (1H, d, J = 8.52 Hz, H-4), 8.40 (1H, d, J = 8.77 Hz, H-3), 8.32 (1H, s, H-11), 8.22 (1H, d, J = 7.80 Hz, H-8), 8.18 (1H, d, J = 7.80 Hz, H-5), 7.98 (1H, d, J = 7.80 Hz, H-7), 7.76 (1H, d, J = 7.80 Hz, H-6), and 7.42–7.36 (4H, m, H-15/19, and H-16/18) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 151.6 (1C, C-2), 142.3 (1C, C-4), 142.1 (1C, C-14), 139.1 (1C, C-9), 133.1 (1C, C-7), 129.4 (2C, C-16/18), 128.9 (1C, C-5), 128.3 (1C, C-6), 126.8 (1C, C-10), 125.7 (1C, C-17), 128.2 (1C, C-11), 121.3 (1C, C-8), 117.8 (1C, C-3), and 115.4 (2C, C-15/19) ppm. MS: m/z 282 (M+, 100%).
- Quinoline-2-carbaldehyde-4-methylphenylhydrazone (14)
Yellow solid; m.p. 181–195 °C; 1H NMR (DMSO-d6, 600 MHz): δ 10.81 (1H, s, NH), 8.28 (1H, d, J = 8.91 Hz, H-4), 8.11 (1H, d, J = 8.43 Hz, H-3), 7.99 (1H, s, H-11), 7.95–7.91 (2H, m, H-5, and H-8), 7.72 (1H, ddd, J = 8.45, 6.74, 1.56 Hz, H-7), 7.54 (1H, ddd, J = 8.36, 6.72, 1.11 Hz, H-6), 7.09 (4H, m, H-15/19, and H-16/18), and 2.24 (3H, s, H-20) ppm. The 13C NMR (DMSO-d6, 150 MHz): δ 155.2 (1C, C-2), 147.5 (1C, C-9), 142.8 (1C, C-14), 136.0 (1C, C-11), 135.9 (1C, C-4), 129.7 (3C, C-7, and C-16/18), 128.4 (1C, C-17), 128.3 (1C, C-8), 127.8 (1C, C-5), 127.1 (1C, C-10), 126.0 (1C, C-6), 117.2 (1C, C-3), 112.5 (2C, C-15/19), and 20.3 (1C, C-20) ppm. MS: m/z 262 (M+, 100%).
3. Results and Discussion
Twelve choline chloride-based deep eutectic solvents (DESs) were used for the synthesis of hydrazones via the conventional (Table 1) and ultrasound (Table 2) methods. Pyridoxal, pyridine-4-carbaldehyde, and quinoline-2-carbaldehyde were used as electrophile and differently substituted phenylhydrazines (R1 and/or R2 = −H, −Cl, −F, −CH3, −NO2) were used as nucleophiles (Scheme 1), resulting in the preparation of 14 different hydrazone derivatives (Figure 1). The eutectic solvents in which the reaction was carried out were already known and characterized via physicochemical methods [62].
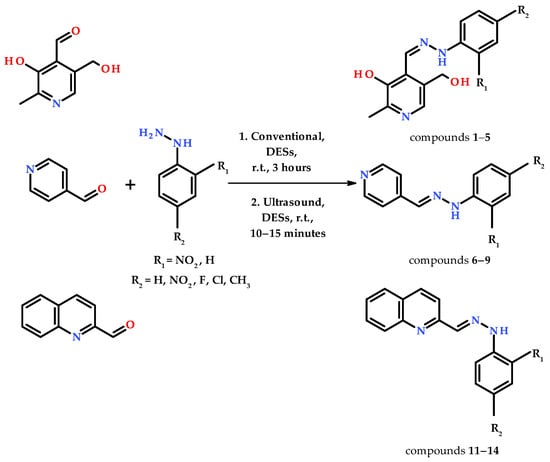
Scheme 1.
Synthetic routes for the preparation of hydrazone derivatives of pyridoxal, pyridine-4-carbaldehyde, and quinoline-2-carbaldehyde.
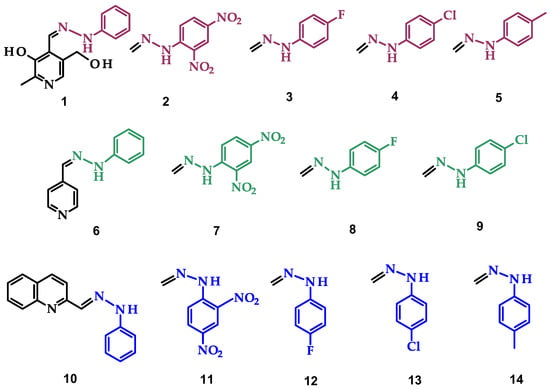
Figure 1.
Structures of all synthesized hydrazones in different DESs. The permanent part of the molecules is marked in black (1, 6, 10, and double bond for other compounds). The part of the molecule that changes is colored in plum (1–5), green (6–9), and blue (10–14).
The structure of the synthesized hydrazone was characterized using 1D and 2D NMR spectroscopy and MS spectrometry (Supplementary Data, Figures S1–S28).
All recorded NMR spectra show a set of signals in the spectrum (Supplementary Data, Figures S1–S14). The important cross peaks in the 2D HMBC NMR spectra (marked with purple arrows and ovals) confirming the successful synthesis of the desired compounds are shown in Figure 2. The m/z values obtained via mass spectrometry also confirm the formation of all the compounds (1–14).
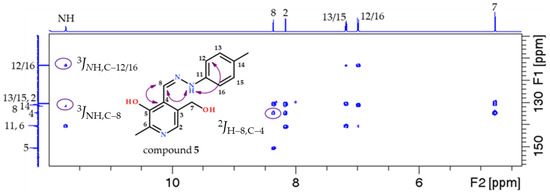
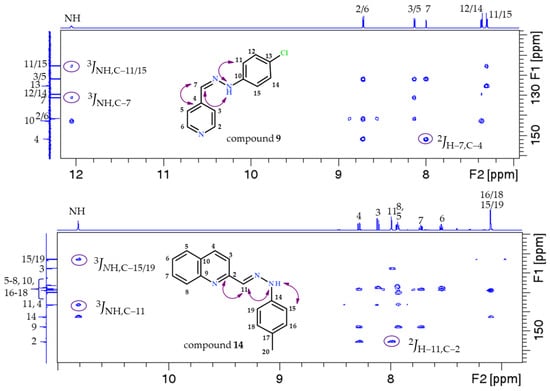
Figure 2.
Enumeration scheme for the assignment of NMR spectra to the examples of compounds 5, 9, and 14. The cross peaks found in the 1H-13C HMBC spectra that confirmed the structure of the compounds are marked in the figure. Part of the 600 MHz 1H NMR spectrum is shown at the top and part of the 150 MHz 13C NMR spectrum is shown at the left.
3.1. Preparation of DESs and Water Content
In order to design the most suitable solvent for the hydrazone formation reaction, 12 different types of deep eutectic solvents with different natures of HBDs were prepared using a relatively simple, economical, and environmentally friendly method. The most common method involves heating and stirring to form a stable liquid. Because of the very simple preparation, purity testing is often omitted. However, studies of eutectic solvents based on choline chloride and organic acids such as HBD have shown that DESs with 5 to 30% impurities can be prepared via this method [63].
The influence of water on DESs has been studied in numerous scientific papers. The influence of water on the physicochemical properties of DESs is extremely important because it affects the structural characteristics and has important practical implications. Studies have shown that even a small amount of water (0–15% w/w) alters the heterogeneous 3D structure of the pure components [64,65]. The water content of the synthesized DESs was determined using the Karl Fischer titration method. The eutectic solvent ChCl:glycerol (1:2) had the lowest water content (0.76%), while the eutectic solvent ChCl:lactic acid (1:2) had the highest water content in the total mass (6.58%).
3.2. Conventional Synthesis
The conventional method required a reaction time of 3 h and compounds 1–14 were generally obtained in low-to-moderate yield (Table 1). A total of 30% of the compounds had a low yield of 11–40%, 69% had a moderate yield of 41–80%, and only 1% had a high yield (82%). In the conventional method, the highest yield was obtained for compound 2 (82%) in the eutectic solvent ChCl:levulinic acid. Four choline chloride-based DESs with HBDs:malonic, oxalic, levulinic, and trans-cinnamic acid were found to be the best medium for hydrazone formation via the conventional method. Nayyar et al. [66] prepared compound 12, quinoline-2-carbaldehyde-4-fluorophenylhydrazone, using the conventional method by heating to 80 °C in abs. ethanol with a reaction yield of 70%. For this compound, four DESs proved to be excellent alternative media in our research: ChCl:urea, ChCl:N-methylurea, ChCl:glycerol, and ChCl:oxalic acid, and the highest yield was obtained in the deep eutectic solvent ChCl:glycerol (87%). Puskullu et al. [67] prepared quinoline-2-carbaldehyde derivatives via conventional synthesis by mixing aldehyde and hydrazine in ethanol with the addition of sodium acetate. They prepared compound 11, quinoline-2-carbaldehyde-2,4-dinitrophenylhydrazone, in a yield of only 12% and compound 13, quinoline-2-carbaldehyde-4-chlorophenylhydrazone, in a yield of 60%. In our studies, compound 11 was obtained in almost quantitative yield (98%) in DES ChCl:oxalic acid, and the highest yield (85%) for compound 13 was obtained in DES ChCl:levulinic acid. All these results indicate that certain eutectic solvents can be an excellent alternative to the classical volatile organic solvents.
3.3. Ultrasound Synthesis
A shorter reaction time of 10–15 min was required for ultrasound synthesis. The results of the synthesis performed showed higher yields. A total of 5% of the compounds had a low yield of 32–40%, 79% had a medium yield of 41–80%, and 15% had a high yield (81–98%). The highest yield of product 11 (98%) was obtained in the deep eutectic solvent ChCl:oxalic acid. The best yield in the preparation of hydrazones via the ultrasound method was obtained in DES ChCl:levulinic acid, where the yield was >50% for almost all prepared compounds (except for 12 and 14). Nguyen et al. 2019 [58] reported the preparation of 2-hydroxy-5-iodo-N′-(1-arylethylidene)benzohydrazide compounds in high yield (98%) using the DESs of ChCl and oxalic acid.
The results obtained via conventional and ultrasound methods show that four DESs are most effective for the preparation of pyridine and quinoline hydrazones—ChCl:malonic, ChCl:oxalic, ChCl:levulinic, and ChCl:trans-cinnamic acid (Figure 3).
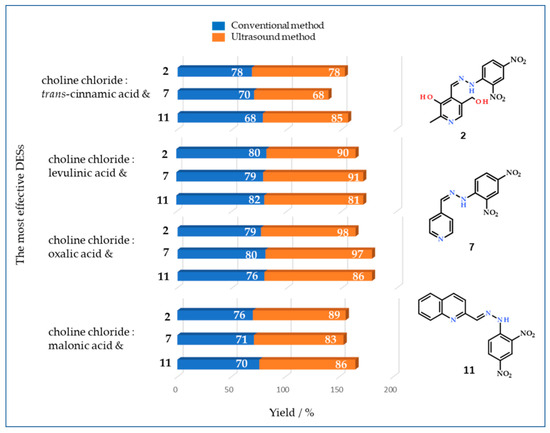
Figure 3.
Yields (%) of compounds 2, 7, and 11 obtained via the conventional (blue) and ultrasound (orange) methods in the four most efficient eutectic solvents (choline chloride:trans-cinnamic acid, levulinic acid, oxalic acid, and malonic acid). Note that the yield depends on the solvent chosen, but an increase was observed when ultrasound method was used.
3.4. Recycling and Reuse of DESs Choline Chloride:Oxalic Acid
Reuse of DESs was performed using choline chloride:oxalic acid (1:1) for the model reaction quinoline-2-carbaldehyde and 2,4-dinitrophenylhydrazine via ultrasound method at room temperature. The DES was recycled by adding water to the reaction mixture, the product crystallized out and was separated via vacuum filtration, and then the water was evaporated. The recycled DES was then reused for another reaction cycle. For each subsequent run, the reaction mixture of qinoline-2-carbaldehyde and 2,4-dinitrophenylhydrazine was run in the same stoichiometric ratio under the same conditions. The results are shown in Figure 4. The yields were very stable after four recycling cycles, indicating that the DES choline chloride:oxalic acid has the potential for easy recycling.
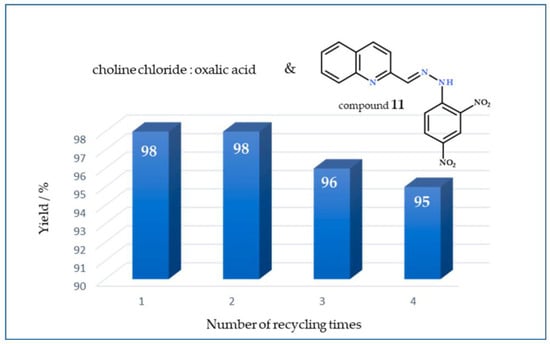
Figure 4.
Monitoring of yield (%) value after reuse of choline chloride:oxalic acid for the model reaction of quinoline-2-carbaldehyde and 2,4-dinitrophenylhydrazine (11) via ultrasound method.
We have confirmed that during the synthesis of eutectic solvents containing carboxylic acids, their esterification can occur. NMR spectra of the eutectic solvent after heating at 80 °C for 3 h showed the presence of ester and water (Figure S29 Supplementary Information). The esterification reaction took place between oxalic acid and the hydroxyl group of alcoholic choline chloride. The yield of esterification was calculated from the value of the integral in the 1H NMR spectrum and was about 30% (data in Supplementary Information). Although the esterification of the solvent occurred during heating, in this case, when the eutectic solvent ChCl:oxalic acid was used, the highest yields were obtained in the synthesis of hydrazones. The hydrazone formation reaction was carried out at room temperature. The presence of water was not confirmed after recycling (Figure S30 Supplementary Information), and references indicate that esterification at higher temperatures requires prolonged heating. Therefore, we assume that esterification does not occur or is negligible after the first cycle [68].
4. Conclusions
All reactions in DESs were successful, so the deep eutectic solvents used can be considered a good alternative to conventional volatile solvents. Low-to-moderate yields were obtained with the conventional method. Higher yields were obtained with shorter reaction time using the ultrasound method. ChCl:malonic, oxalic, levulinic, and trans-cinnamic acid were found to be the most effective solvents for the reaction in both methods tested. Moreover, DESs can be recycled and reused, and the yield did not change after four recycling cycles, indicating the potential of DESs for future applications in the laboratory and industry. The advantages of DESs include their biodegradability, nontoxicity, ease of preparation, and need for low-cost starting materials. Since DESs are environmentally friendly solvents and belong to the green solvents, the syntheses were carried out according to the principles of green chemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10110551/s1, Figure S1: Compound 1 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S2: Compound 2 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S3: Compound 3 A) 1H and B) 13C{1H} NMR spectra (δ/ppm); Figure S4: Compound 4 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S5: Compound 5 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S6: Compound 6 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S7: Compound 7 A) 1H and B) 13C{1H} NMR spectra (δ/ppm); Figure S8: Compound 8 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S9: Compound 9 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S10: Compound 10 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S11: Compound 11 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S12: Compound 12 A) 1H and B) 13C{1H} NMR spectra (δ/ppm); Figure S13: Compound 13 A) 1H and B) 13C{1H} NMR spectra (δ/ppm); Figure S14: Compound 14 A) 1H and B) 13C (APT) NMR spectra (δ/ppm); Figure S15: MS spectra of compound 1; Figure S16: MS spectra of compound 2; Figure S17: MS spectra of compound 3; Figure S18: MS spectra of compound 4; Figure S19: MS spectra of compound 5; Figure S20: MS spectra of compound 6; Figure S21: MS spectra of compound 7; Figure S22: MS spectra of compound 8; Figure S23: MS spectra of compound 9; Figure S24: MS spectra of compound 10; Figure S25: MS spectra of compound 11; Figure S26: MS spectra of compound 12; Figure S27: MS spectra of compound 13; Figure S28: MS spectra of compound 14; Figure S29: 600 MHz1H NMR spectra of oxalic acid: ChCl. Peaks marked in green were added manually. The esterification yield was calculated from the value of the integral as given in the equation above and is approximately 30%; Figure S30: 600 MHz1H NMR spectra of oxalic acid: ChCl after the 1st repetition cycle. Peaks marked in green were added manually. The esterification yield was calculated from the value of the integral as given in the equation above and is approximately 8%.
Author Contributions
Conceptualization, V.B. and D.G.-S.; methodology, V.B. and D.G.-S.; synthesis; V.B. and D.G.-S.; writing—original draft preparation V.B. and D.G.-S.; S.R. analyzed and interpreted NMR data, writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project UIP-2017-05-6593. This work was supported by Croatian Science Foundation and the Green Technologies in Synthesis of Heterocyclic Compounds Project UIP-2017-05-6593.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sevim, R.; Küçükgüzel, G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Kumar, Y.; Sinha, R.; Kumar, R.; Stables, J. Menthone aryl acid hydrazones: A new class of anticonvulsants. Med. Chem. 2011, 7, 56–61. [Google Scholar] [CrossRef]
- Tributino, J.L.; Duarte, C.D.; Corrêa, R.S.; Doriguetto, A.C.; Ellena, J.; Romeiro, N.C.; Castro, N.G.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A. Novel 6-methanesulfonamide-3, 4-methylenedioxyphenyl-N-acylhydrazones: Orally effective anti-inflammatory drug candidates. Bioorg. Med. Chem. 2009, 17, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Gil-Longo, J.; Laguna, M.D.L.R.; Verde, I.; Castro, M.E.; Orallo, F.; Fontenla, J.A.; Calleja, J.M.; Ravina, E.; Teran, C. Pyridazine derivatives. XI: Antihypertensive activity of 3-hydrazinocycloheptyl [1, 2-c] pyridazine and its hydrazone derivatives. J. Pharm. Sci. 1993, 82, 286–290. [Google Scholar] [CrossRef][Green Version]
- Liu, W.Y.; Li, H.Y.; Zhao, B.X.; Shin, D.S.; Lian, S.; Miao, J.Y. Synthesis of novel ribavirin hydrazone derivatives and anti-proliferative activity against A549 lung cancer cells. Carbohydr. Res. 2009, 344, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Pal, R.; Dwivedi, A.; Hajela, K. Synthesis of some new diaryl and triaryl hydrazone derivatives as possible estrogen receptor modulators. Arzneimittelforsch 2002, 52, 39–44. [Google Scholar] [CrossRef]
- Terzioğlu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b]-[1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Lilliu, V.; Onnis, V. Synthesis and in vitro antitumoral activity of new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg. Med. Chem. 2005, 14, 366–372. [Google Scholar] [CrossRef]
- Gürsoy, E.; Güzeldemirci-Ulusoy, N. Synthesis and primary cytotoxicity evaluation of new imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 2007, 42, 320–326. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Wahab, A.E.; El-Dewellawy, M.A. Cyanoacetic acid hydrazones of 3-(and 4-) acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 2006, 339, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.A.; Telvekar, V.N. Synthesis and evaluation of novel chloropyrrole molecules designed by molecular hybridization of common pharmacophores as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2010, 20, 5681–5685. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Blache, Y.; Güven, K. Synthesis and antimicrobial activity of some imidazo-[1,2-a]pyridine-2-carboxylic acid arylidenehydrazide derivatives. Boll. Chim. Farm. 2001, 140, 397–400. [Google Scholar]
- Fattorusso, C.; Campiani, G.; Kukreja, G.; Persico, M.; Butini, S.; Romano, M.P.; Altarelli, M.; Ros, S.; Brindisi, M.; Savini, L. Design, synthesis, and structure–activity relationship studies of 4-quinolinyl-and 9-acrydinylhydrazones as potent antimalarial agents. J. Med. Chem. 2008, 51, 1333–1343. [Google Scholar] [CrossRef]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D.B.; Gordeuk, V.R.; Richardson, D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Let. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Ma, X.D.; Yang, S.Q.; Gu, S.X.; He, Q.Q.; Chen, F.E.; De Clercq, E.; Balzarini, J.; Pannecouque, C. Synthesis and anti-HIV activity of Aryl-2-[(4-cyanophenyl) amino]-4-pyrimidinone hydrazones as potent non-nucleoside reverse transcriptase inhibitors. Chem. Med. Chem. 2011, 6, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Kremer, L.; Louw, S.; Guéradel, Y.; Chibale, K.; Biot, C. Synthesis and in vitro antitubercular activity of ferrocene-based hydrazones. Bioorg. Med. Chem. Lett. 2011, 21, 2866–2868. [Google Scholar] [CrossRef]
- Bukowski, L.; Janowiec, M. 1-Methyl-1H-2-imidazo[4,5-b]pyridinecarboxylic acid and some of derivatives with suspected antituberculotic activity. Pharmazie 1996, 51, 27–30. [Google Scholar]
- Bukowski, L.; Janowiec, M.; Zwolska-Kwiek, Z.; Andrzejczyk, Z. Synthesis and some reactions of 2- acetylimidazo[4,5-b]pyridine. Antituberculotic activity of the obtained compounds. Pharmazie 1999, 54, 651–654. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazole-2-ylthio]acetic acid arylidene-hydrazide derivatives. Farmaco 2001, 56, 587–592. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E.; Scialino, G. Synthesis and antimycobacterial activity of (3,4-diaryl-3H-thiazole-2-ylidene)hydrazide derivatives. Farmaco 2003, 58, 631–637. [Google Scholar] [CrossRef]
- Musad, E.A.; Mohamed, R.; Saeed, B.A.; Vishwanath, B.S.; Rai, K.L. Synthesis and evaluation of antioxidant and antibacterial activities of new substituted bis (1, 3, 4-oxadiazoles), 3, 5-bis (substituted) pyrazoles and isoxazoles. Bioorg. Med. Chem. Lett. 2011, 21, 3536–3540. [Google Scholar] [CrossRef]
- El-Sabbagh, O.; Shabaan, M.A.; Kadry, H.H.; Al-Din, E.S. New octahydroquinazoline derivatives: Synthesis and hypotensive activity. Eur. J. Med. Chem. 2010, 45, 5390–5396. [Google Scholar] [CrossRef]
- De Oliveira, K.N.; Costa, P.; Santin, J.R.; Mazzambani, L.; Bürger, C.; Mora, C.; Nunes, R.J.; De Souza, M.M. Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg. Med. Chem. 2011, 19, 4295–4306. [Google Scholar] [CrossRef] [PubMed]
- Ergenç, N.; Günay, N.S. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar] [CrossRef]
- Shtyrlin, V.; Khaziev, R.M.; Shtyrlin, V.G.; Gilyazetdinov, E.M.; Agafonova, M.N.; Usachev, K.S.; Islamov, D.R.; Klimovitskii, A.E.; Vinogradova, T.I.; Dogonadze, M.Z.; et al. Isonicotinoyl hydrazones of pyridoxine derivatives: Synthesis and antimycobacterial activity. Med. Chem. Res. 2021, 30, 952–963. [Google Scholar] [CrossRef]
- Aljuhani, A.; Rezki, N.; Al-Sodies, S.; Messali, M.; ElShafei, G.M.S.; Hagar, M.; Aouad, M.R. Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations. Molecules 2022, 27, 2492. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Abid, S.; Tahir, N.M.; Ashfaq, M.; Kanwal, F.; Lu, C.; Rehman, M.F. Green Synthesis, SC-XRD, Non-Covalent Interactive Potential and Electronic Communication via DFT Exploration of Pyridine-Based Hydrazone. Crystals 2020, 10, 778. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Abid, S.; Iqbal, J.; Tahir, N.M.; Raza, A.R.; Zukerman-Schpector, J.; Paixão, M.W. Facile synthesis, crystal growth, characterization and computational study of new pyridine-based halogenated hydrazones: Unveiling the stabilization behavior in terms of noncovalent interactions. Appl. Organometal Chem. 2020, 34, e5399. [Google Scholar] [CrossRef]
- Nogueira, T.C.M.; Cruz, L.S.; Lourenço, M.C.; de Souza, M.V.N. Design, Synthesis and Anti-tuberculosis Activity of Hydrazones and N-acylhydrazones Containing Vitamin B6 and Different Heteroaromatic Nucleus. Lett. Drug Des. Discov. 2019, 16, 792–798. [Google Scholar] [CrossRef]
- Alptüzün, V.; Parlar, S.; Taşli, H.; Erciyas, E. Synthesis and antimicrobial activity of some pyridinium salts. Molecules 2009, 14, 5203–5215. [Google Scholar] [CrossRef]
- Parlar, S.; Erzurumlu, Y.; Ilhan, R.; Ballar, P.; Kırmızıbayrak, P.B.; Alptüzün, V.; Erciyas, E. Synthesis and evaluation of pyridinium-hydrazone derivatives as potential antitumoral agents. Chem. Biol. Drug Des. 2018, 92, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, T.; Milton, M.D. Logic gate based novel phenothiazine-pyridylhydrazones: Halochromism in solid and solution state. Dye. Pigment. 2019, 164, 305–318. [Google Scholar] [CrossRef]
- Mali, S.N.; Thorat, B.R.; Gupta, D.R.; Pandey, A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Eng. Proc. 2021, 11, 21. [Google Scholar]
- Mandewale, M.C.; Thorat, B.; Nivid, Y.; Jadhav, R.; Nagarsekar, A.; Yamgar, R. Synthesis, structural studies and antituberculosis evaluation of new hydrazone derivatives of quinolone and their Zn(II) complexes. J. Saudi Chem. Soc. 2018, 22, 218–228. [Google Scholar] [CrossRef]
- Pisk, J.; Đilović, I.; Hrenar, T.; Cvijanović, D.; Pavlović, G.; Vrdoljak, V. Effective methods for the synthesis of hydrazones, quinazolines, and Schiff bases: Reaction monitoring using a chemometric approach. RCS Adv. 2020, 10, 38566–38577. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Dugoni, G.C.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. 2023, 30, 17268–17279. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline and reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kudlak, B.; Owczarek, K.; Namiesnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents-a review. Environ. Sciand Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Intensification of biotransformations using deep eutectic solvents: Overview and outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Thermal Instability of Choline Chloride-Based Deep Eutectic Solvents and Its Influence on Their Toxicity─Important Limitations of DESs as Sustainable Materials. Ind. Eng. Chem. Res. 2022, 61, 11288–11300. [Google Scholar] [CrossRef]
- Chen, W.J.; Xue, Z.M.; Xue, J.F.; Wang, J.Y.; Jiang, X.H.; Zhao, T.C.; Mu, T. Investigation on the Thermal Stability of Deep Eutectic Solvents. Acta Phys.-Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- García-Álvarez, J. Deep Eutectic Mixtures: Promising Sustainable Solvents for Metal-Catalysed and Metal-Mediated Organic Reactions. Eur. J. Inorg. Chem. 2015, 2015, 5147–5157. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Lorenzo Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green. Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.R.S.; Silvestre, A.J.D.; Freire, M.G. Deep Eutectic Solvents and Pharmaceuticals. Encyclopedia 2021, 1, 942–963. [Google Scholar] [CrossRef]
- Sheldon, R.A. Biocatalysis and Biomass Conversion in Alternative Reaction Media. Chem.—A Eur. J. 2016, 22, 12984–12999. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green. Chem. 2018, 20, 3468–3475. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green. Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef]
- Massolo, E.; Palmieri, S.; Benaglia, M.; Capriati, V.; Perna, F.M. Stereoselective organocatalysed reactions in deep eutectic solvents: Highly tunable and biorenewable reaction media for sustainable organic synthesis. Green. Chem. 2016, 18, 792–797. [Google Scholar] [CrossRef]
- Millia, L.; Dall’Asta, V.; Ferrara, C.; Berbenni, V.; Quartarone, E.; Perna, F.M.; Capriati, V.; Mustarelli, P. Bio-inspired choline chloride-based deep eutectic solvents as electrolytes for lithium-ion batteries. Solid State Ion. 2018, 323, 44–48. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, C.T.; Tran, P.H. Synthesis of a new series of 2-hydroxy-5-iodo-N’-(1-arylethylidene)benzohydrazides using a deep eutectic solvent as solvent/catalyst under sonication. Heliyon 2019, 5, e02353. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. Engl. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Baskevich, A.S.; Korniy, S.A.; Danilov, F.I. Electrodeposition of chromium coatings from a choline chloride based ionic liquid with the addition of water. J. Chem. Technol. Metall. 2018, 53, 906–915. [Google Scholar]
- McCalman, D.C.; Sun, L.; Zhang, Y.; Brennecke, J.F.; Maginn, E.J.; Schneider, W.F. Speciation, conductivities, diffusivities, and electrochemical reduction as a function of water content in mixtures of hydrated chromium chloride/choline chloride. J. Phys. Chem. B 2015, 119, 6018–6023. [Google Scholar] [CrossRef] [PubMed]
- Bušić, V.; Gašo-Sokač, D. Menshutkin Reaction in Choline Chloride-based Deep Eutectic Solvents. Org. Prep. Proced. Int. 2022, 55, 160–166. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Nolasco, M.N.; Pedro, S.N.; Vilela, C.; Vaz, P.D.; Ribeiro-Claro, P.; Rudić, S.; Parker, S.F.; Freire, C.R.S.; Freire, M.G.; Silvestre, A.D.J. Water in Deep Eutectic Solvents: New Insights from Inelastic Neutron Scattering Spectroscopy. Front. Phys. 2022, 10, 834571. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of Water Addition on Choline Chloride/glycol Deep Eutectic Solvents: Characterization of Their Structural and Physicochemical Properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Nayyar, A.; Malde, A.; Coutinho, E.; Jain, R. Synthesis, antituberculosis activity, and 3D-QSAR study of ring-substituted-2/4-quinolinecarbaldehyde derivatives. Bioorg. Med. Chem. 2006, 14, 7302–7310. [Google Scholar] [CrossRef] [PubMed]
- Puskullu, M.O.; Shirinzadeh, H.; Nenni, M.; Gurer-Orhan, H.; Suzen, S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehyde hydrazone derivatives: Bioisosteric melatonin analogues. J. Enzym. Inhib. Med. Chem. 2016, 31, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Rodriguez, N.; van den Bruinhorst, A.; Kollau, J.B.M.L.; Kroon, C.M.; Binnemans, K. Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2019, 7, 11521–11528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).