Ultrasonic (US)-Assisted Electrocoagulation (EC) Process for Oil and Grease (O&G) Removal from Restaurant Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Sampling and Collection
2.2. Efficiency of US-Assisted EC Treatment Method
2.3. Statistical Optimisation Utilising RSM
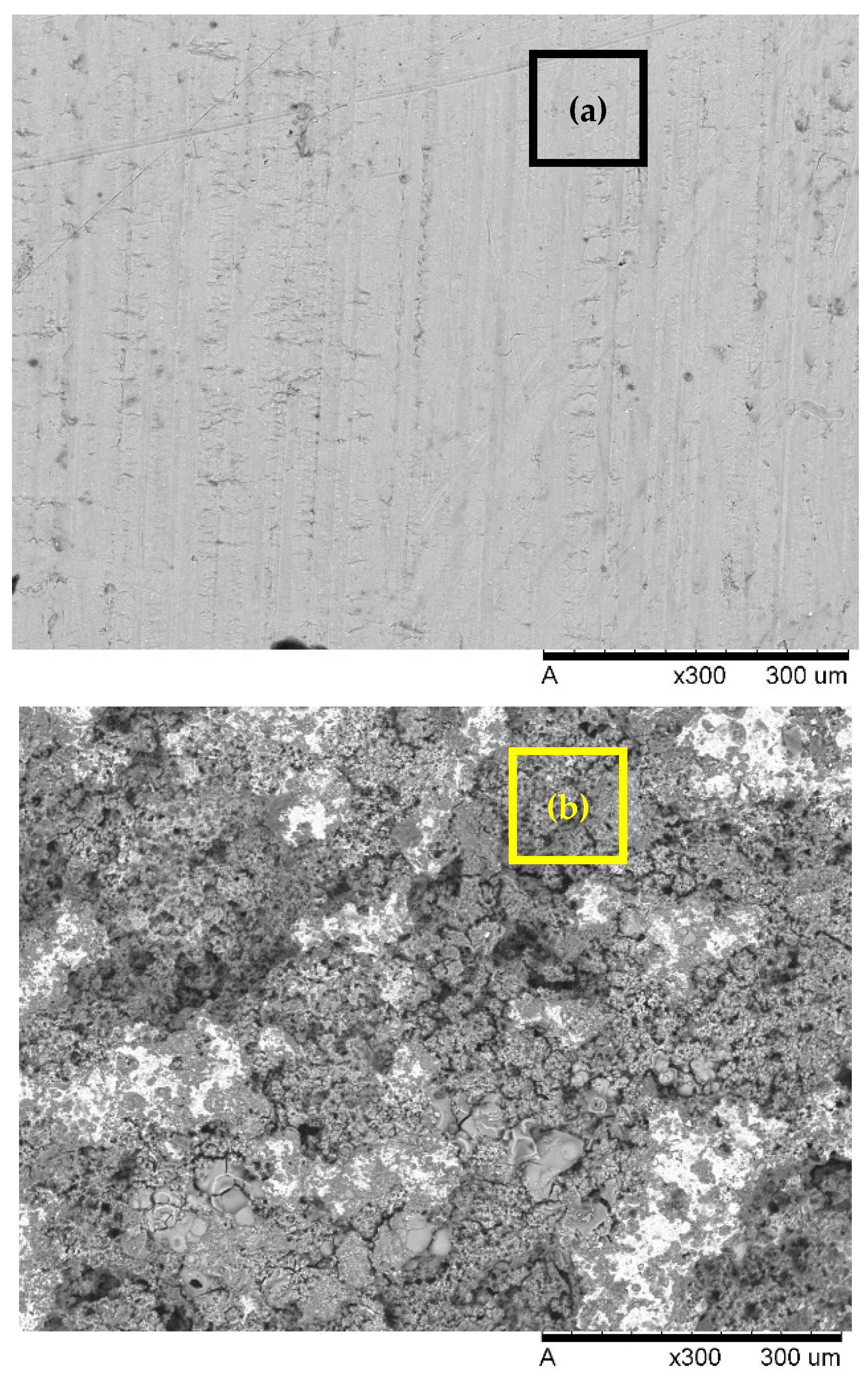
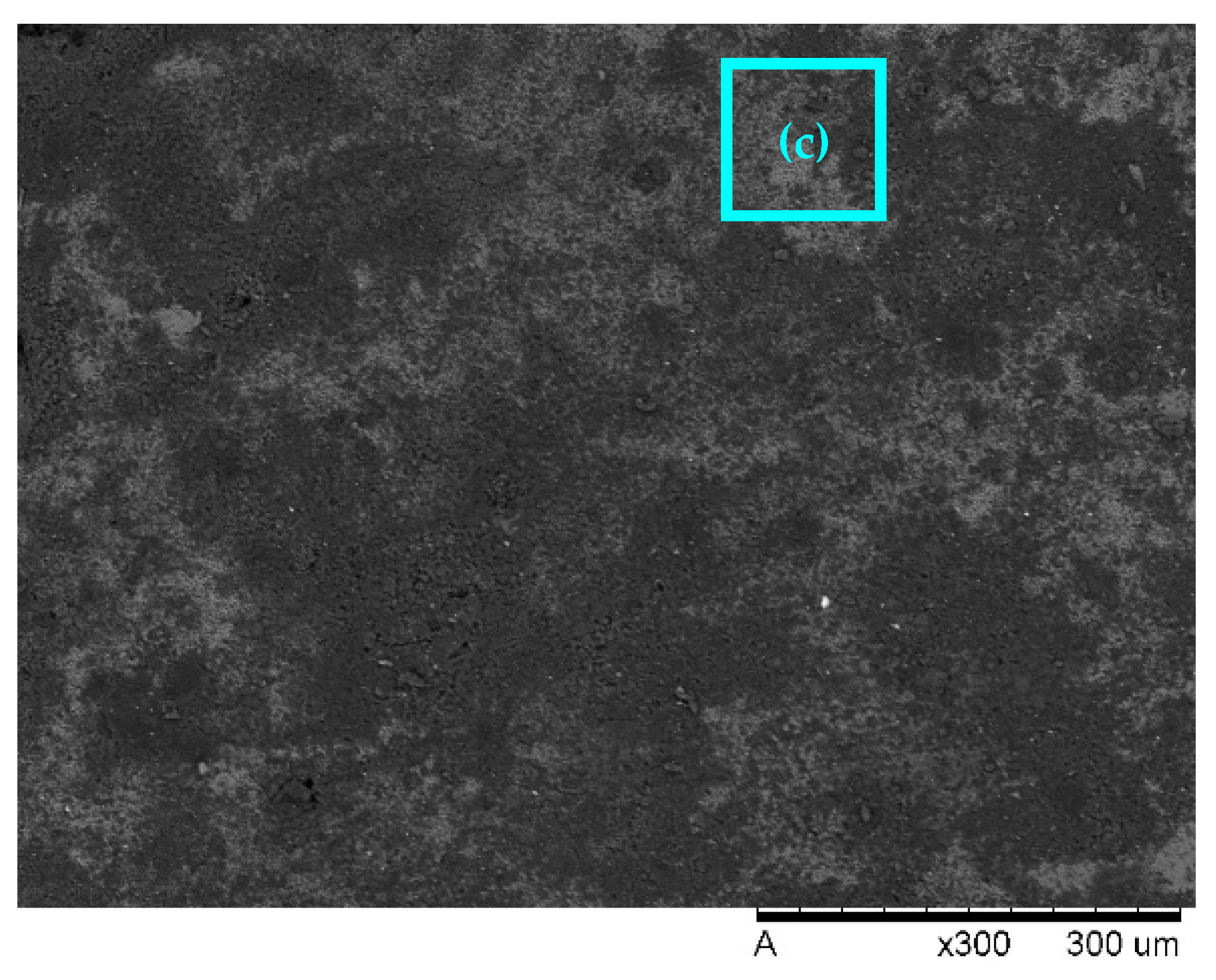
2.4. SEM
3. Results and Discussion
3.1. Batch Experiments for the EC Treatment Method
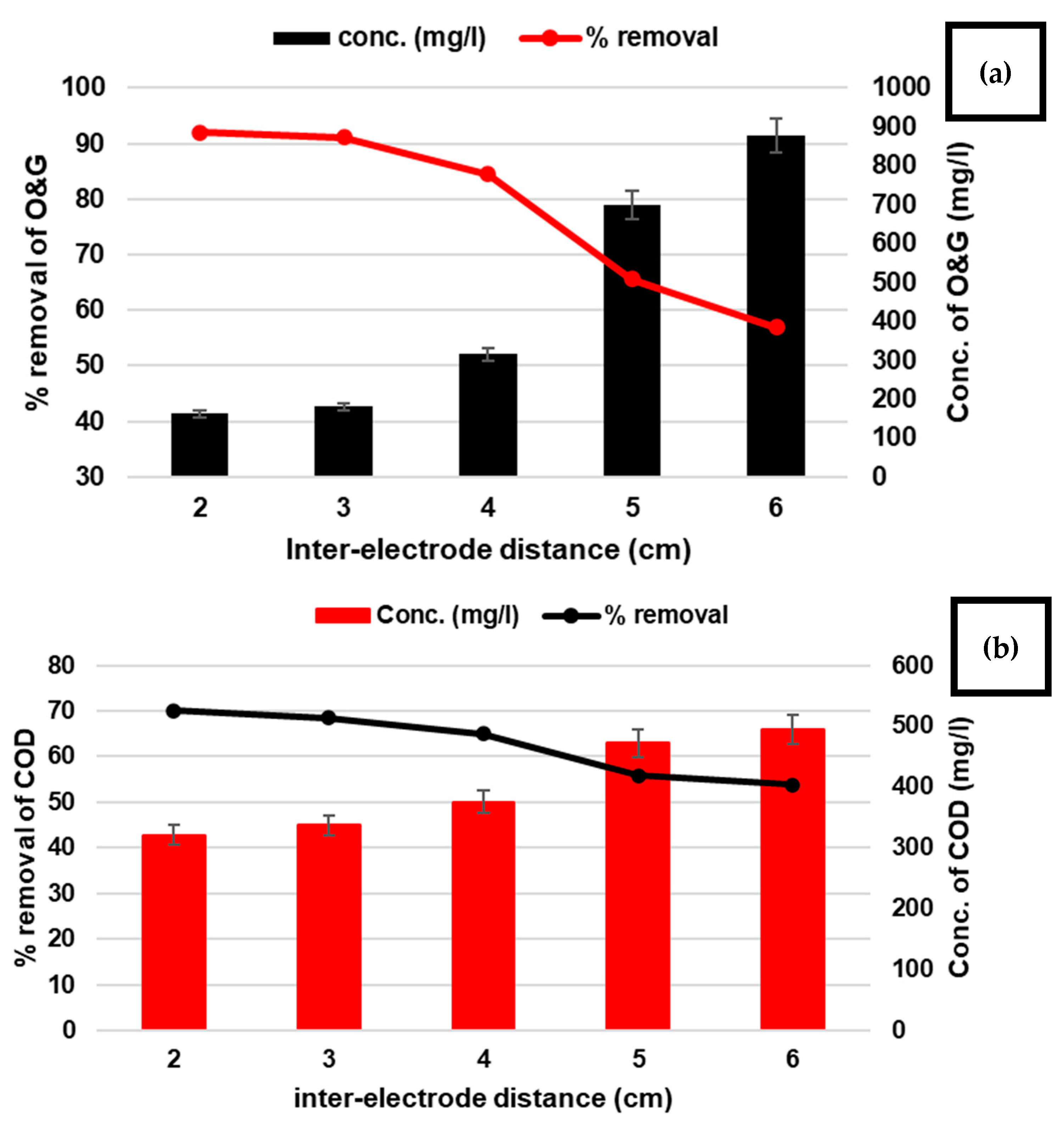
3.1.1. ID Factor
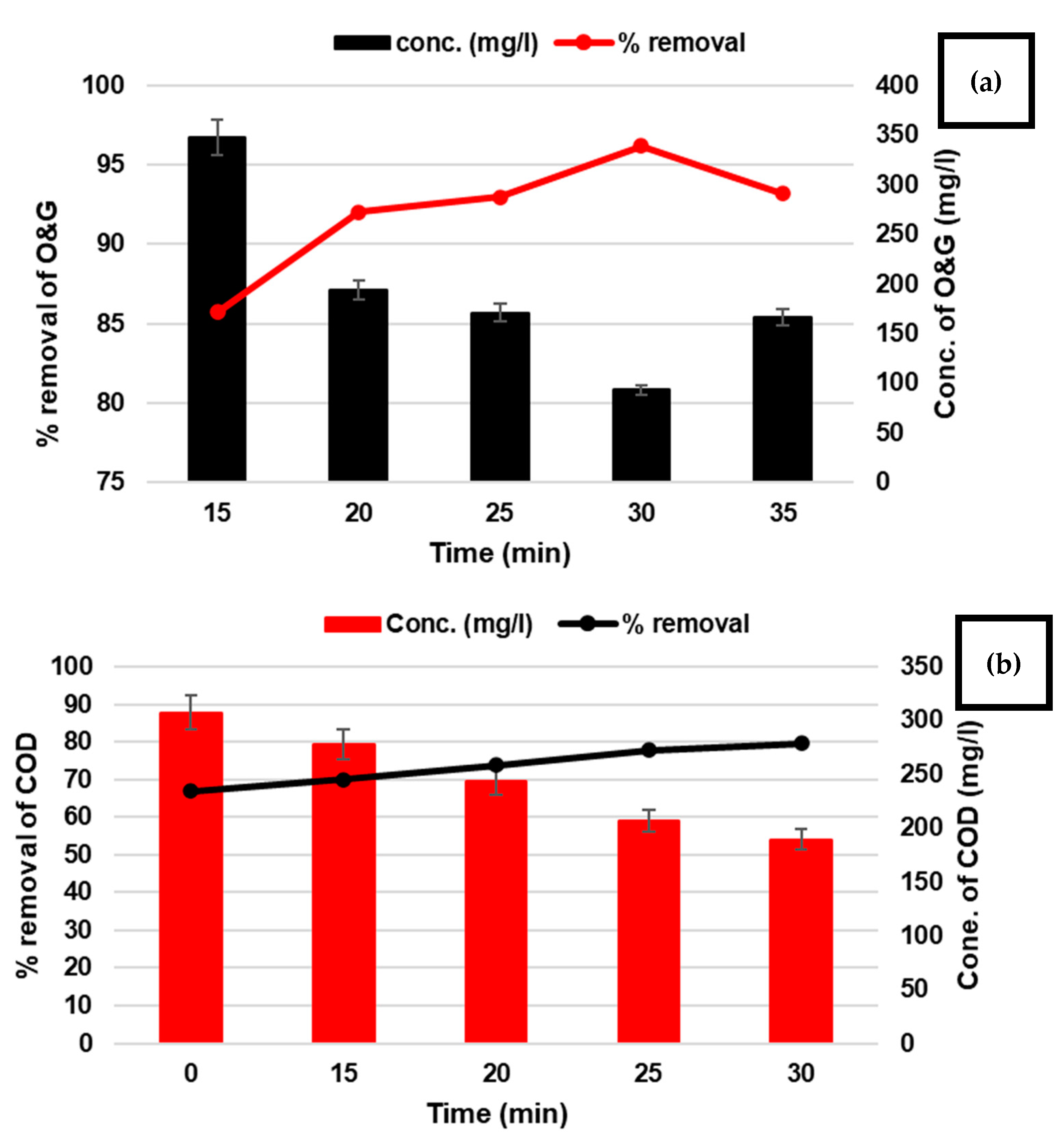
3.1.2. T Factor
3.1.3. CD Factor
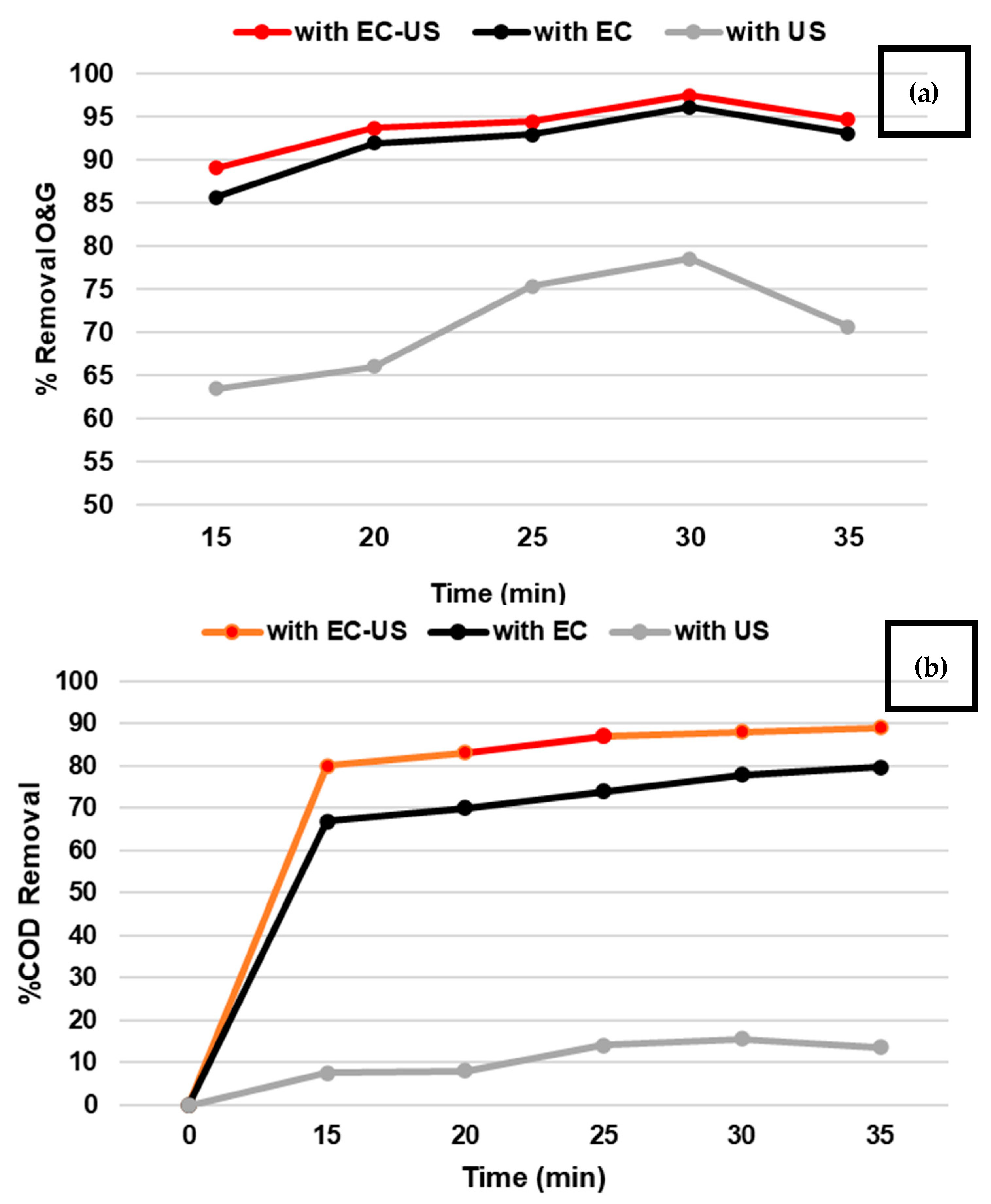
3.1.4. US, EC, and US-EC
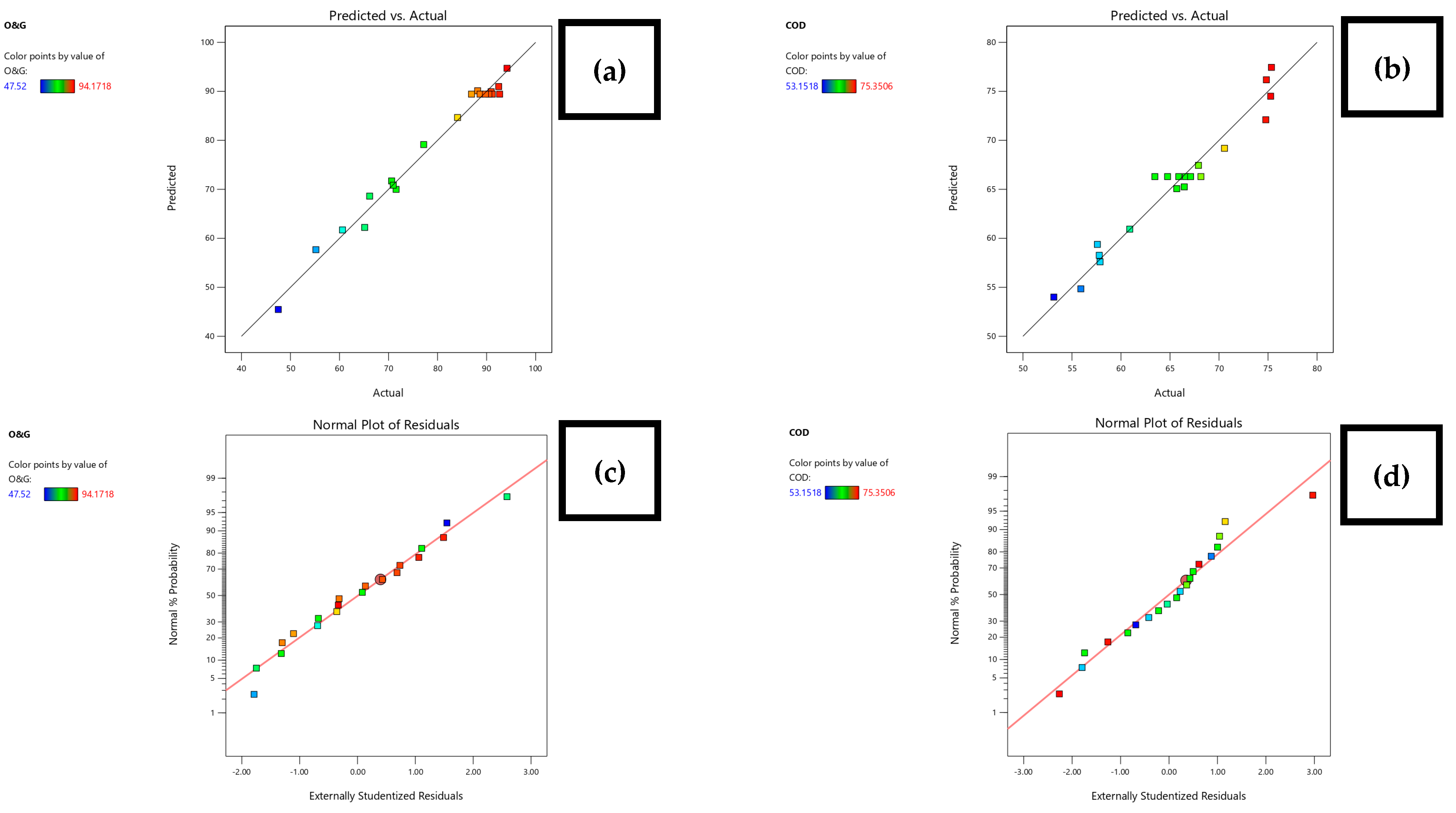
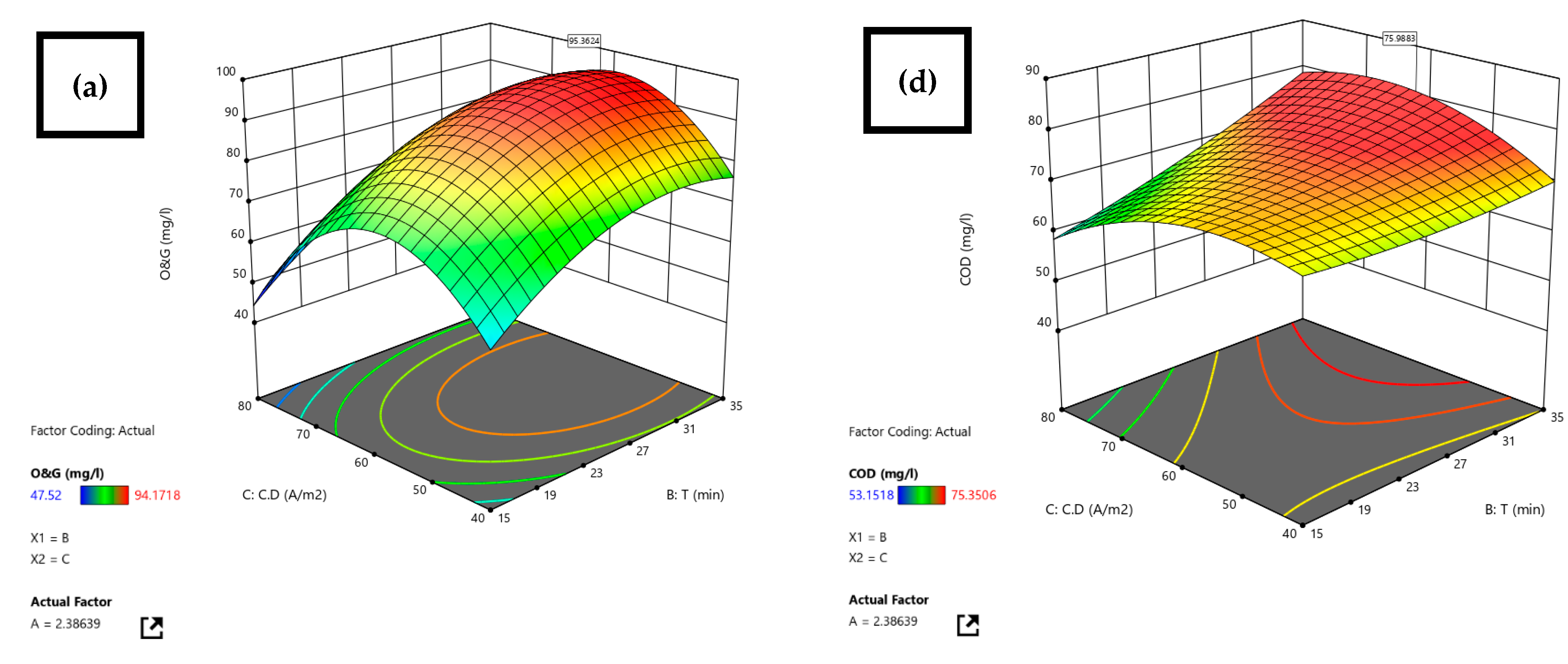
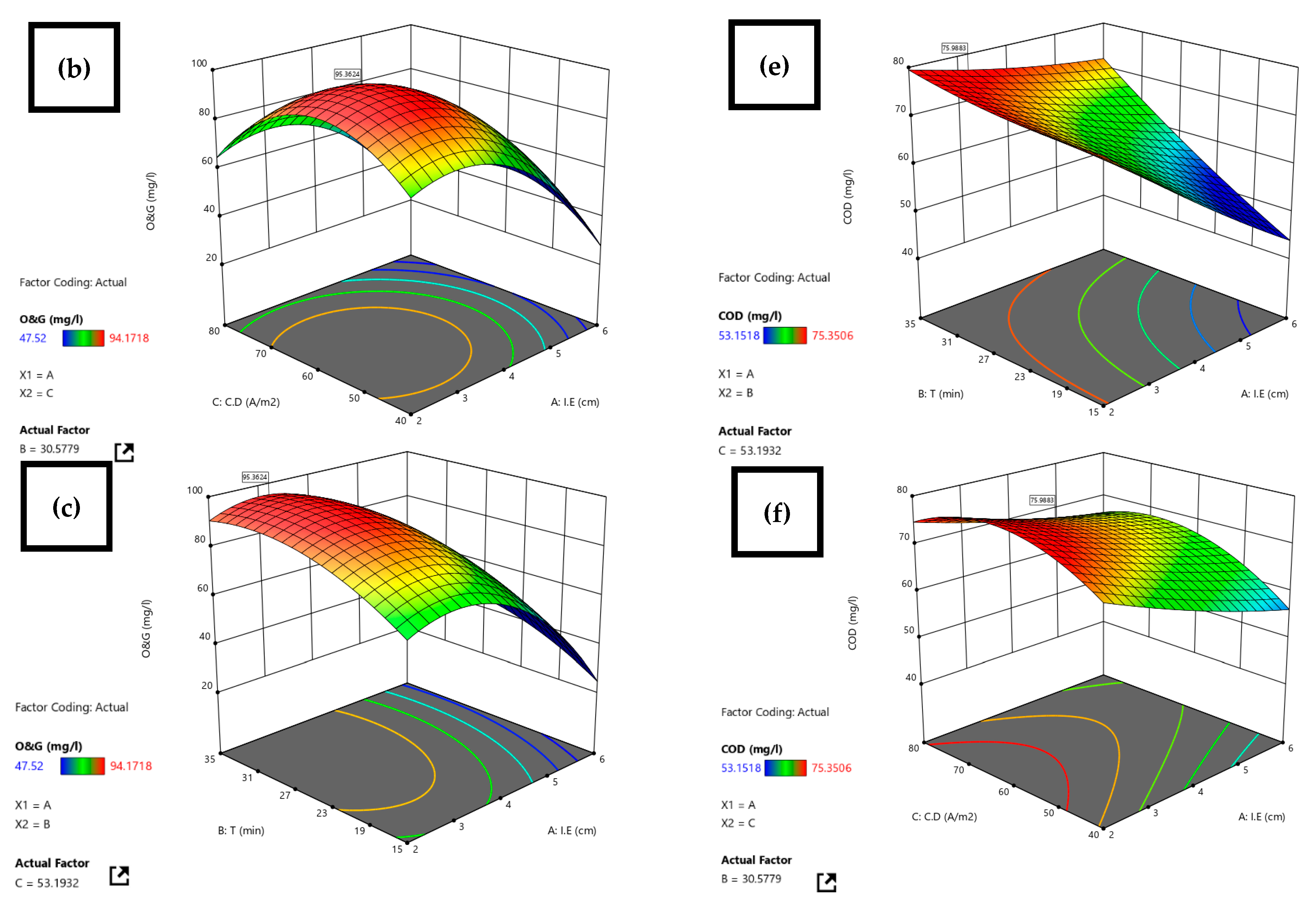
3.2. Statistical Analysis by RSM
3.3. SEM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Electrocoagulation | RSM | Response surface methodology |
| US | Ultrasonic | CCD | Central composite design |
| O&G | Oil and grease | ID | Interelectrode distance |
| COD | Chemical oxygen demand | T | Electrolysis time |
| BOD | Biological oxygen demand | CD | Current density |
| TSS | Total suspended Solids | SEM | Scanning electron microscopy |
| DO | Dissolved oxygen | Fe | Iron |
| TDS | Total dissolved solids | SS | stainless steel |
| TN | Total nitrogen | Al | Aluminium |
| C.V | Coefficient of variation | R2 | Coefficient of determination |
References
- El-Ezaby, K.H.; El-Gammal, M.I.; Shaaban, Y.A. Electrocoagulation Treatment for Wastewaters from some Restaurants in New Damietta City-Egypt. J. Environ. Sci. Mansoura Univ. 2020, 49, 87–98. [Google Scholar] [CrossRef]
- Yau, Y.-H.; Rudolph, V.; Lo, C.C.-M.; Wu, K.-C. Restaurant oil and grease management in Hong Kong. Environ. Sci. Pollut. Res. 2021, 28, 40735–40745. [Google Scholar] [CrossRef]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total. Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Fard, M.S. A critical review in electrocoagulation technology applied for oil removal in industrial wastewater. Chemosphere 2021, 288, 132355. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.; Gibbons, D.; O’Dwyer, M.; Curran, T.P. International evolution of fat, oil and grease (FOG) waste management—A review. J. Environ. Manag. 2017, 187, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; O’Hara, P.; Bertazzon, S.; Morgan, K.; Underwood, F.; Paquet, P. A preliminary spatial assessment of risk: Marine birds and chronic oil pollution on Canada’s Pacific coast. Sci. Total. Environ. 2016, 573, 799–809. [Google Scholar] [CrossRef]
- Gao, L.-L.; Lu, Y.-C.; Zhang, J.-L.; Li, J.; Zhang, J.-D. Biotreatment of restaurant wastewater with an oily high concentration by newly isolated bacteria from oily sludge. World J. Microbiol. Biotechnol. 2019, 35, 179. [Google Scholar] [CrossRef] [PubMed]
- Kalla, S. Use of membrane distillation for oily wastewater treatment–a review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Shi, Z.; Huang, P.; Li, J.; Zhang, J.; Wang, J. Separation of Pollutants from Oil-Containing Restaurant Wastewater by Novel Microbubble Air Flotation and Traditional Dissolved Air Flotation. Sep. Sci. Technol. 2015, 50, 2568–2577. [Google Scholar] [CrossRef]
- Sravanth, T.; Ramesh, S.; Gandhimathi, R.; Nidheesh, P.V. Continuous treatability of oily wastewater from locomotive wash facilities by electrocoagulation. Sep. Sci. Technol. 2019, 55, 583–589. [Google Scholar] [CrossRef]
- Jiang, W.-M.; Chen, Y.-M.; Chen, M.-C.; Liu, X.-L.; Liu, Y.; Wang, T.; Yang, J. Removal of emulsified oil from polymer-flooding sewage by an integrated apparatus including EC and separation process. Sep. Purif. Technol. 2018, 211, 259–268. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.-S.; Amin, N.; Fouad, Y.; Hamad, H. Intensification of a new electrocoagulation system characterized by minimum energy consumption and maximum removal efficiency of heavy metals from simulated wastewater. Chem. Eng. Process.—Process. Intensif. 2020, 154, 108026. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-flow electrocoagulation (EC) process for iron removal from water: Experimental, statistical and economic study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef]
- Hamid, M.A.A.; Aziz, H.A.; Yusoff, M.S.; Rezan, S.A. Optimization and Analysis of Zeolite Augmented Electrocoagulation Process in the Reduction of High-Strength Ammonia in Saline Landfill Leachate. Water 2020, 12, 247. [Google Scholar] [CrossRef]
- Shabestar, M.P.; Moghaddam, M.R.A.; Karamati-Niaragh, E. Evaluation of energy and electrode consumption of Acid Red 18 removal using electrocoagulation process through RSM: Alternating and direct current. Environ. Sci. Pollut. Res. 2021, 28, 67214–67223. [Google Scholar] [CrossRef] [PubMed]
- Bharath, M.; Krishna, B.; Kumar, B.M. A Review of Electrocoagulation Process for Wastewater Treatment. Int. J. ChemTech Res. 2018, 11, 289–302. [Google Scholar] [CrossRef]
- Nigri, E.M.; Santos, A.L.A.; Rocha, S.D.F. Removal of organic compounds, calcium and strontium from petroleum industry effluent by simultaneous electrocoagulation and adsorption. J. Water Process. Eng. 2020, 37, 101442. [Google Scholar] [CrossRef]
- He, C.-C.; Hu, C.-Y.; Lo, S.-L. Integrating chloride addition and ultrasonic processing with electrocoagulation to remove passivation layers and enhance phosphate removal. Sep. Purif. Technol. 2018, 201, 148–155. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Emerick, T.; Vieira, J.L.; Silveira, M.H.L.; João, J.J. Ultrasound-assisted electrocoagulation process applied to the treatment and reuse of swine slaughterhouse wastewater. J. Environ. Chem. Eng. 2020, 8, 104308. [Google Scholar] [CrossRef]
- Benekos, A.K.; Zampeta, C.; Argyriou, R.; Economou, C.N.; Triantaphyllidou, I.-E.; Tatoulis, T.I.; Tekerlekopoulou, A.; Vayenas, D.V. Treatment of table olive processing wastewaters using electrocoagulation in laboratory and pilot-scale reactors. Process. Saf. Environ. Prot. 2019, 131, 38–47. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martinez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Brahmi, K.; Bouguerra, W.; Hamrouni, B.; Elaloui, E.; Loungou, M.; Tlili, Z. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem. 2019, 12, 1848–1859. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process. Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, C. Paper industry wastewater treatment by electrocoagulation and aspect of sludge management. J. Clean. Prod. 2022, 360, 131970. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Balderas-Hernández, P.; Bilyeu, B. Electrocoagulation: Fundamentals and prospectives. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 61–76. [Google Scholar]
- Safari, S.; Aghdam, M.A.; Kariminia, H. Electrocoagulation for COD and diesel removal from oily wastewater. Int. J. Environ. Sci. Technol. 2016, 13, 231–242. [Google Scholar] [CrossRef]
- Verma, A.K. Treatment of textile wastewaters by electrocoagulation employing Fe-Al composite electrode. J. Water Process. Eng. 2017, 20, 168–172. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Alardhi, S.M.; Ahmed, S.A.; Salman, A.D.; Juzsakova, T.; Cretescu, I.; Le, P.-C.; Chung, W.; Chang, S.; Nguyen, D. Can electrocoagulation technology be integrated with wastewater treatment systems to improve treatment efficiency? Environ. Res. 2022, 214, 113890. [Google Scholar] [CrossRef] [PubMed]
- Demirci, Y.; Pekel, L.; Alpbaz, M. Investigation of different electrode connections in electrocoagulation of textile wastewater treatment. Int. J. Electrochem. Sci. 2015, 10, 2685–2693. [Google Scholar]
- Neto, S.L.d.C.; Viviani, J.C.T.; Weschenfelder, S.E.; Cunha, M.d.F.R.d.; Junior, A.E.O.; Costa, B.R.D.S.; Mazur, L.P.; Marinho, B.A.; da Silva, A.; de Souza, A.A.U.; et al. Evaluation of petroleum as extractor fluid in liquid-liquid extraction to reduce the oil and grease content of oilfield produced water. Process. Saf. Environ. Prot. 2022, 161, 263–272. [Google Scholar] [CrossRef]
- Oktiawan, W.; Samadikun, B.P.; Junaidi; Bramahesa, I.G.N.; Taqiyya, T.A.; Amrullah, M.R.; Basyar, C. Effect of electrode configuration and voltage variations on electrocoagulation process in surfactant removal from laundry wastewater. IOP Conf. Series: Earth Environ. Sci. 2021, 896, 012049. [Google Scholar] [CrossRef]
- Almukdad, A.; Hafiz, M.A.; Yasir, A.T.; Alfahel, R.; Hawari, A.H. Unlocking the application potential of electrocoagulation process through hybrid processes. J. Water Process. Eng. 2021, 40, 101956. [Google Scholar] [CrossRef]
- Asaithambi, P.; Aziz, A.R.A.; Sajjadi, B.; Daud, W.M.A.B.W. Sono assisted electrocoagulation process for the removal of pollutant from pulp and paper industry effluent. Environ. Sci. Pollut. Res. 2017, 24, 5168–5178. [Google Scholar] [CrossRef]
- Dizge, N.; Akarsu, C.; Ozay, Y.; Gulsen, H.E.; Adiguzel, S.K.; Mazmanci, M.A. Sono-assisted electrocoagulation and cross-flow membrane processes for brewery wastewater treatment. J. Water Process. Eng. 2018, 21, 52–60. [Google Scholar] [CrossRef]
- Al-Rubaiey, N.A.; Al-Barazanjy, M.G. Ultrasonic Technique in Treating Wastewater by Electrocoagulation. Eng. Technol. J. 2018, 36, 54–62. [Google Scholar] [CrossRef]
- Moradi, M.; Vasseghian, Y.; Arabzade, H.; Khaneghah, A.M. Various wastewaters treatment by sono-electrocoagulation process: A comprehensive review of operational parameters and future outlook. Chemosphere 2021, 263, 128314. [Google Scholar] [CrossRef]
- Prajapati, A.K. Sono-assisted electrocoagulation treatment of rice grain based distillery biodigester effluent: Performance and cost analysis. Process. Saf. Environ. Prot. 2021, 150, 314–322. [Google Scholar] [CrossRef]
- Ohrdes, H.; Ille, I.; Twiefel, J.; Wallaschek, J.; Nogueira, R.; Rosenwinkel, K.-H. A control system for ultrasound devices utilized for inactivating E. coli in wastewater. Ultrason. Sonochem. 2018, 40, 158–162. [Google Scholar] [CrossRef]
- Hashim, K.S.; Ali, S.S.M.; AlRifaie, J.K.; Kot, P.; Shaw, A.; Al Khaddar, R.; Idowu, I.; Gkantou, M. Escherichia coli inactivation using a hybrid ultrasonic–electrocoagulation reactor. Chemosphere 2020, 247, 125868. [Google Scholar] [CrossRef]
- Ritesh, P.; Srivastava, V.C. Understanding of ultrasound enhanced electrochemical oxidation of persistent organic pollutants. J. Water Process. Eng. 2020, 37, 101378. [Google Scholar] [CrossRef]
- Manojkumar, N.; Muthukumaran, C.; Sharmila, G. A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. J. King Saud Univ.—Eng. Sci. 2022, 34, 198–208. [Google Scholar] [CrossRef]
- Hanrahan, G.; Lu, K. Application of Factorial and Response Surface Methodology in Modern Experimental Design and Optimization. Crit. Rev. Anal. Chem. 2006, 36, 141–151. [Google Scholar] [CrossRef]
- Khorram, A.G.; Fallah, N. Treatment of textile dyeing factory wastewater by electrocoagulation with low sludge settling time: Optimization of operating parameters by RSM. J. Environ. Chem. Eng. 2018, 6, 635–642. [Google Scholar] [CrossRef]
- Deveci, E.; Akarsu, C.; Gönen, Ç.; Özay, Y. Enhancing treatability of tannery wastewater by integrated process of electrocoagulation and fungal via using RSM in an economic perspective. Process. Biochem. 2019, 84, 124–133. [Google Scholar] [CrossRef]
- Bajpai, M.; Katoch, S.S.; Kadier, A.; Ma, P.-C. Treatment of pharmaceutical wastewater containing cefazolin by electrocoagulation (EC): Optimization of various parameters using response surface methodology (RSM), kinetics and isotherms study. Chem. Eng. Res. Des. 2021, 176, 254–266. [Google Scholar] [CrossRef]
- Abbasi, S.; Mirghorayshi, M.; Zinadini, S.; Zinatizadeh, A.A. A novel single continuous electrocoagulation process for treatment of licorice processing wastewater: Optimization of operating factors using RSM. Process. Saf. Environ. Prot. 2020, 134, 323–332. [Google Scholar] [CrossRef]
- Lakshmi, P.M.; Sivashanmugam, P. Treatment of oil tanning effluent by electrocoagulation: Influence of ultrasound and hybrid electrode on COD removal. Sep. Purif. Technol. 2013, 116, 378–384. [Google Scholar] [CrossRef]
- Ji, M.; Jiang, X.; Wang, F. A mechanistic approach and response surface optimization of the removal of oil and grease from restaurant wastewater by electrocoagulation and electroflotation. Desalination Water Treat. 2015, 55, 2044–2052. [Google Scholar] [CrossRef]
- Yusoff, M.S.; Azwan, A.M.; Zamri, M.F.M.A.; Aziz, H.A. Removal of colour, turbidity, oil and grease for slaughterhouse wastewater using electrocoagulation method. AIP Conf. Proc. 2017, 1892, 040012. [Google Scholar] [CrossRef]
- Naser, G.F.; Mohammed, T.J.; Abbar, A.H. Treatment of Al-Muthanna Petroleum Refinery Wastewater by Electrocoagulation Using a Tubular batch Electrochemical Reactor. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012094. [Google Scholar] [CrossRef]
- Priya, M.; Jeyanthi, J. Removal of COD, oil and grease from automobile wash water effluent using electrocoagulation technique. Microchem. J. 2019, 150, 104070. [Google Scholar] [CrossRef]
- Ghahrchi, M.; Rezaee, A.; Adibzadeh, A. Study of kinetic models of olive oil mill wastewater treatment using electrocoagulation process. Desalination Water Treat. 2021, 211, 123–130. [Google Scholar] [CrossRef]
- Adegoke, A.T.; Abayomi, E.T. A preliminary study on the treatment of restaurant wastewater using electrocoagulation technique. J. Degraded Min. Lands Manag. 2020, 7, 2029–2033. [Google Scholar] [CrossRef]
- Nasrullah, M.; Ansar, S.; Krishnan, S.; Singh, L.; Peera, S.G.; Zularisam, A. Electrocoagulation treatment of raw palm oil mill effluent: Optimization process using high current application. Chemosphere 2022, 299, 134387. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA, 2017; Volume 91. [Google Scholar]
- Zhao, S.; Huang, G.; Cheng, G.; Wang, Y.; Fu, H. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to Reverse Osmosis membranes. Desalination 2014, 344, 454–462. [Google Scholar] [CrossRef]
- Zargazi, M.; Entezari, M.H. Sono-electrodeposition of novel bismuth sulfide films on the stainless steel mesh: Photocatalytic reduction of Cr (VI). J. Hazard. Mater. 2020, 384, 121300. [Google Scholar] [CrossRef]
- Carroll Croarkin and Paul TobiasNist/Sematech, Engineering Statistics Handbook; National Institute of Standards and Technology: Washington, DC, USA, 2012.
- Breig, S.J.M.; Luti, K.J.K. Response surface methodology: A review on its applications and challenges in microbial cultures. Mater. Today Proc. 2021, 42, 2277–2284. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process. Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Akkaya, G.K. Treatment of petroleum wastewater by electrocoagulation using scrap perforated (Fe-anode) and plate (Al and Fe-cathode) metals: Optimization of operating parameters by RSM. Chem. Eng. Res. Des. 2022, 187, 261–275. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Li, X.; Li, P.; Zhang, T.; Lv, X.; Liu, L.; Dong, J.; Zheng, D. Optimized removal of natural organic matter by ultrasound-assisted coagulation of recycling drinking water treatment sludge. Ultrason. Sonochem. 2018, 48, 171–180. [Google Scholar] [CrossRef]
- Dahlan, I.; Azhar, E.E.M.; Hassan, S.R.; Aziz, H.A.; Hung, Y.-T. Statistical Modeling and Optimization of Process Parameters for 2,4-Dichlorophenoxyacetic Acid Removal by Using AC/PDMAEMA Hydrogel Adsorbent: Comparison of Different RSM Designs and ANN Training Methods. Water 2022, 14, 3061. [Google Scholar] [CrossRef]
- Ciggin, A.S.; Sarica, E.S.; Doğruel, S.; Orhon, D. Impact of ultrasonic pretreatment on Fenton-based oxidation of olive mill wastewater—Towards a sustainable treatment scheme. J. Clean. Prod. 2021, 313, 127948. [Google Scholar] [CrossRef]
- He, C.-C.; Hu, C.-Y.; Lo, S.-L. Evaluation of sono-electrocoagulation for the removal of Reactive Blue 19 passive film removed by ultrasound. Sep. Purif. Technol. 2016, 165, 107–113. [Google Scholar] [CrossRef]
- Noor, M.H.M.; Azli, M.F.Z.M.; Ngadi, N.; Inuwa, I.M.; Opotu, L.A.; Mohamed, M. Optimization of sonication-assisted synthesis of magnetic Moringa oleifera as an efficient coagulant for palm oil wastewater treatment. Environ. Technol. Innov. 2022, 25, 102191. [Google Scholar] [CrossRef]
- Patidar, R.; Srivastava, V.C. Ultrasound-assisted enhanced electroxidation for mineralization of persistent organic pollutants: A review of electrodes, reactor configurations and kinetics. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1667–1701. [Google Scholar] [CrossRef]



| Wastewater Type | Electrode Combination | Optimum pH | IE (cm) | T (Min) | CD | Treatment Efficiency (%) | Optimisation Study | SEM Image(s) on the Electrode | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Oil Tanning Effluent | Fe-Fe | n.a. | 1.5 | - | 20 mA/cm2 | COD 89.6 | Linear regression analysis | n.a. | [48] |
| Restaurant Wastewater | Al-SS | 5–6 | 3.6 | 34 | 43 A/m2 | O&G 95 | RSM | [49] | |
| Slaughterhouse | Al-Fe | n.a. | 0.5 | 30 | - | 98 O&G 92 colour 91 turbidity | n.a. | n.a. | [50] |
| Petroleum Refinery Plant | AL-SS | 7 | 2 | 45 | 15 mA/cm2 | COD 95.3 | n.a. | n.a. | [51] |
| Automobile Wash | Cu (anode)–Al (cathode) | 6 | 5 | 40 | 25 A/m2 | COD 95.1 O&G 92.5 | n.a. | n.a. | [52] |
| Olive Oil Mill Wastewater | Al-SS | 7 | 1 | 60 | 12.5 mA/cm2 | COD 99 | n.a. | n.a. | [53] |
| Restaurant Wastewater | carbon electrodes | n.a. | 1 | 90 | 1.0 Amp | PP 40.75 PO43− 33 P2O5 32.83 COD 25.83 | n.a. | n.a. | [54] |
| Restaurants WW | Al-Al | 7.03 | 2 | 60 | 40 mA/cm2 | COD 84.6, 74.5, 89.15, 68.5, 92.79% O&G 100%, 100%, 92.42, 94.5, 87.76% | n.a. | n.a. | [1] |
| Palm Oil Mill Effluent | steel wool | 4.37 | 0.86 | 44.97 | 542 mA/cm2 | COD 97.21 BOD 99.26 Suspended solid 99.00 | n.a. | n.a. | [55] |
| Restaurant WW | Al-Al | 7 | 2.4 | 30.5 | 53.2 | COD 75.9 O&G 95.4 | RSM | Before treatment, after EC, after US-EC treatment | Current research |
| Parameters | Unit | Value | Limit Value from the Environmental Quality (Regulation 2009) |
|---|---|---|---|
| Temperature | °C | 30 | 40 |
| PH | - | 6 | 6.0–9.0 |
| Electrical conductivity | µs/cm | 332.4 | - |
| DO | mg/L | 2.1 | - |
| DO | % | 28 | |
| TDS | mg/L | 268 | - |
| BOD | mg/L | 379 | 250 |
| TSS | mg/L | 373.9 | 300 |
| COD | mg/L | 955 | 500 |
| TN | mg/L | 22.6 | 50 |
| O&G | mg/L | 3434 | 50 |
| Sign | Independent Factors | Unit | Factors Level | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| A (ID) | interelectrode distance | cm | 2 | 3 | 4 | 5 | 6 |
| B (T) | electrolysis time | min | 15 | 20 | 25 | 30 | 35 |
| C (CD) | current density | A/m2 | 40 | 50 | 60 | 70 | 80 |
| Run | Point Type | Factors | O&G Removal (%) | COD Removal (%) | ||||
|---|---|---|---|---|---|---|---|---|
| ID (cm) | T (min) | CD (A/m2) | Experiment Value | Predicted Value | Experiment Value | Predicted Value | ||
| 1 | star | 4 | 35 | 60 | 92.47 | 90.98 | 75.35 | 77.44 |
| 2 | Factorial | 5 | 30 | 50 | 70.63 | 71.71 | 66.46 | 65.25 |
| 3 | Factorial | 3 | 30 | 50 | 94.17 | 94.71 | 74.77 | 72.1 |
| 4 | Center | 4 | 25 | 60 | 90.47 | 89.43 | 66.59 | 66.3 |
| 5 | star | 6 | 25 | 60 | 47.52 | 45.48 | 57.78 | 58.26 |
| 6 | Factorial | 3 | 20 | 70 | 77.17 | 79.13 | 65.69 | 65.07 |
| 7 | Factorial | 5 | 30 | 70 | 66.15 | 68.61 | 70.56 | 69.19 |
| 8 | Center | 4 | 25 | 60 | 88.67 | 89.43 | 64.76 | 66.3 |
| 9 | Factorial | 3 | 20 | 50 | 84.08 | 84.66 | 67.9 | 67.45 |
| 10 | Center | 4 | 25 | 60 | 92.65 | 89.43 | 65.89 | 66.3 |
| 11 | star | 4 | 25 | 40 | 70.97 | 70.84 | 57.59 | 59.38 |
| 12 | Factorial | 3 | 30 | 70 | 88.17 | 90.11 | 75.27 | 74.5 |
| 13 | Center | 4 | 25 | 60 | 89.76 | 89.43 | 63.46 | 66.3 |
| 14 | Center | 4 | 25 | 60 | 86.92 | 89.43 | 67.1 | 66.3 |
| 15 | star | 2 | 25 | 60 | 90.92 | 89.93 | 74.83 | 76.18 |
| 16 | Center | 4 | 25 | 60 | 91.15 | 89.43 | 68.15 | 66.3 |
| 17 | star | 4 | 25 | 80 | 65.14 | 62.23 | 60.89 | 60.94 |
| 18 | Factorial | 5 | 20 | 50 | 60.61 | 61.71 | 55.91 | 54.85 |
| 19 | Factorial | 5 | 20 | 70 | 55.19 | 57.68 | 53.15 | 54 |
| 20 | star | 4 | 15 | 60 | 71.55 | 70 | 57.87 | 57.6 |
| Fit Summary Model for O&G Removal (%) | |||||
| Source | Model p-Value | Lack of Fit p-Value | Adjusted R2 | Predicted R2 | |
| Design Model | 0.0008 | 0.0011 | 0.6605 | 0.5671 | |
| Linear | 0.0007 | 0.0007 | 0.5814 | 0.4808 | |
| 2FI | 0.9995 | 0.0004 | 0.4854 | 0.2949 | |
| Quadratic | <0.0001 | 0.2107 | 0.9691 | 0.9043 | Suggested |
| Cubic | 0.9006 | 0.0332 | 0.9559 | −0.8096 | Aliased |
| Model summary | R2 | Adj. R2 | C.V | ||
| 98.38% | 96.91% | 3.18% | |||
| Fit Summary Model for COD Removal (%) | |||||
| Source | Model p-value | Lack of Fit p-value | Adjusted R2 | Predicted R2 | |
| Design Model | <0.0001 | 0.0767 | 0.8228 | 0.7778 | |
| Linear | <0.0001 | 0.0657 | 0.8032 | 0.6937 | |
| 2FI | 0.3785 | 0.0610 | 0.8073 | 0.6894 | |
| Quadratic | 0.0097 | 0.2938 | 0.9161 | 0.7559 | Suggested |
| Cubic | 0.6464 | 0.0928 | 0.9025 | −1.9485 | Aliased |
| Model summary | R2 | Adj. R2 | C.V | ||
| 95.58% | 91.61% | 2.98% | |||
| ANOVA for Quadratic Model for O&G | ||||||
| Source | S.S | d.f | M.S | F-Value | p-Value | |
| Model | 3783.92 | 9 | 420.44 | 67.29 | <0.0001 | significant |
| A-I. E | 1975.97 | 1 | 1975.97 | 316.27 | <0.0001 | |
| B-T | 440.18 | 1 | 440.18 | 70.45 | <0.0001 | |
| C-C. D | 74.29 | 1 | 74.29 | 11.89 | 0.0062 | |
| AB | 0.0014 | 1 | 0.0014 | 0.0002 | 0.9883 | |
| AC | 1.12 | 1 | 1.12 | 0.1794 | 0.6809 | |
| BC | 0.4297 | 1 | 0.4297 | 0.0688 | 0.7984 | |
| A2 | 741.93 | 1 | 741.93 | 118.75 | <0.0001 | |
| B2 | 125.69 | 1 | 125.69 | 20.12 | 0.0012 | |
| C2 | 823.91 | 1 | 823.91 | 131.87 | <0.0001 | |
| Residual | 62.48 | 10 | 6.25 | |||
| Lack of Fit | 42.63 | 5 | 8.53 | 2.15 | 0.2107 | not significant |
| Pure Error | 19.85 | 5 | 3.97 | |||
| Cor Total | 3846.40 | 19 | ||||
| Final Equation | ||||||
| ANOVA for Quadratic Model for COD | ||||||
| Source | S.S | d.f | M.S | F-Value | p-Value | |
| Model | 821.61 | 9 | 91.29 | 24.04 | <0.0001 | significant |
| A-I. E | 320.95 | 1 | 320.95 | 84.51 | <0.0001 | |
| B-T | 393.74 | 1 | 393.74 | 103.67 | <0.0001 | |
| C-C. D | 2.43 | 1 | 2.43 | 0.6395 | 0.4425 | |
| AB | 16.55 | 1 | 16.55 | 4.36 | 0.0634 | |
| AC | 1.17 | 1 | 1.17 | 0.3092 | 0.5904 | |
| BC | 11.44 | 1 | 11.44 | 3.01 | 0.1133 | |
| A2 | 1.34 | 1 | 1.34 | 0.3526 | 0.5659 | |
| B2 | 2.36 | 1 | 2.36 | 0.6214 | 0.4488 | |
| C2 | 59.24 | 1 | 59.24 | 15.60 | 0.0027 | |
| Residual | 37.98 | 10 | 3.80 | |||
| Lack of Fit | 23.75 | 5 | 4.75 | 1.67 | 0.2938 | not significant |
| Pure Error | 14.23 | 5 | 2.85 | |||
| Cor Total | 859.58 | 19 | ||||
| Final Equation | ||||||
| ID (cm) | T (min) | CD (A/m2) | O&G Removal (%) | Error (%) | COD Removal (%) | Error (%) | ||
|---|---|---|---|---|---|---|---|---|
| Predicted | Experiment | Predicted | Experiment | |||||
| 2.4 | 30.5 | 53.2 | 95.4 | 95.9 | 0.52 | 75.9 | 75.3 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, S.O.A.; Yusoff, M.S.; Halim, H.; Mokhtar Kamal, N.H.; Bashir, M.J.K.; Manan, T.S.B.A.; Aziz, H.A.; Mojiri, A. Ultrasonic (US)-Assisted Electrocoagulation (EC) Process for Oil and Grease (O&G) Removal from Restaurant Wastewater. Separations 2023, 10, 61. https://doi.org/10.3390/separations10010061
Nassar SOA, Yusoff MS, Halim H, Mokhtar Kamal NH, Bashir MJK, Manan TSBA, Aziz HA, Mojiri A. Ultrasonic (US)-Assisted Electrocoagulation (EC) Process for Oil and Grease (O&G) Removal from Restaurant Wastewater. Separations. 2023; 10(1):61. https://doi.org/10.3390/separations10010061
Chicago/Turabian StyleNassar, Shefaa Omar Abu, Mohd Suffian Yusoff, Herni Halim, Nurul Hana Mokhtar Kamal, Mohammed J. K. Bashir, Teh Sabariah Binti Abd Manan, Hamidi Abdul Aziz, and Amin Mojiri. 2023. "Ultrasonic (US)-Assisted Electrocoagulation (EC) Process for Oil and Grease (O&G) Removal from Restaurant Wastewater" Separations 10, no. 1: 61. https://doi.org/10.3390/separations10010061
APA StyleNassar, S. O. A., Yusoff, M. S., Halim, H., Mokhtar Kamal, N. H., Bashir, M. J. K., Manan, T. S. B. A., Aziz, H. A., & Mojiri, A. (2023). Ultrasonic (US)-Assisted Electrocoagulation (EC) Process for Oil and Grease (O&G) Removal from Restaurant Wastewater. Separations, 10(1), 61. https://doi.org/10.3390/separations10010061








