Abstract
Removal of organic pollutants and metal ions from produced water by adsorption, using prepared activated carbon (AC) from sewage sludge, with chemical activations using NaOH, KOH and ZnCl2 separately and pyrolysis at different temperatures (500, 600 and 700 °C). Pure sludge and prepared ACs were analyzed using FTIR and XRD. The results showed 18% crystallinity compared to that of commercial AC, which has 44% crystallinity. The results of FTIR demonstrate that the properties of the post-treated affect the final products depending on the method used and that it contains similar functional groups to those present in the commercial AC, but at a higher peak intensity. Adsorption treatments were carried out at 25, 35 and 45 °C solution temperatures. The results showed that the removal of pollutants from produced water using prepared AC with all types of chemical activations reached 99.5%, such as commercial AC with 0.06 g dosage of adsorbent at pyrolysis temperatures of 500 and 600 °C and a solution temperature of 25 °C. The obtained results refer to the mechanism of exothermic reaction and physical adsorption. It was observed that despite the lower dosage of adsorbent of 0.01 g, a sufficient treatment of pollutants was achieved. This reveals the effectiveness of using sewage sludge as a cheap adsorbent. Also, using pure sewage sludge, the adsorption data showed a 95.2% removal of the pollutants. This result indicated that pure sludge has an efficient adsorption capacity and can be utilized as a cheap and environmentally friendly material. For the removal of manganese and cadmium metal ions from the produced water, the resultant data showed that more than 90% of manganese was adsorbed and more than 97% of cadmium was adsorbed, especially when using pure sewage sludge and prepared activated carbon with NaOH chemical activation at pyrolysis temperatures of 500 °C and 600 °C.
1. Introduction
Oil production is associated with a huge amount of produced water. Globally, the production of produced water from oilfields is expected to increase over the next 10 years from about 158,900 million barrels/day to 243,000 million barrels/day, due to increased demand for oil [,]. More than 40% of produced water is discharged into the environment []. In Oman a large amount of produced water, around 900,000 m3, is generated each day as a byproduct of its 135,000 m3 of oil production per day. In other words, for every barrel of oil there will be nine barrels of produced water []. A similar scenario occurred in Nigeria in which the oil–to–water ratio could be 1:10 []. Produced water is a waste byproduct of the oil and gas industry that contains relatively high concentrations of hydrocarbons, including dissolved and dispersed oils, grease, heavy metals, radionuclides, treating chemicals, formation solids, salts, dissolved gases, scale products, waxes, microorganisms, and dissolved oxygen []. Currently, the treatment of produced water is being performed through various methods, including physical, chemical, and biological methods [,,]. The importance of the treatment of produced water encourages many researchers to focus on the topic and several treatment methods were investigated for optimal and economical methods, such as Humidification-dehumidification []; Ozonation []; Electrochemical and Electrocoagulation techniques [,]; Filtration and reverse osmosis []; Advanced oxidation process []; Chemical coagulation []; etc.
The major component of sewage sludge is organic material, which comprises between 60% and 80% of sewage sludge [,], the sludge is utilized for producing activated carbon. There are many sources to produce activated carbon [,,,], but producing activated carbon from sewage sludge will have many economic and environmental advantages. Activated carbon-based sludge can be prepared by either direct pyrolysis or pyrolysis followed by a physical activation process, chemical activation process, or physical-chemical activation process [,,]. Gupta and Garg stated that the poor management of sewage sludge, which is a by-product from sewage treatment plants, is a major environmental issue. Being carbonaceous in nature, the waste residue can be converted into activated carbon for utilization in wastewater treatment plants to remove toxic and persistent organic pollutants. This study investigated the performance of sewage sludge-derived adsorbents for the removal of recalcitrant lignin (found in paper and pulp mill effluents) from synthetic wastewater []. The adsorbent was prepared from primary sewage sludge using a chemical activation process employing a strong base, KOH. Aliakbari, et al. [] prepared activated carbons from sewage sludge by chemical activation with phosphoric acid (H3PO4), acid (HCl, HF), and base (NaOH) as post-treatment steps, which were employed to eliminate the mineral matter and other impurities of AC based sewage-sludge. Microwave radiation could be utilized to produce activated carbon from sewage sludge []. Zaker Ali et al. [] published a detailed review paper on the microwave-assisted pyrolysis of sewage sludge. They have reported that the main advantages of the microwave pyrolysis process lie in reducing energy consumption and processing time during microwave heating. They have also mentioned that the quality and properties of microwave-assisted pyrolysis products are dependent on many factors, including sewage sludge composition and conditions employed, such as the process temperature, heating rate, and time. Yu-Huan Li, et al. [] reported a method for preparing sludge-activated carbon (SAC) by activating sludge pyrolysis carbon by zinc chloride. The effect of ZnCl2 concentration, activation temperature, and activation time on the activated carbon were investigated. The prepared activated carbons were characterized (TGA, XRD, FTIR, SEM, and N2 adsorption-desorption) and tested for their adsorption ability of iodine and methylene blue. According to the data obtained, sludge pyrolysis char is a suitable precursor for activated carbon preparation, and the obtained SAC could be used as a low-cost adsorbent with favorable surface properties. Dos Res et al., [] have reported a comparison study of activated carbon prepared from sewage sludge by using two types of pyrolysis: conventional furnace and microwave. In their experiments, it was reported that the activated carbon made by conventional pyrolysis possessed higher surface area values overall than the samples synthesized using the microwave. The optimum parameters for the preparation of activated carbon using conventional pyrolysis have been identified as a pyrolysis temperature of 500 °C, a holding time of 15 min, and a ratio of ZnCl2: sludge of 0.5. Microwave pyrolysis is found to be optimal when operating at 980 W for 12 min. The analysis of nitrogen adsorption/desorption isotherms revealed the presence of micro- and mesopores in the activated carbon.
In this study, the treatment of produced water was carried out using prepared activated carbon-based sewage sludge and chemical-physical activation using NaOH, KOH, and ZnCl2 separately. Pyrolysis was performed at different temperatures (500, 600, and 700 °C), followed by physical activation using carbon dioxide. The treatment was compared to that of using commercial AC and sewage sludge.
2. Materials and Methods
Sewage sludge samples were collected from an aeration tank at Nizwa Sewage Treatment Plant (STP). Activation chemical agent: 1 M of each solution, KOH, NaOH, and ZnCl2, were used. Chemicals were provided by the chemistry lab at the University of Nizwa.
The produced water sample was provided by Petroleum Development Oman (PDO). The properties of the produced water were as follows: UV absorption: 0.59; Conductivity: 5.47 , and pH: 7.6. The sample was used to evaluate the potential efficiency of the developed adsorbents for the removal of pollutants from produced water.
Surface morphology and chemical structure of treated sludge were investigated using XRD (BRUKER, model D8 DISCOVER, Karlsruhe, Germany, with CuKα radiation, and their diffraction patterns were recorded between diffraction angles of 0° and 90°) and FTIR (model TENSOR 37-UK). Both devices are available at Daris laboratory at the University of Nizwa.
2.1. Preparation of Sewage Sludge Sample for Activated Carbon
Two liters of sewage sludge samples were filtrated using a piece of cloth, then, air-dried in a clean place for two days. Samples of 30 g of dried sludge were chemically activated using 100 mL of 1 M NaOH, KOH, and ZnCl2 separately. The solution was stirred using an orbital shaker at 200 rpm for 24 h at room temperature. Then, samples were thoroughly washed using distilled water several times until the pH reached 8, then samples were dried in an electrical oven at 105 °C for one hour.
Pyrolyzed materials were obtained from the dried sewage sludge (10 g) by using thermal treatment in a tubular furnace under nitrogen gas at different temperatures (500, 600 and 700 °C) for two hours with a heating rate of 10 °C/min. Carbon dioxide gas was used for physical activation at 400 °C for one hour. The resulting adsorbent was crushed and sieved to 300 μm.
2.2. Adsorption Study
Experiments of batch adsorption were carried out using different dosages; 0.1, 0.2, 0.4, and 0.6 g of the prepared activation carbon for treatment of 50 mL of produced water at different temperatures: 25, 35, and 45 °C, respectively. Samples were mixed using an Orbital shaker at 200 rpm for 8 h (an equilibrium time). Then, samples were filtered through filter paper. The pollutants in the produced water were determined using the UV absorption technique. The percentage of removal of pollutants was calculated as follows:
where UV° and UV are the ultraviolet absorption measurements of initial and treated produced water samples.
Also, to test the capability of the prepared activated carbon adsorbents to adsorb metal ions from produced water, the availability of metal ions in the sample of produced water was determined using the 8000-ICP-OES double monochromator (PerkinElmer, Inc., Waltham, MA, USA) device available at the University of Nizwa-Daris Center. Table 1 shows the concentration of metals present in the produced water. Two metals were selected to perform the adsorption test: cadmium and manganese. The adsorption test was performed, and batch experimental tests were carried out using 0.05 g of adsorbent with 50 mL of produced water at a temperature of 25 °C and contact time of 8 h.

Table 1.
Concentration of some metals available in produced water.
3. Results and Discussion
A comparative study of the prepared sewage sludge adsorbents at different pyrolysis temperatures was performed and compared with that of commercial activated carbon and pure non-treated sewage sludge.
3.1. Characterization of Prepared Activated Carbon-Based Sewage Sludge
The XRD was examined for prepared AC at a pyrolysis temperature of 500 °C and the results are presented in Table 2. The results showed that the crystallinity of the commercial AC shows a crystallinity content of 44.6% and the rest are amorphous structures, while the prepared activated carbon from pure sludge has 15.2% crystallinity. Most of the prepared AC with or without chemical activation showed a similar percentage of crystallinity, the highest crystallinity was obtained for that which was chemically activated using NaOH, of which the crystallinity is 23.6%.

Table 2.
XRD of prepared and commercial activated carbon.
To identify the functional groups of the prepared activated carbon-based sewage sludge with and without chemical activations, pure sludge, or commercial activated carbon, which are responsible for the adsorption of pollutants from produced water, Fourier transform infrared spectroscopy (FTIR) spectrum of all samples were recorded. Figure 1, Figure 2 and Figure 3 show the results of the FTIR spectra. The presence of functional groups in the activated carbon mainly depends on the starting precursor and the activation method used. FTIR spectra of the commercial activated carbon is presented in Figure 1 and compared with that of the chemically activated carbon. The commercial activated carbon exhibited two characteristic bands at 2360 and 2332 cm−1, which corresponds to the presence of O=C=O. The FTIR of sewage sludge samples (Figure 2a,b) exhibit wave-number range 990–1000 cm−1, which corresponds to the C=C bending, and wavenumbers in the range 1210–1370 cm−1 correspond to the C-N stretching. The chemically activated carbon samples have exhibited similar absorption patterns in the range of 2300–2350 cm−1 to that of commercial activated carbon (Figure 3a–c). The CO2 functional group existed due to the physical treatment carried out in the presence of CO2 gas. The absorption in the range of 1730–1780 cm−1 was observed in chemically activated carbon samples due to the presence of aromatic C=O stretching. The wavenumber range 990–1000 cm−1 corresponds to the C=C bending. The wavenumbers in the range 1210–1370 cm−1 correspond to the C-N stretching. The activated carbon prepared from the sewage sludge contains heavy metals and other impurities which are corresponding to the many miscellaneous peaks.
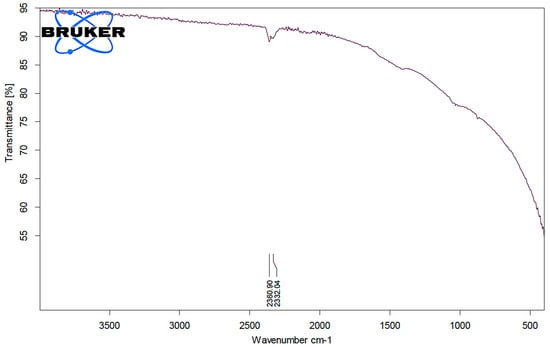
Figure 1.
FTIR analysis of commercial activated carbon.
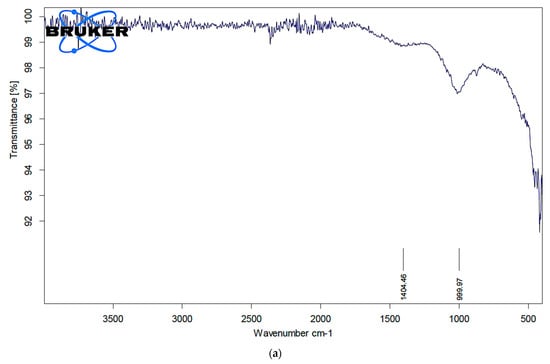
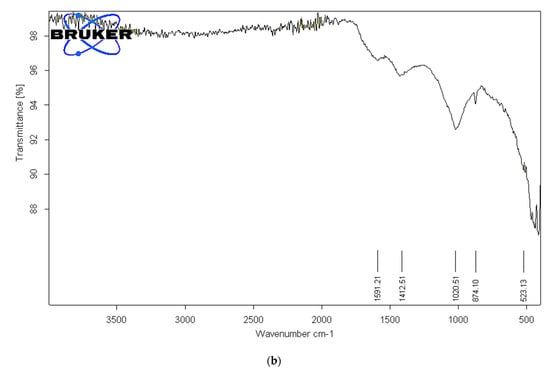
Figure 2.
(a). FTIR analysis of pure sewage sludge. (b). FTIR analysis of activated carbon from sludge without chemical activation at 500 °C.
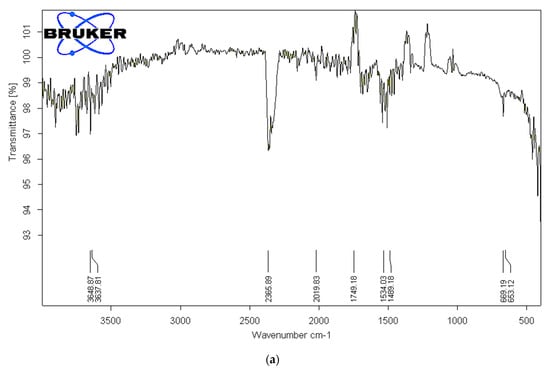
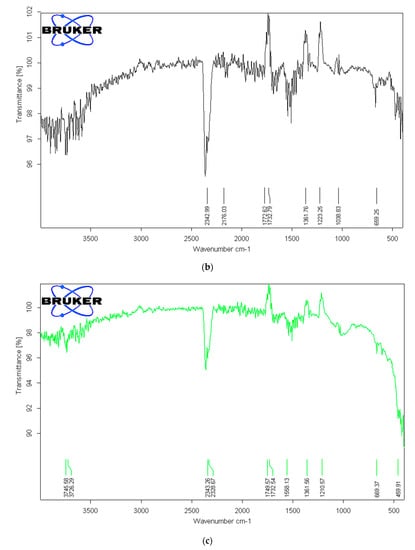
Figure 3.
(a). FTIR analysis of activated carbon from sludge chemically activated with ZnCl2 at 500 °C. (b). FTIR analysis of activated carbon from sludge chemically activated with NaOH at 500 °C. (c). FTIR analysis of activated carbon from sludge chemically activated with KOH at 500 °C.
3.2. Produced Water Treatment Using Commercial AC
The adsorption data for the removal of pollutants from produced water by using commercial AC adsorbent at the optimized experiment conditions is represented in Figure 4. The adsorption data show that with the increase of the adsorbent dosage from 0.01 to 0.06 g the removal percentage of the pollutants was increased from 98.8% to 99.6%, especially at a solution temperature of 25 °C. Similar results were obtained at a solution temperature of 35 °C, while the removal percentage was much lower at a solution temperature of 45 °C (increased from 15% to 34.7%). This means that the highest adsorption affinity occurred at lower solution temperatures. The solution temperature has the highest effects on the adsorption process, which affects the rate of diffusion of the sorbate within the pores and may affect the number of sorption sites generated due to the breaking of some internal bonds near the edge of active surface sites of sorbent []. The decrease of the sorption capacities with the increase in solution temperature indicates an exothermic reaction followed by physical adsorption. These results agree with all previous results [,].
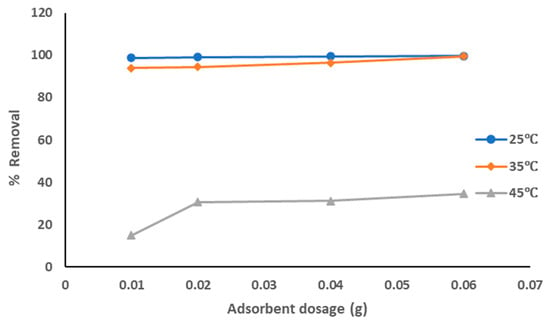
Figure 4.
Removal of pollutants from produced water using commercial AC.
3.3. Produced Water Treatment Using Pure Sewage Sludge
The adsorption data for the removal of pollutants from produced water by pure sewage sludge adsorbent at the optimized experiment conditions is represented in Figure 5. The adsorption data show that with the increase of the adsorbent dosage from 0.01 to 0.06 g the removal percentage of the pollutants was increased from 91% to 95.2%, especially at a solution temperature of 25 °C. The removal percentage was much lower at solution temperatures of 35 °C and 45 °C (around 10–20%). The highest adsorption affinity occurred at a lower solution temperature as temperature has the highest effects on the adsorption process, as explained above. The decrease of the sorption capacity with the increase of solution temperature refers to an exothermic reaction. The results showed that pure sewage sludge has an efficient capacity for adsorption at room temperature.
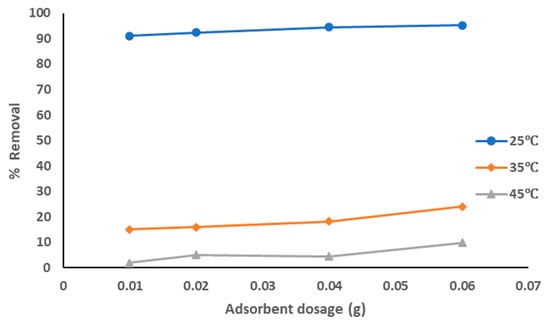
Figure 5.
Removal of pollutants from produced water using pure sewage sludge.
3.4. Produced Water Treatment Using Prepared AC from Sludge without Chemical Activation
The adsorption data for the removal of pollutants from produced water by prepared AC from sludge without chemical activation adsorbent at the optimized experiment conditions is represented in Figure 6a–c at different pyrolysis temperatures. The adsorption data show that with the increase of the adsorbent dosage from 0.01 to 0.06 g the removal percentage of the pollutants was increased: from 94% to 97.5% with pyrolysis temperature 500 °C; from 96 to 100% with pyrolysis temperature 600 °C from 88% to 100% with pyrolysis temperature 700 °C, especially at a solution temperature of 25 °C and similar results at 35 °C. The removal percentage was much lower at a solution temperature of 45 °C (around 10–26% with all pyrolysis temperatures). Also, the highest adsorption affinity occurred at lower solution temperatures as the temperature has the highest effects on the adsorption process as explained above. The decrease of the sorption capacities with the increase of solution temperature refers to an exothermic reaction. The results showed that pure sewage sludge has an efficient capacity for adsorption at room temperature. These results clearly show that the best treatment conditions were at room temperature with a pyrolysis temperature of 500–600 °C and most dosages of prepared AC. The obtained results agreed with the previous results [].
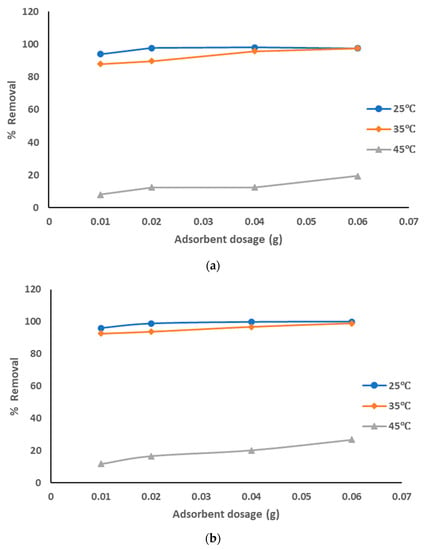
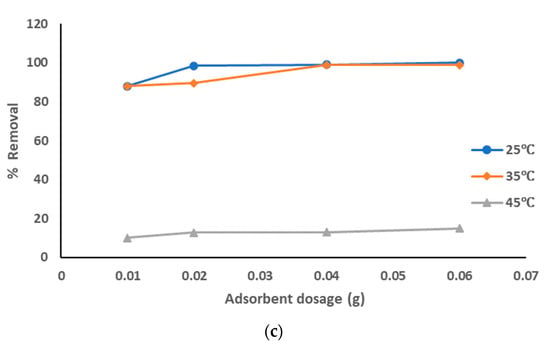
Figure 6.
(a). Removal of pollutants from produced water using prepared AC without chemical activation at a pyrolysis temperature of 500 °C. (b). Removal of pollutants from produced water using prepared AC without chemical activation at a pyrolysis temperature of 600 °C. (c). Removal of pollutants from produced water using prepared AC without chemical activation at a pyrolysis temperature of 700 °C.
3.5. Produced Water Treatment Using Prepared AC from Sludge Activated with ZnCl2
The adsorption data for the removal of pollutants from produced water, by using prepared AC from sludge with ZnCl2 chemical activation adsorbent at the optimized experiment conditions, is represented in Figure 7a–c at different pyrolysis temperatures. The adsorption data show that with the increase of the adsorbent dosage from 0.01 to 0.06 g, the removal percentage of the pollutants was increased: from 96 to 99% with pyrolysis temperature 500 °C; from 98 to 99% with pyrolysis temperatures 600 °C and 700 °C, especially at solution temperature of 25 °C. However, results of the removal of pollutants were lower at both 35 °C (around 25 to 55% with all pyrolysis temperatures) and 45 °C (around 12 to 25% with all pyrolysis temperatures). It is clear that the highest adsorption affinity occurred at lower solution temperatures. The decrease of the sorption capacities with the increase of solution temperature refers to an exothermic reaction. The results showed that pure sewage sludge has an efficient capacity for adsorption at room temperature. These results show that the best treatment conditions are at room temperature with pyrolysis temperatures of 500–700 °C, using the highest dosage of prepared AC that activated using ZnCl2. The activation using ZnCl2 suppressed the release of small organic molecules and enhanced the functional groups, hence increasing the quality of prepared AC.
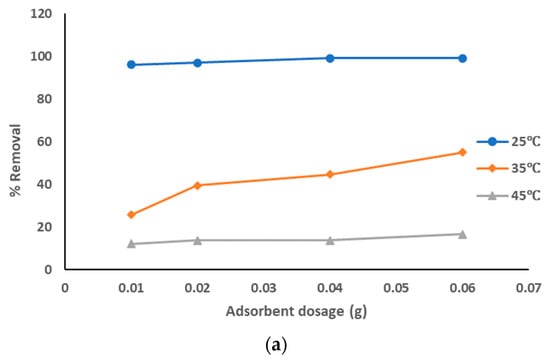
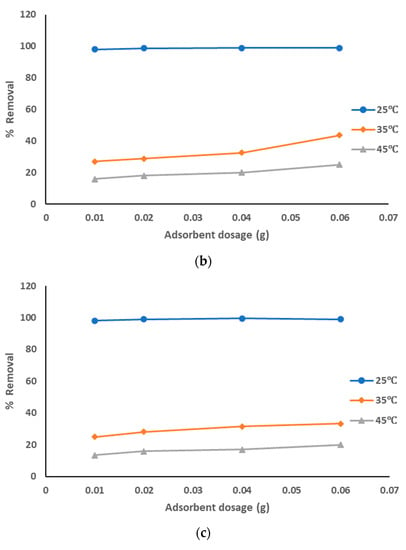
Figure 7.
(a). Removal of pollutants from produced water using prepared AC with ZnCl2 at a pyrolysis temperature of 500 °C. (b). Removal of pollutants from produced water using prepared AC with ZnCl2 at a pyrolysis temperature of 600 °C. (c). Removal of pollutants from produced water using prepared AC with ZnCl2 at a pyrolysis temperature of 700 °C.
3.6. Produced Water Treatment Using Prepared AC from Sludge Activated with NaOH
The adsorption data for the removal of pollutants from produced water by using prepared AC from sludge with NaOH chemical activation adsorbent at the optimized experiment conditions is represented in Figure 8a–c at different pyrolysis temperatures. The adsorption data show that with the increase of the adsorbent dosage from 0.01 to 0.06 g, the removal percentage of the pollutants was increased: from 90 to 98% with a pyrolysis temperature of 500 °C; from 92 to 94% with pyrolysis temperatures 600 °C and 700 °C, especially at solution temperature of 25 °C. However, results of the removal of pollutants were lower at both 35 °C (around 35 to 55% with all pyrolysis temperatures) and 45 °C (around 6 to 17% with all pyrolysis temperatures). The highest adsorption affinity occurred at lower solution temperatures. The decrease of the sorption capacities with the increase of solution temperature refers to an exothermic reaction. The results showed that pure sewage sludge has an efficient capacity for adsorption at room temperature. These results show the best treatment conditions were at room temperature with all pyrolysis temperatures of 500 °C and using the highest dosages of prepared AC that activated using NaOH. The activation using NaOH suppressed the release of small organic molecules and enhanced the functional groups, hence increasing the quality of prepared AC.
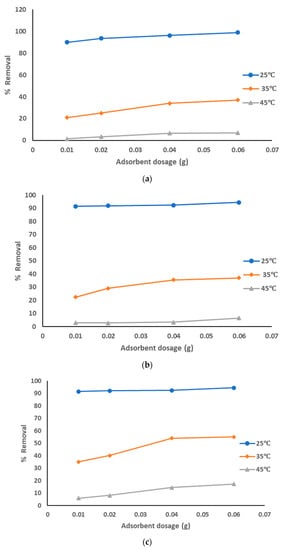
Figure 8.
(a). Removal of pollutants from produced water using prepared AC with NaOH at a pyrolysis temperature of 500 °C. (b). Removal of pollutants from produced water using prepared AC with NaOH at a pyrolysis temperature of 600 °C. (c). Removal of pollutants from produced water using prepared AC with NaOH at a pyrolysis temperature of 700 °C.
3.7. Produced Water Treatment Using Prepared AC from Sludge Activated with KOH
The adsorption data for the removal of pollutants from produced water, using prepared AC from sludge with KOH chemical activation adsorbent at the optimized experiment conditions, is represented in Figure 9a–c at different pyrolysis temperatures. The adsorption data show that with the increase of the adsorbent dosage from 0.01 to 0.06 g, the removal percentage of the pollutants was increased from 91 to 95% with all pyrolysis temperatures 500–700 °C; from 92 to 94% with pyrolysis temperatures 600 °C and 700 °C, especially at a solution temperature of 25 °C. However, results of the removal of pollutants were lower at both 35 °C and 45 °C (around 5 to 15% with all pyrolysis temperatures). The highest adsorption affinity occurred at lower solution temperatures. The decrease of the sorption capacities with the increase of solution temperature refers to an exothermic reaction. The results showed that pure sewage sludge has an efficient capacity for adsorption at room temperature. These results show that the best treatment conditions were at room temperature with all pyrolysis temperatures of 500–700 °C and with the highest dosage of prepared AC that activated using KOH. The activation using KOH suppressed the release of small organic molecules and enhanced the functional groups, hence increasing the quality of prepared AC.
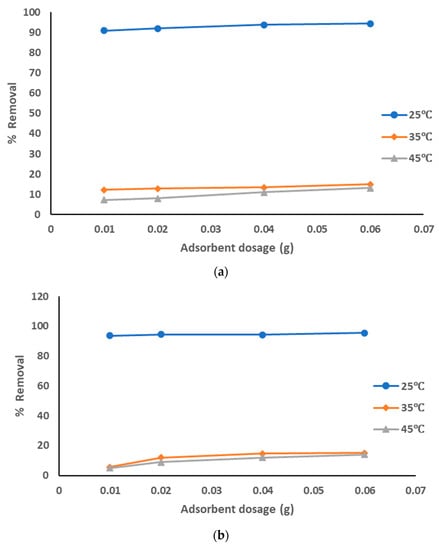
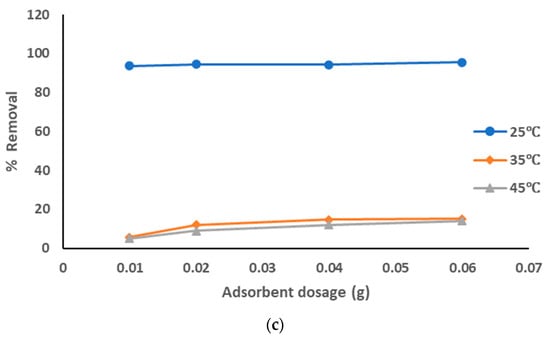
Figure 9.
(a). Removal of pollutants from produced water using prepared AC with KOH at a pyrolysis temperature of 500 °C. (b). Removal of pollutants from produced water using prepared AC with KOH at a pyrolysis temperature of 600 °C. (c). Removal of pollutants from produced water using prepared AC with KOH at a pyrolysis temperature of 700 °C.
3.8. Adsorption of Metals in Produced Water
The concentration of manganese and cadmium ions after adsorption and percentage removal are shown in Figure 10 and Figure 11. Despite the considerable contents of metals available in the sludge sample, the adsorption data show that more than 90% of manganese was adsorbed and more than 97% of cadmium was adsorbed, especially when using pure sewage sludge and prepared activated carbon with NaOH chemical activation and pyrolysis temperatures of 500 °C and 600 °C.
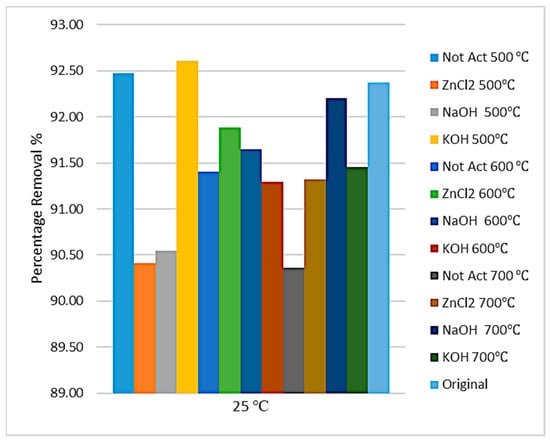
Figure 10.
Removal of manganese ion from produced water at 25 °C.
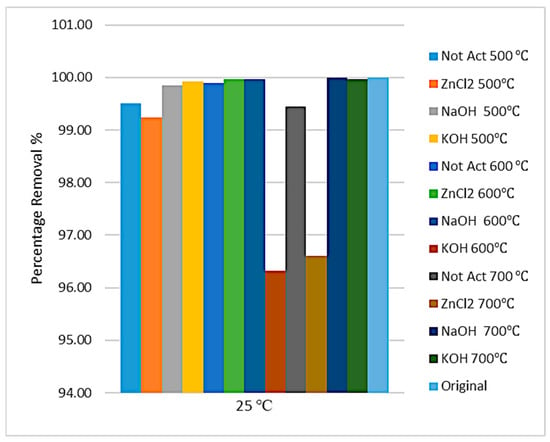
Figure 11.
Removal of cadmium ion from produced water at 25 °C.
Using pure sewage sludge, the adsorption data showed the removal of 93% manganese and 100% cadmium. This result indicated that pure sludge has an efficient adsorption capacity and can be utilized as a cheap and environmentally friendly material.
4. Conclusions
The aim of this project was to investigate the feasibility of the effect of using activated carbon prepared from sewage sludge with chemical activation using 1 M of NaOH, KOH, and ZnCl2 at different pyrolysis temperatures: 500 °C, 600 °C and 700 °C for the treatment of produced water at different temperatures: 25, 35 and 45 °C. It was evident from the conducted experiments that removal of pollutants from produced water using prepared AC reached 99.5% with all types of chemical activations. Similar results were obtained using commercial AC. The optimum adsorption results were obtained at pyrolysis temperatures of 500 °C and 600 °C and solution temperature of 25 °C. Lower removal of pollutants was indicated at a solution temperature higher than 25 °C, which describes the properties of exothermic reaction and physical adsorption. XRD was found on the best crystallinity at chemical-activated pyrolysis at 500 °C NaOH ratio of 23%, compared with the commercial ratio of 44.6%. The results of metals removal from the produced water (manganese and cadmium ions) indicated 90% manganese and 97% cadmium, especially when using pure sewage sludge and prepared activated carbon with NaOH chemical activation and pyrolysis temperatures of 500 °C and 600 °C.
Author Contributions
Conceptualization, S.K.A.D., M.K.A.-S. and M.S.A.D.; Data curation, S.H.K.A.; Formal analysis, S.K.A.D. and G.M.S.A.M.; Funding acquisition, R.H.H. and H.N.H.; Methodology, M.K.A.-S. and G.M.S.A.M.; Project administration, R.H.H. and A.A.; Resources, M.K.A.-S., G.M.S.A.M. and S.H.K.A.; Software, S.H.K.A. and M.S.A.D.; Supervision, H.N.H. and A.A.; Validation, S.K.A.D.; Writing—original draft, S.K.A.D. and M.K.A.-S.; Writing—review & editing, G.M.S.A.M., M.S.A.D. and R.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Khalid University under grant number RGP.2/34/44.
Data Availability Statement
Not applicable.
Acknowledgments
The author extends their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Group Research Project under grant number RGP.2/34/44.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blanchard, R. The Status of Global Oil Production: 2023 Update. 2023. Available online: https://www.resilience.org/stories/2023-04-19/the-status-of-global-oil-production-2023-update/ (accessed on 3 March 2023).
- Produced Water Society. Global Hydrocarbon Production. 2020. Available online: https://www.producedwatersociety.com/ (accessed on 18 December 2019).
- Duraisamy, R.T.; Beni, A.H.; Henni, A. State of the Art Treatment of Produced Water, 1st ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Al Dawery, S.; AbdulMajeed, W.; Al Shukaili, S.; Thotireddy, C.; Al Amri, I. Produced Water Deoxygenation: Investigation of Nitrogen PurgingScheme-Parametric Study. Eng. Technol. J. 2022, 40, 1090–1104. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Ogolo, N.A.; Angelis-Dimakis, A.; Albert, O. Physicochemical assessment and treatment of produced water: A case study in Niger delta Nigeria. Pet. Res. 2023, 8, 87–95. [Google Scholar] [CrossRef]
- Alipour, Z.; Azari, A. COD removal from industrial spent caustic wastewater: A review. J. Environ. Chem. Eng. 2022, 8, 103678. [Google Scholar] [CrossRef]
- Global Water Intelligence. 2020. Available online: https://producedwatermiddleeast.com/index.php/2019/11/08/five-things-i-learned-at-pws-middle-east-2019/ (accessed on 10 October 2022).
- Abbas, A.J.; Gzar, H.A.; Rahi, M.N. Oilfield-produced water characteristics and treatment technologies: A mini-review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022; Volume 1058, p. 12063. [Google Scholar]
- Li, X.; Muraleedaaran, S.; Li, L.; Lee, R. A humidification-dehumidification process for produced water purification. Desalination Water Treat. 2010, 20, 51–59. [Google Scholar] [CrossRef]
- Martínez, S.B.; Pérez-Parra, J.; Suay, R. Use of ozone in wastewater treatment to produce water suitable for irrigation. Water Resour. Manag. 2011, 25, 2109–2124. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, G.; Cheng, G.; Wang, Y.; Fu, H. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to reverse osmosis membranes. Desalination 2014, 344, 454–462. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.; Sadrzadeh, M.; Pernitsky, D.; Bhattacharjee, S.; Hajinasiri, J. Treatment of an in situ oil sands produced water by polymeric membranes. Desalination Water Treat. 2016, 57, 14869–14887. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of produced water in the permian basin for hydraulic fracturing: Comparison of different coagulation processes and innovative filter media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Rio, S.; Faur-Brasquet, C.; Le Coq, L.; Le Cloirec, P. Production and characterization of adsorbent materials from industrial waste. Adsorption 2005, 11, 793–798. [Google Scholar] [CrossRef]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: Contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 10. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar Derived from Non-customized Matamba Fruit Shell as an Adsorbent for Wastewater Treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Yao, N.; Wang, X.; Yang, Z.; Zhao, P.; Meng, X. Characterization of solid and liquid carbonization products of polyvinyl chloride (PVC) and investigation of the PVC-derived adsorbent for the removal of organic compounds from water. J. Hazard. Mater. 2023, 456, 131687. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.; Ansari, Z.; Anwer, A.H.; Kim, S.H.; Nasar, A.; Shoeb, M.; Mashkoor, F. A review on metal-organic frameworks for the removal of hazardous environmental contaminants. Sep. Purif. Technol. 2023, 305, 122416. [Google Scholar]
- Bian, Y.; Yuan, Q.; Zhu, G.; Ren, B.; Hursthouse, A.; Zhang, P. Recycling of waste sludge: Preparation and application of sludge-based activated carbon. Int. J. Polym. Sci. 2018, 2018, 8320609. [Google Scholar] [CrossRef]
- Biswas, S.; Mishra, U. Treatment of Copper Contaminated Municipal Wastewater by Using UASB Reactor and Sand-Chemically Carbonized Rubber Wood Sawdust Column. BioMed Res. Int. 2016, 2016, 5762781. [Google Scholar] [CrossRef]
- Nageeb, M.; Rashed, M.S. Heavy Metals Removal from Wastewater by Adsorption on Modified Physically Activated Sewage Sludge. Arch. Org. Inorg. Chem. Sci. 2018, 1, 18–25. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Recycling of Sewage Sludge as Adsorbent for the Purification of Wastewater, Centre for Environmental Science and Engineering, Indian Institute of Technology Bombay, Powai, India. In Proceedings of the 5th International Conference on Sustainable Energy and Environmental Sciences (SEES 2016), Singapore, 22–23 February 2016. [Google Scholar]
- Aliakbari, Z.; Younesi, H.; Ghoreyshi, A.A.; Bahramifar, N.; Heidari, A. Production and characterization of sewage-sludge based activated carbons under different post-activation conditions. Waste Biomass Valorization 2018, 9, 451–463. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Advancement in the production of activated carbon from biomass using microwave heating. J. Teknol. 2017, 79, 79–88. [Google Scholar]
- Zaker, A.; Chen, Z.; Wang, X.; Zhang, Q. Microwave-assisted pyrolysis of sewage sludge: A review. Fuel Process. Technol. 2019, 187, 84–104. [Google Scholar] [CrossRef]
- Li, Y.H.; Chang, F.M.; Huang, B.; Song, Y.P.; Zhao, H.Y.; Wang, K.J. Activated carbon preparation from pyrolysis char of sewage sludge and its adsorption performance for organic compounds in sewage. Fuel 2020, 266, 117053. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Wilhelm, M.; de Almeida Silva, T.C.; Rezwan, K.; Sampaio, C.H.; Lima, E.C.; de Souza SM, G.U. The use of design of experiments for the evaluation of the production of surface rich activated carbon from sewage sludge via microwave and conventional pyrolysis. Appl. Therm. Eng. 2016, 93, 590–597. [Google Scholar] [CrossRef]
- Boudrahem, F.; Aissani Benissad, F.; Ait Amar, H. Batch sorption dynamics and equilibrium for the removal of lead ions from aqueous phase using activated carbon developed from coffee residue activated with zinc chloride. J. Environ. Manag. 2009, 90, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.L.; Lin, Y.; Chai, L.; Min, X.; Wang, Y.; Yan, F.; Pu, W. Biosorption behaviors of Cu2+, Zn2+, Cd2+ and mixture by waste activated sludge. Trans. Nonferrous Met. Soc. China 2006, 16, 1431–1435. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Xia, S.; Zhao, J.; Chovelon, J.M.; Renault, N.J. Biosorption of Cu (II) and Pb (II) from aqueous solutions by dried activated sludge. Miner. Eng. 2006, 19, 968–971. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration participation of calcium. Environ. Pollut. 2021, 287, 11756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).