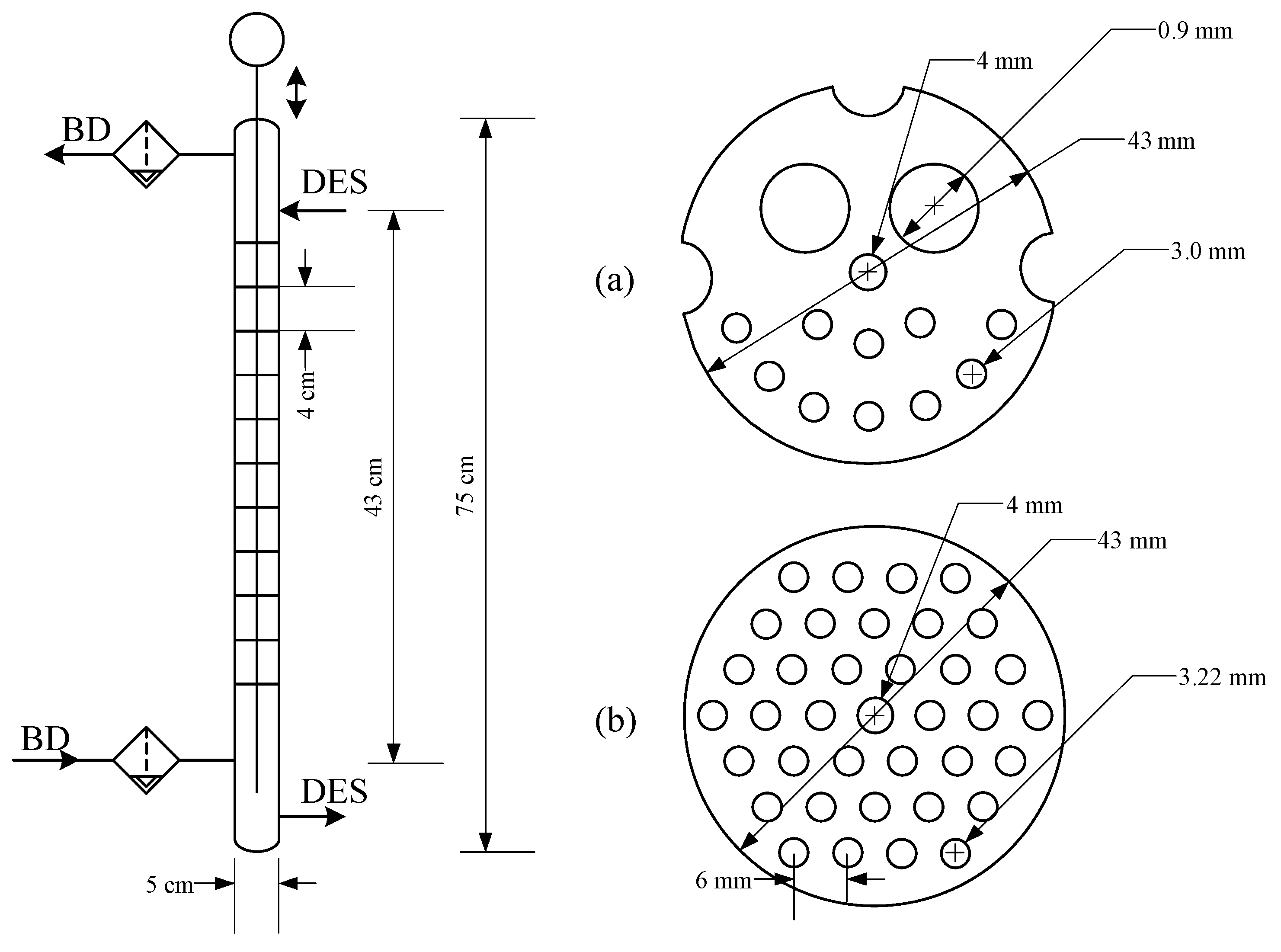
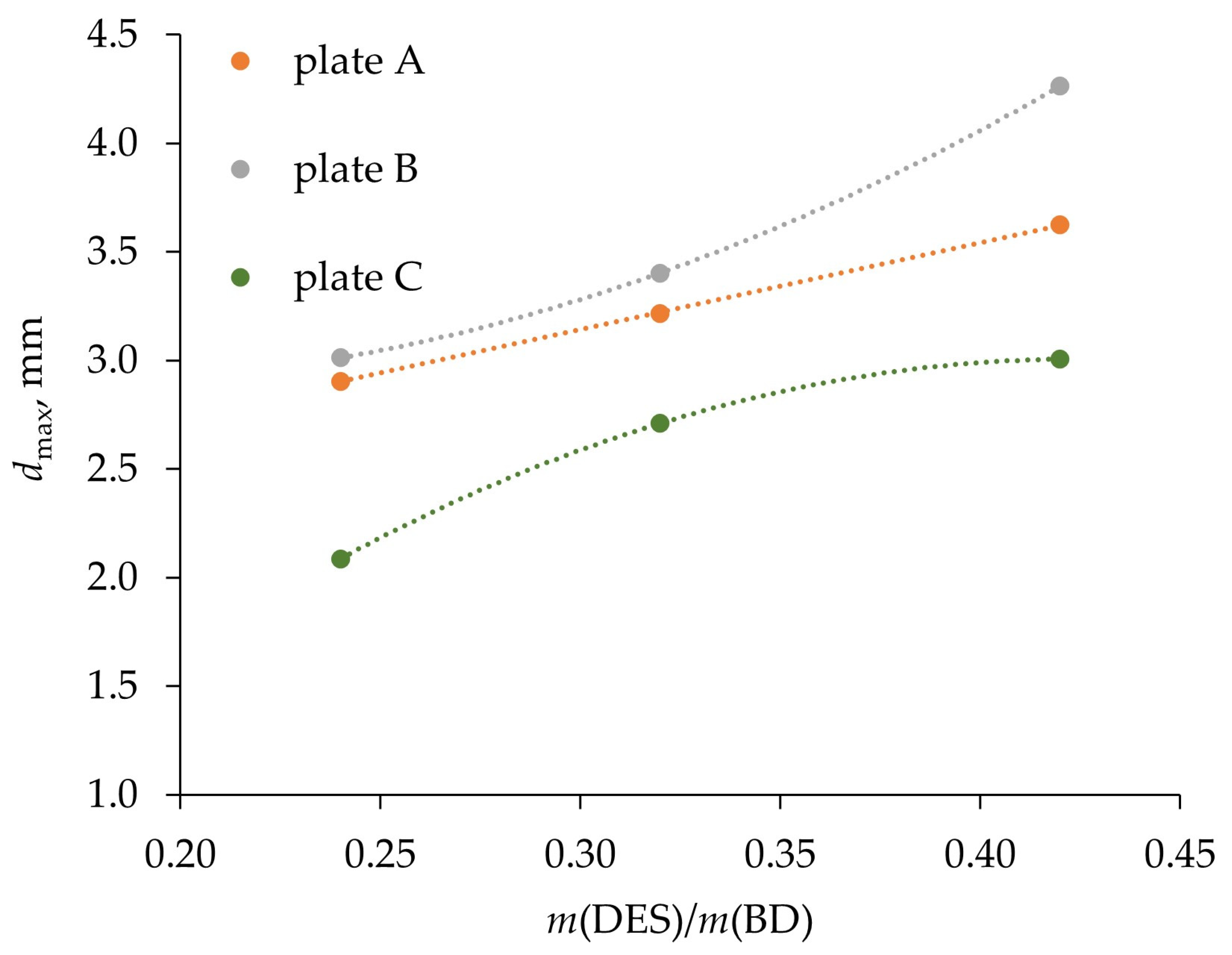
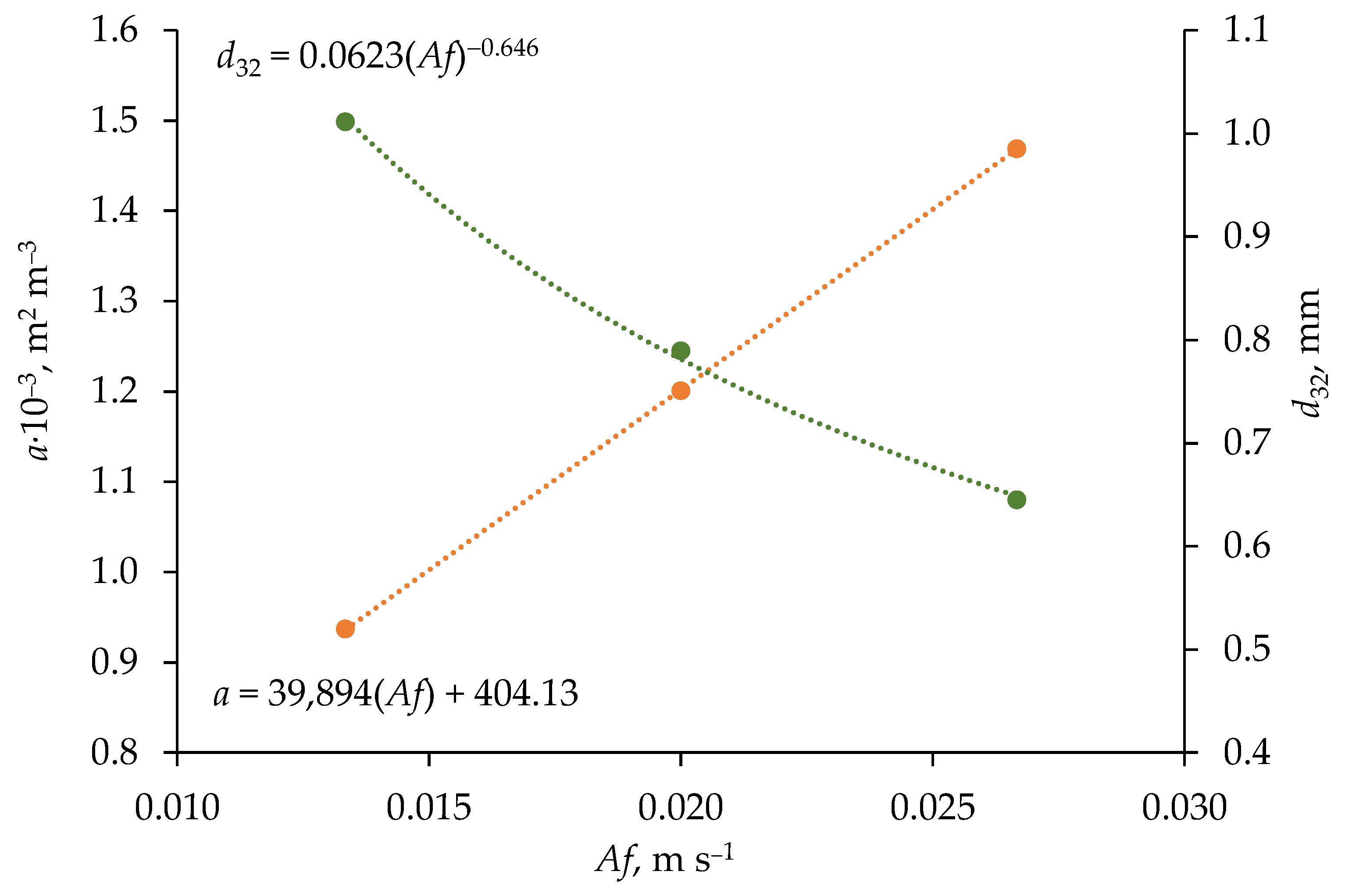3.1. Physical and Surface Properties of Phases
The measured values of density,
ρ, viscosity,
μ, surface tension,
σ, and interfacial tension,
γ, are given in
Table 2. The density difference was large enough to assure phase separation after extraction, the viscosity of the DES was low enough not to interfere with mass transfer, and based on prior experience with the DES the system is prone to coalescence, which also allows for phase separation at the bottom and the top of the extraction column [
9]. Low interfacial tension ensures the easy formation of droplets.
Contact angle measurements were taken to determine the surface free energy (SFE) of steel and PETG by a sessile drop method. Measurements were conducted at 23 °C with deionized water (
κ = 2.0 μS/cm) as a polar liquid, diiodomethane (99%, Aldrich, St. Louis, MO, USA) as a non-polar liquid, and formamide (p.a. 99.5%, Fluka, Charlotte, NC, USA) with properties in between. The drop volume of 2 μL was used for the measurements. The contact angles were determined at the same time, 80 ms after the droplet touched the surface of the tested material. Arithmetic mean values of five contact angles were calculated for each sample. The surface free energies of steel and PETG were calculated using three models: Owens–Wendt (OW), Wu, and Acid-base (AB),
Table 3. Higher surface free energy was observed for PETG plates (SFE (steel) = 33.0 mJ m
−2, SFE (PETG) = 46.2 mJ m
−2), which indicates the slightly better wettability of PETG. The spreading ability of both liquids was determined by measuring the contact angle of both liquids on steel and PETG. A drop volume of 10 μL was used for the measurements. The contact angles were determined at the same time, 90 ms and 2000 ms, after the drop touched the surface of the tested materials,
Table 4. The contact angle of biodiesel after 2 s is much lower compared to DES. Both the lower contact angle and its reduction indicate a better spreading ability of biodiesel. Based on the obtained results, biodiesel was chosen as the continuous phase.
The surface free energy and contact angle measurements of steel and PETG showed that both steel and PETG have similar surface properties (
Table 3). Water contact angles around 75° indicate that both materials are slightly hydrophilic. The total surface free energy of PETG is slightly higher, mainly due to the higher dispersive component of the surface free energy. For both materials, the polar component of the surface free energy is very similar and is inherently basic. These results indicate that the liquids will have the same spreading ability on both materials.
3.3. Extraction Efficiency
To analyze the behavior of the system, batch extraction experiments at different hydrodynamics conditions, durations, and mass ratios of biodiesel and DES have been performed. The
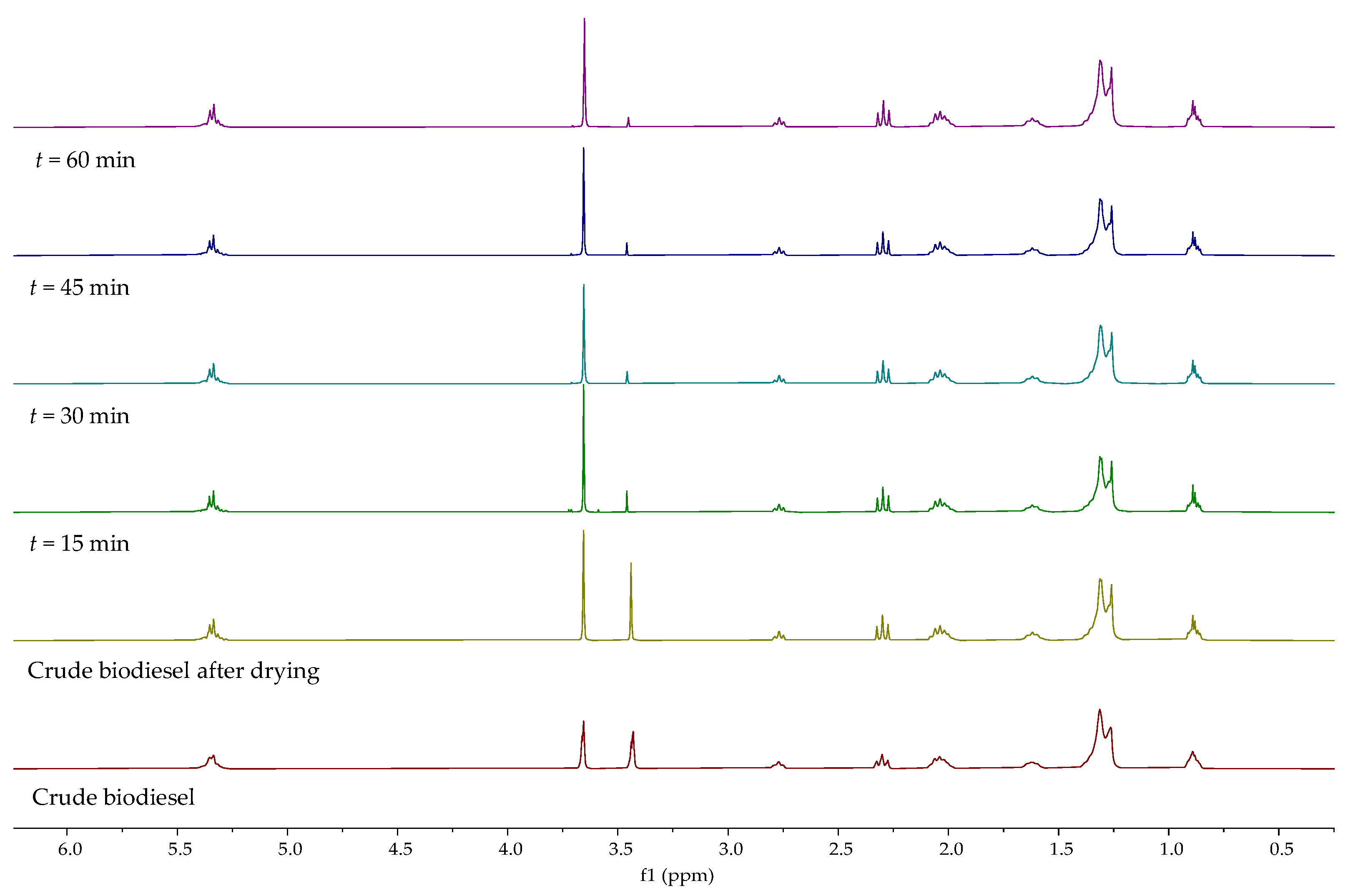
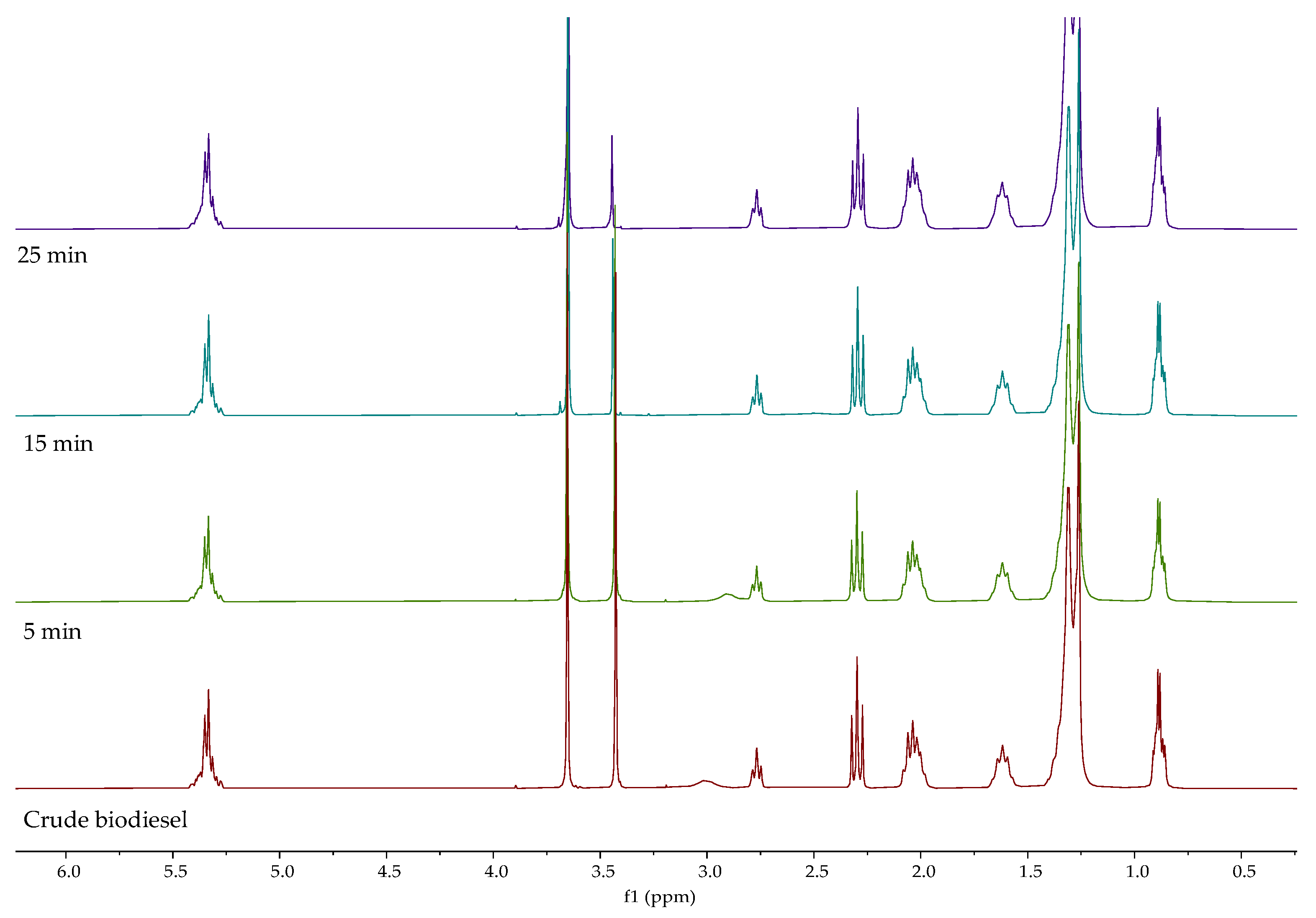
1H NMR spectra of biodiesels obtained after different extraction times is presented in
Figure 4. Signal characteristic for the methyl ester group at 3.65 ppm can be observed at all process conditions. The intensity of the hydroxyl group signal at around 3.44 ppm was significantly reduced, which can indicate that the used DES is suitable for the extraction of glycerol. Similar results were obtained with increasing mixing rate and DES to biodiesel mass ratio,
Figures S2 and S3. When comparing the spectra of crude and dried biodiesel, the downfield effect of hydroxylic proton can be observed on the spectrum of the dried sample (after removal of methanol). Considering that the signal at 3.4 ppm belongs to hydroxyl protons of glycerol and methanol, the ratio of signal surfaces belonging to methyl esters,
A(Me), and alcohols,
A(OH), was calculated. The increase in this ratio from 1.08 to 1.58 is attributed to the removal of methanol. A further increase can be observed with increasing mixing rate, extraction time, and DES to biodiesel mass ratio, as a result of the decreasing concentration of glycerol in biodiesels,
Table 5.
To evaluate the fatty acid methyl ester (FAME) content in biodiesel, data obtained by
1H NMR was used (integration values of signals at 3.66 ppm—methyl ester (
AMe) and 2.30 ppm—glyceridic signal in triglycerides (
ACH
2)) [
18]:
Crude and dried (after removal of methanol) biodiesels contain 94.61% FAME and 96.26% FAME, respectively. The results obtained by batch extraction are shown in
Table 5.
Based on the obtained results, it can be concluded that hydrodynamic conditions have a significant influence on the efficiency of biodiesel purification. At lower mixing rates,
n, (400 and 600 rpm) a smaller interfacial surface is formed and, due to the higher resistances, the rate of mass transfer is reduced. For mixing rates higher than 600 rpm, the FAME content complies with the biodiesel quality standard [
19].
The same biodiesel was also used in the first set of experiments in the Karr column equipped with plate A (steel). The influence of extraction time, DES to biodiesel mass ratio, and agitation rate was analyzed. Samples were collected during extraction after 5, 15, and 25 min. To demonstrate the influence of the extraction time in the Karr column, the
1H NMR spectra of one experimental setup is presented in
Figure 5. As extraction proceeds, the ratio
A(Me)/
A(OH) increases so residual methanol and glycerol are successfully extracted from crude biodiesel. A broad signal at 3 ppm (before extraction) can be attributed to methanol (OH), just as in the one at 2.9 ppm (after 5 min of extraction) [
20]. This signal disappears after 15 min.
The evaluated FAME content and the ratio
A(Me)/
A(OH) for all process conditions are given in
Table 6. All experiments resulted with a satisfactory FAME content (>96.5). At a higher pulsation rate and DES to biodiesel mass ratio (or volume fraction of the dispersed phase,
φD), an increase in the extraction efficiency of glycerol and methanol can be observed. FAME content also slightly decreases, which means that methyl esters are sparingly soluble in DES.
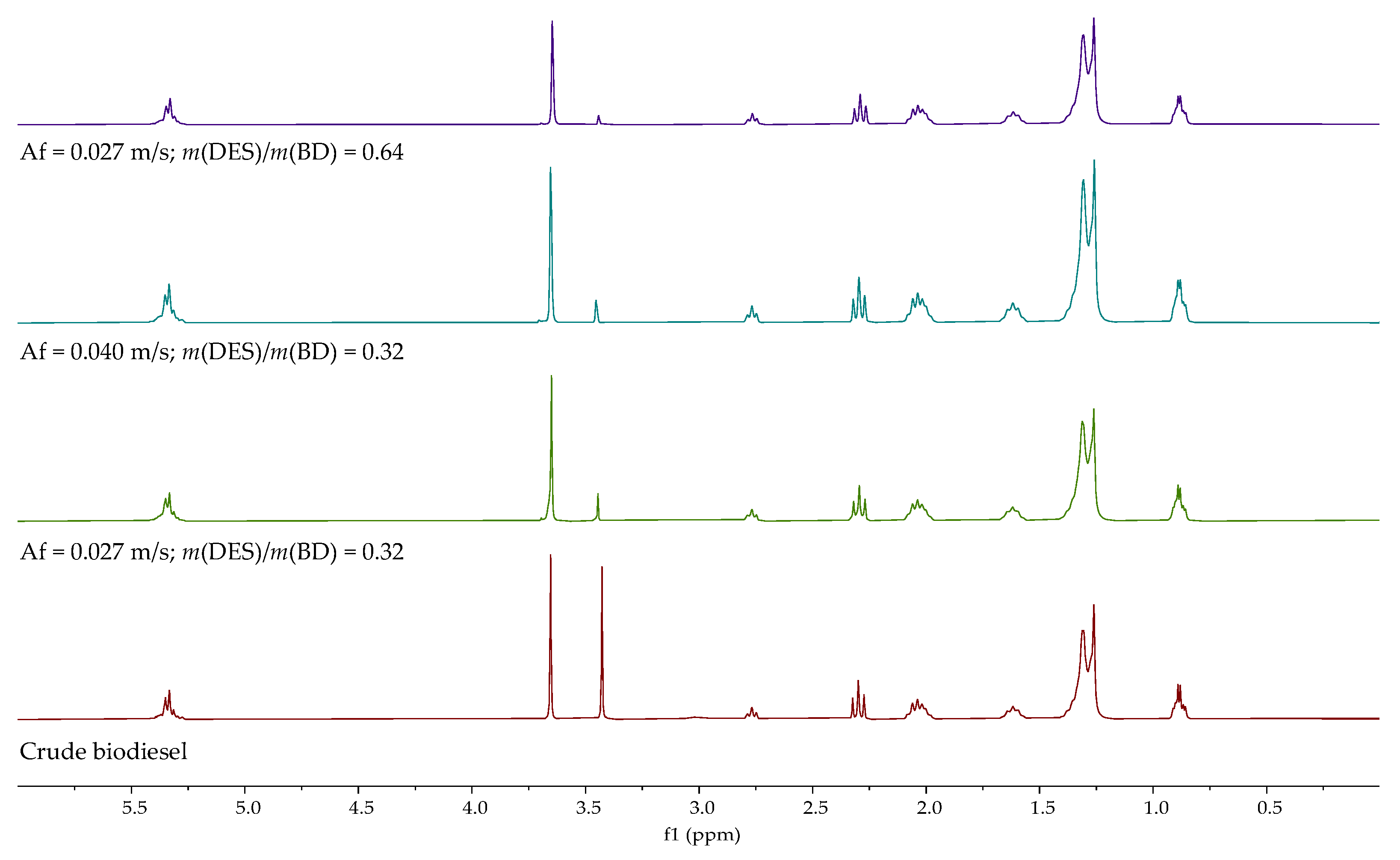
If the
1H NMR spectra of purified biodiesels after 25 min are compared, the most favorable conditions for the purification of biodiesel are a lower pulsation rate and a higher DES to biodiesel ratio,
Figure 6.
After the preliminary experiments were conducted with biodiesel 1 and analyzed by
1H NMR, the following experiments were carried with biodiesel 2 and analyzed by FTIR (
Figure 7),
1H NMR (
Figure 8),
13C NMR (
Figure 9), two-dimensional HMQC (
Figure 10), and GC.
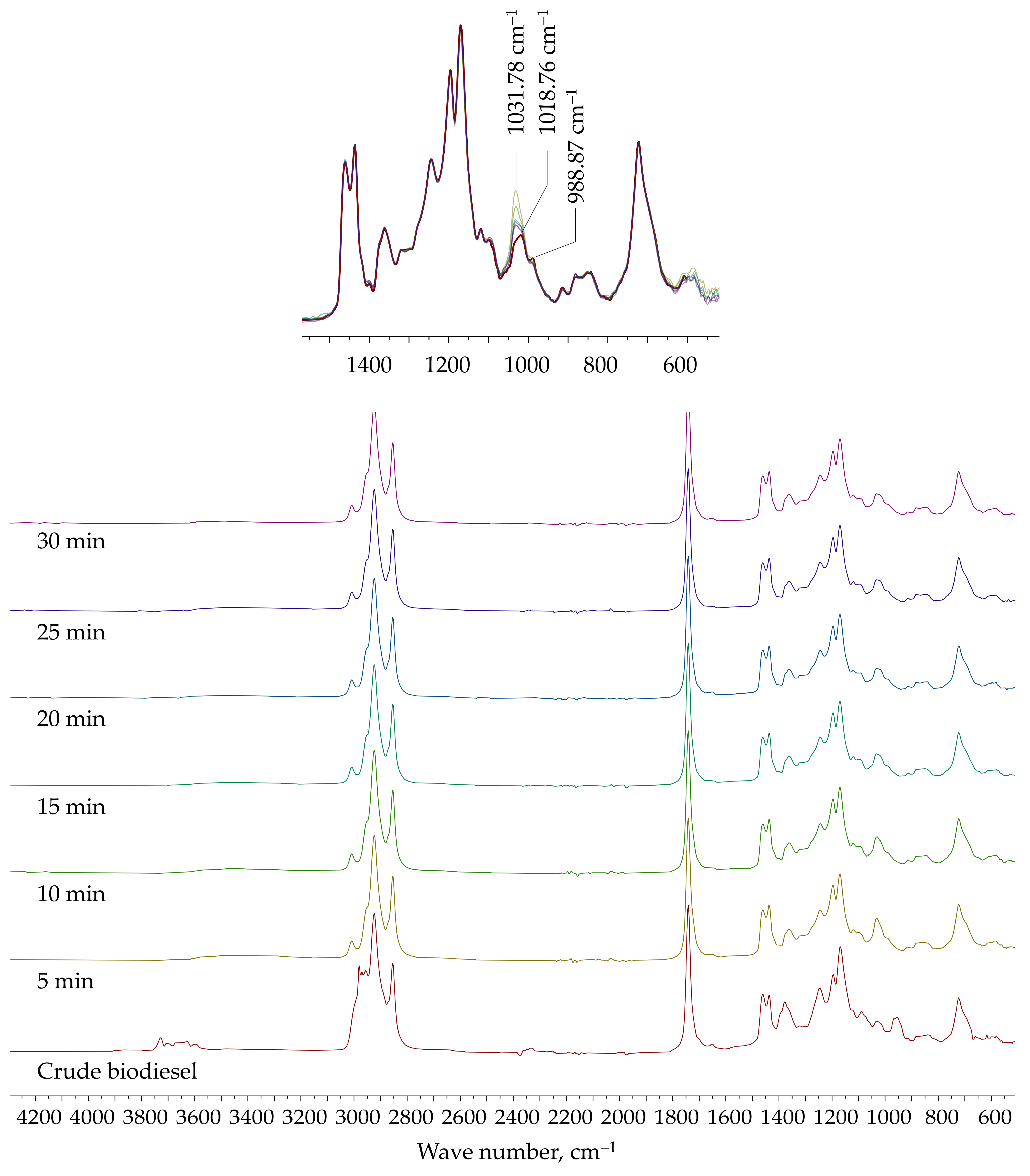
The FTIR spectra of crude biodiesel and samples of purified biodiesel collected during extraction are shown in
Figure 7 (plate A,
φV = 0.2;
Af = 0.02 m/s). Signals characteristic of fatty-acid methyl esters are clearly visible: 1742.43 cm
−1 (C=O stretching), 1435.81 cm
−1 (CH
3 bending), and 1195.71 cm
−1 (-OCH
3 stretching). The broad signal between 3000 and 3600 cm
−1 corresponds to the OH group in glycerol and methanol, while the signal at 1031.78 cm
−1 corresponds to the C-O stretching of methanol [
21]. In the fingerprint region, a decrease in the intensity of the signal at 1031.78 cm
−1 (methanol) and the disappearance of the signal at 988.87 cm
−1 (glycerol) can be observed as extraction proceeds [
22].
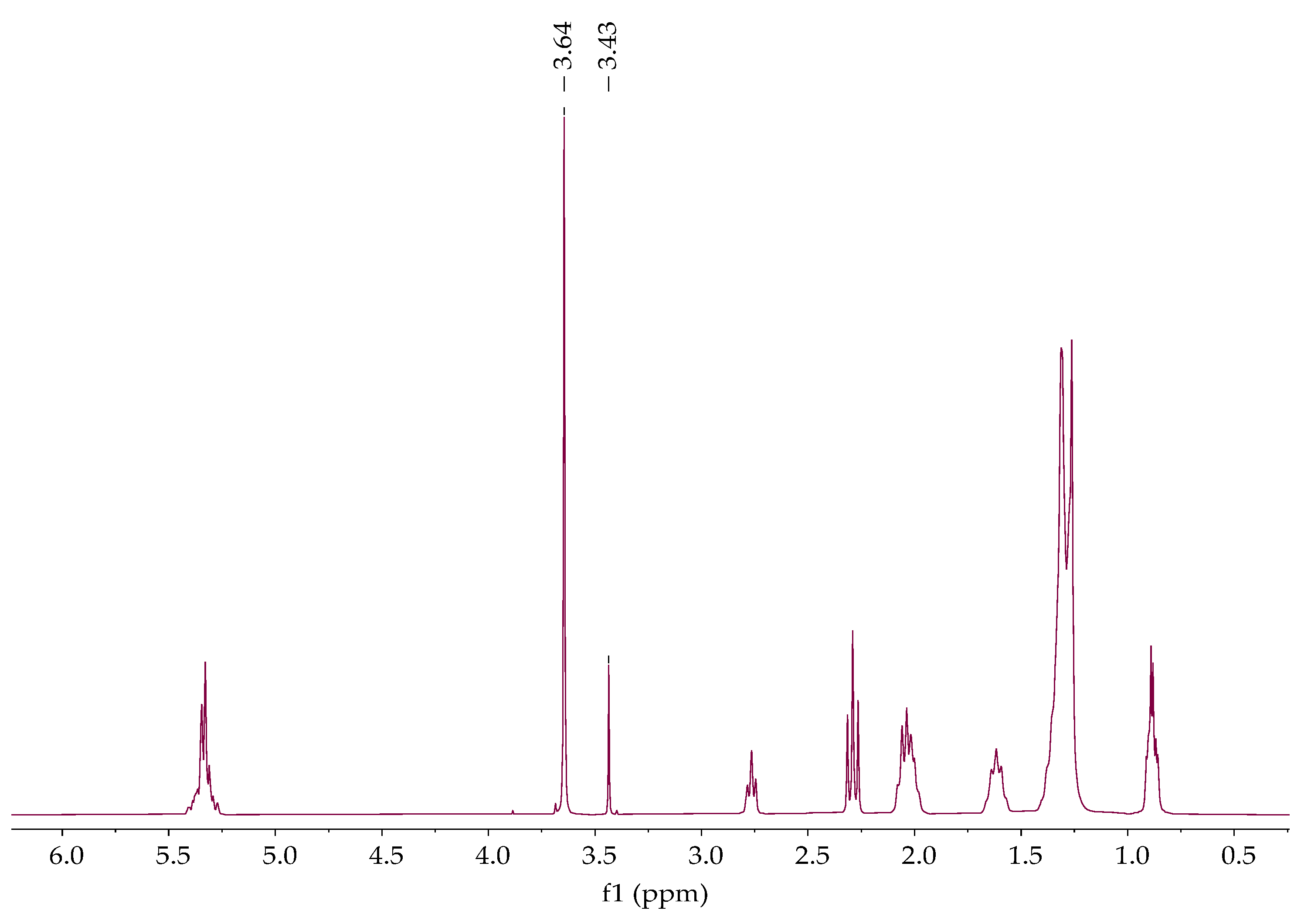
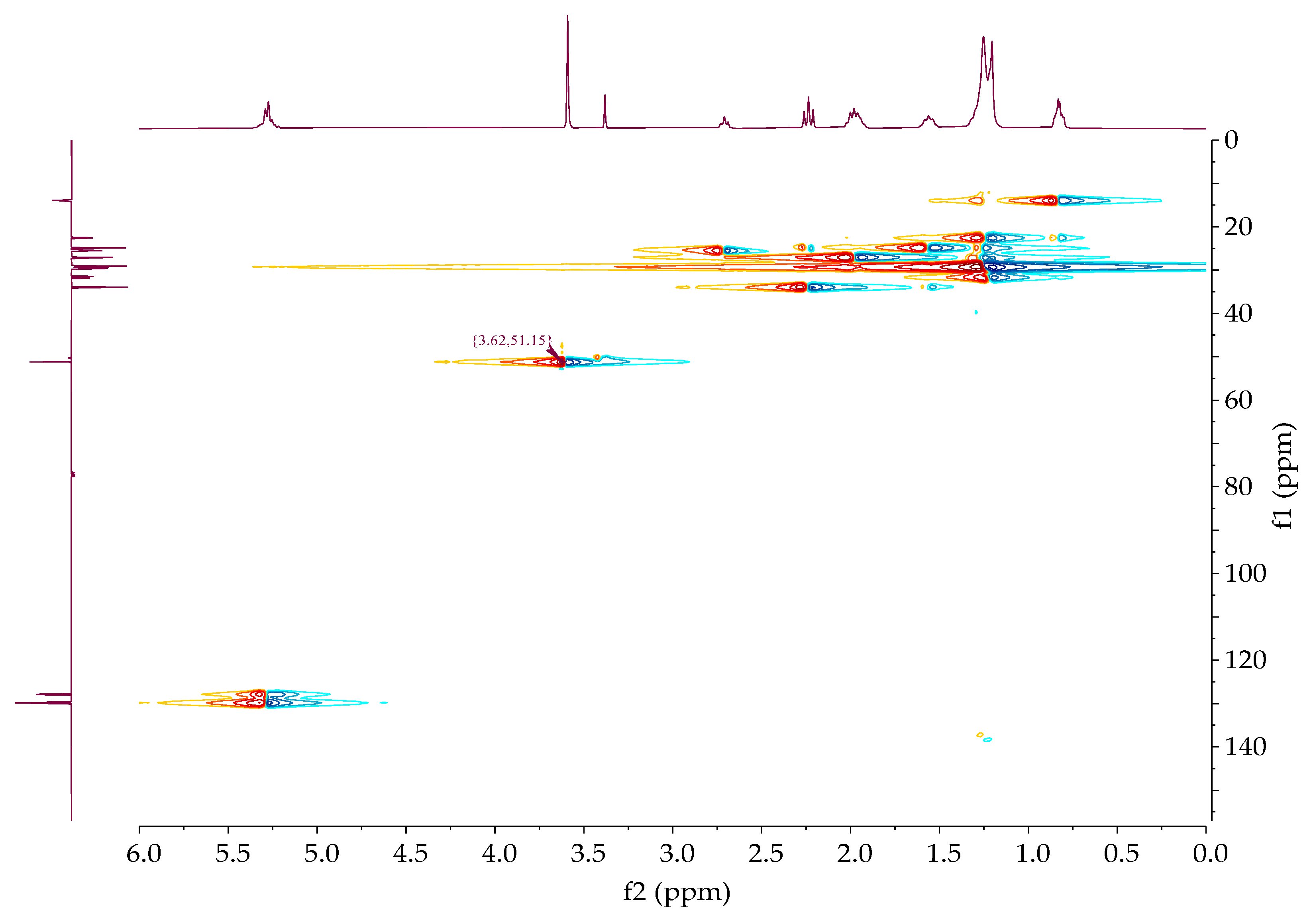
The chemical shifts of the proton and carbon NMR spectra of all samples were obtained by the combined use of one
1H,
13CAPT, and two-dimensional HMQC spectra according to the previously published data [
23]. The proton spectra of the sample showed a signal that resonated at ~3.60 ppm, which was assigned to the methyl group of methanol,
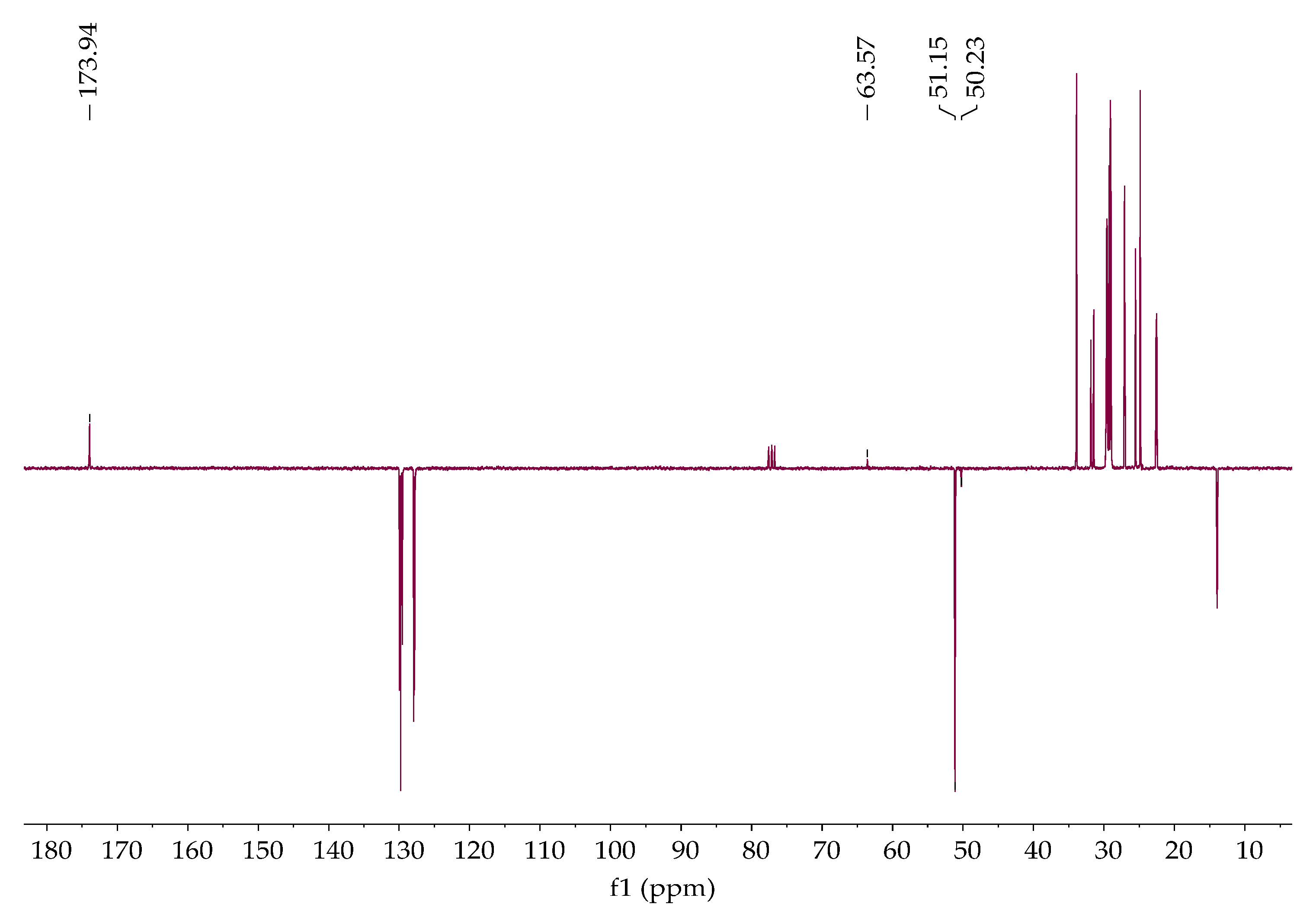
Figure 8. Methyl ester proton and carbon were found at 3.62 ppm and 51.15 ppm, respectively,
Figure 10. The signal observed at 63.84 ppm indicates the presence of glycerol carbon atoms,
Figure 9.
Concentrations (in mass fraction) of linoleic acid methyl ester, FAME, monoglycerides (MG), diglycerides (DG), triglycerides (TG), and free and total glycerol in crude biodiesel (BD) are 0.30, 96.9, 0.28, 0.06, <0.01, 0.13, and 0.21, respectively. Concentrations of linoleic acid methyl ester and triglycerides did not change during extraction. The results obtained by the GC analysis of purified biodiesels are presented in
Table 7.
For all selected process conditions, concentration of glycerol was reduced. It was also observed that, at some process conditions, FAME, MG, and DG are also soluble in DES. The highest amount of glycerol was removed with steel plates and the highest DES to biodiesel mass flowrates ratio. At those conditions, the concentration of MG and DG was also reduced. For the PTFE plates of the same geometry, the highest reduction of glycerol was obtained at the highest pulsation rate. However, at those conditions, concentration of FAME was reduced below 96.5% (standard). Solubility of DG was also observed. The lowest extraction efficiency for plate C was obtained with the lowest DES to biodiesel mass flowrates ratio. When disperse phase flowrate was maintained constant, the flowrate of the continuous phase did not have an impact on the extraction efficiency for this type of plate. The increase in FAME and/or lower glyceride (MG and DG) content may be attributed to the transfer of glycerol from biodiesel to DES.
Higher pulsation rates may result in backmixing and consequently lower extraction efficiency [
24]. Generally speaking, an increase in mixing intensity should produce a larger interphase surface area, which in turn enhances the rate of mass transfer. At larger volume fractions of the dispersed phase, in this case the solvent, a larger surface area for mass transfer is also formed. However, at some point there will be too many droplets and some of them will be in close contact, so the actual mass transfer area will be significantly smaller. Coalescence is also possible if the interfacial energy is low enough.
The presence of surface-active compounds in biodiesel strongly influences the droplet formation. Surfactants act as emulsifiers and dispersants by lowering the interfacial tension, which results in reduction of droplet size. Partial glycerides (monoglycerides, MG, and diglycerides, DG) are non-ionic surfactants [
25], while fatty acids are anionic surfactants [
26]. Biodiesel produced from waste cooking oil contains low concentrations of glycerides and free fatty acids as well as some other compounds transferred from food during frying.
As extraction proceeds, the compositions of both phases change, which in turn affects the mass transfer interface and mass transfer area. All compounds that were transferred from biodiesel to solvent change the composition of the solvent and thus influence the solubility of alcohols and FAME. Previous investigations proved that elements in traces, compounds present in biodiesel produced from waste coffee ground oil [
10], and glycerides [
27] are slightly soluble in the solvent used in this article.
To further put this work into the context of the existing literature,
Table 8 lists all published experiments with the same DES. Shabaz et al. (2010) found that the optimal DES to palm oil biodiesel molar ratio for all synthesized DESs was 2.5:1, which corresponds to approximately 0.71:1 DES to biodiesel mass ratio, for the same DES as the one used in this investigation. The optimum DES to biodiesel molar ratio for the removal of total glycerol was 1:1, which corresponds to approximately 0.29:1 DES to biodiesel mass ratio. When biodiesels were produced from different types of animal fats and subjected to purification with the same DES, the authors concluded that when batch extraction was conducted at an elevated temperature (50 °C), free glycerol content was reduced below the standard (EN 14214) specification [
27]. Extraction efficiency was higher for lower initial glycerol concentration and higher extraction temperature. Petračić et al. investigated the influence of the batch process conditions (extraction time, initial glycerol concentration, mixing rate, and DES to biodiesel mass ratio) as well as the influence of the pulsation rates in the continuous Karr column on the extraction efficiency of glycerol and glycerides from biodiesels synthesized from fresh and used sunflower oils. The optimal DES to biodiesel mass flow rate for batch experiments was found to be 0.50:1 when the concentration of glycerol was reduced below 0.02%. Biodiesel purified in the Karr column at a mixing intensity equal to 0.072 m s
−1 satisfied the quality standard. The higher extraction efficiency of continuous extraction was attributed to the larger DES to biodiesel mass ratio. Extraction of glycerides was also observed [
9]. Batch and continuous extraction of glycerol from biodiesel synthesized from waste coffee ground oils was compared by Sander et al. The free glycerol content of biodiesels purified in the Karr column meets the quality standard. Again, the higher DES to biodiesel mass ratio used in the continuous process resulted in higher extraction efficiency [
10]. Šalić et al. investigated the use of DESs in a microreactor and compared their extraction efficiency to wet washing, with biodiesels from sunflower oil and household waste cooking oil. They used the same DES as in this work and several DESs with choline chloride and glycerol, both with and without water. Their results showed that far less DES was needed when compared to water and that DESs were very efficient at reducing free glycerol content. In their work, DESs with glycerol were found to be even more efficient than DES with ethylene glycol [
8]. It may be that the increased viscosity of the glycerol DES influences hydrodynamics in a microreactor in a different way than when used on a macro scale. Critical analysis of all published data regarding the extraction of glycerol and glycerides leads to the following conclusions: (1) initial glycerol content, feedstock chemical composition, and temperature influence the extraction efficiency of glycerol and glycerides; (2) higher DES to biodiesel mass ratio in the continuous column should be used to enhance the extraction efficiency of glycerol; (3) hydrodynamic conditions show a greater influence for continuous processes.
Continuous processes offer several advantages over batch processes. They provide consistent and uninterrupted production, leading to higher efficiency and throughput. Additionally, they enable real-time monitoring and control, facilitating rapid adjustments to optimize product quality and resource utilization.
The feedstock used in this investigation had a small initial amount of glycerol and the DES to biodiesel mass ratio was significantly smaller than for other continuous experiments found in the literature, which may explain lower glycerol extraction efficiency due to a lower mass transfer driving force.