Abstract
Acral melanocytic neoplasms, including acral melanocytic nevus and acral melanoma, are common melanocytic lesions in Asian populations. Both lesions occur on the volar surface of the hands and feet, and on nail units. Acral melanocytic nevi occur on the arch area of the sole, whereas acral melanomas frequently occur on weight-bearing areas of the sole, and on the fingernails. Therefore, the development of acral melanoma may be associated with chronic pressure, physical stress, or trauma. Dermoscopy is a useful adjunctive diagnostic tool for differential diagnosis. Acral melanocytic nevus is characterized by a parallel furrow pattern, whereas acral melanoma has a parallel ridge pattern. Genetic alterations are also different between the two types of lesion. BRAF and NRAS mutations are common in acral melanocytic nevus, whereas acral melanoma shows lower rates of KIT, NF1, BRAF, and NRAS mutations and remarkable copy number variations in genes such as CCND1, CDK4, hTERT, PAK1, and GAB2. Sentinel lymph node biopsy is important for staging and prognosis. Contemporary treatments for melanoma include targeted therapy for mutations and immunotherapy, such as anti-PD1 inhibitors.
Melanocytic neoplasms in Asian populations are characterized by a predominance of acral melanoma compared with populations from Western countries. Here, we focus on acral melanoma and its benign counterpart, acral melanocytic nevus.
1. Acral Melanocytic Nevus
Acral melanocytic nevus occurs on the palms of the hands, soles of the feet, and nail units.
1.1. Epidemiology
A study performed in the USA in 2010 indicated prevalence rates of acral melanocytic nevus of 23.0% and 42.0%, in White and Black populations, respectively [1]. A more recent large prospective cohort study (performed in 2016) analyzed a much larger number of cases, including Asian and Hispanic populations, and showed that 36% of people in the United States have at least one acral nevus [2]. Interestingly, the total number of melanocytic nevus and their distribution at the anatomic sites are different according to racial populations. A Brazilian study reported that patients with darker skin have fewer overall acquired nevi, but more nevi on the face and acral sites, than patients with lighter skin [3]. In Japan, acral melanocytic nevi was reported in approximately 7% in the population, and 10.9% of subjects in another study had melanocytic nevi on the soles of the feet [4,5]. The rates of acral nevus on the palms and soles in a Korean population were reported to be 15.7% and 9.2%, respectively [6]. In summary, acral melanocytic nevi occur more commonly in Asians and people of darker skin types compared with lighter skinned individuals, even though the overall prevalence of melanocytic nevi is lower in the former than the latter. The limitation of these data is they analyzed only patients who visited dermatologic clinic; therefore, the possibility of selection bias may exist.
1.2. Etiology and Genetics
The etiology of acral melanocytic nevus is still unknown. Numerous genetic studies have been conducted on melanocytic nevi in low-cumulative sun-damaged (low-CSD) skin, but the genetic profiles of those in acral sites are still unclear. Genomic analyses have shown that most nevi on low-CSD skin harbor mutually exclusively mutations in melanoma driver oncogenes, such as BRAF and NRAS [7,8,9]. One author (S.J.Y.) and coworkers also found mutations in five genes in acral melanocytic nevi in Korean subjects, i.e., BRAF (66.7%), NRAS (9.5%), NF1 (9.5%), GNAQ (38.1%), and KIT (14.3%) [10]. BRAFV600E and NRAS mutations were mutually exclusive, whereas GNAQ mutation co-occurred with other gene mutations. Copy number variations (CNVs) are more common in acral melanoma than non-acral cutaneous melanoma [11]. They showed that CNVs were much less common in acral melanocytic nevus than acral melanoma. A mutational survey of acral melanocytic nevi was also performed in a U.S. population [12]. Similarly to the Korean results, BRAF mutation was commonly observed in acral melanocytic nevus. In that study, mutations in BRAF and NRAS were observed in 86% and 10% of acral melanocytic nevi, respectively. These mutational profiles were similar to benign melanocytic nevi in low-CSD skin. Mutations in BRAF and NRAS were mutually exclusive. Very low levels of CNV were observed in acral melanocytic nevus compared with acral melanoma. Therefore, it was concluded that acral melanocytic nevi demonstrated a mutational spectrum similar to nevi on low-CSD skin, suggesting that they are unlikely to be precursor lesions for the majority of acral melanomas. Further large-scale studies are needed to draw definitive conclusions regarding this issue.
1.3. Clinical Features
Acral melanocytic nevus occurs on the palms of the hands, soles of the feet, and nails, all of which are non-hair-bearing sites. These nevi may also occur on the volar surface of the fingers and toes. Acral melanocytic nevi have a different distribution on volar sites than acral melanomas, and primarily affect non-weight-bearing areas [13,14,15]. Most of these lesions present as brown-to-black small (less than 6 mm) macules or papules. They are typically symmetrical and well circumscribed, although they are sometimes asymmetrical and poorly circumscribed. These atypical acral nevi are difficult to diagnose, both clinically and pathologically, because early acral melanoma may have a similar clinical presentation. In such cases, dermoscopy is a useful noninvasive tool for the differential diagnosis of acral pigmented lesions [16,17,18]. Acral melanocytic nevi have three major dermoscopic patterns: a parallel furrow pattern, lattice-like pattern, or fibrillar pattern (Figure 1). The parallel furrow pattern is regarded as the prototype of the three major dermoscopic patterns of acral melanocytic nevus. Melanocytes (nevus cells) are mostly arranged in well-demarcated nests mainly located in the crista profunda limitans underlying the surface furrow [19]. These melanocytic nests form melanin columns in the cornified layer under the sulci of the surface skin. This histopathological finding mirrors the parallel furrow pattern shown by dermoscopy. The fibrillar pattern is caused by an oblique arrangement of melanin pigment in the slanting cornified layer, which is induced by mechanical pressure from the body weight [20]. Therefore, the fibrillar pattern can be regarded as an artifactual expression of the parallel furrow pattern [16].
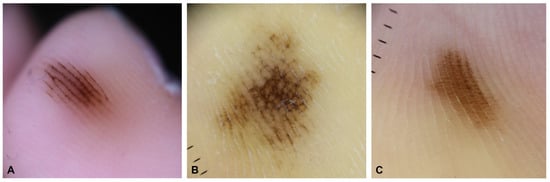
Figure 1.
Three major dermoscopic patterns in acral melanocytic nevus. (A) Parallel furrow pattern. (B) Lattice-like pattern. (C) Fibrillar pattern.
Nail matrix nevus, which is an acral melanocytic nevus located on the nail matrix, manifests as longitudinal melanonychia. This pigmentation of the nail plate is mostly derived from nevus cells on the distal nail matrix (Figure 2). Dermoscopy can be useful for visualizing these findings. This longitudinal band exhibits regularly spaced, thickened, parallel lines on a brownish background, running from the proximal to distal direction on the nail plate. However, nail matrix nevus in children manifests with atypical features, including irregular wide, dark, and multicolored lines (Figure 3) and micro-Hutchinson’s sign [21,22]. The micro-Hutchinson’s sign is defined by the visibility on dermoscopy of a pigmentation of the periungual tissues that could not be seen with the naked eye [23].
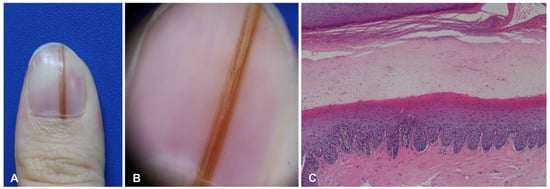
Figure 2.
Nail matrix nevus in a 28-year-old woman. (A) Brownish linear melanonychia of the nail plate. (B) Regular line on dermoscopy. (C) Junctional nests in the epidermis of the nail matrix.
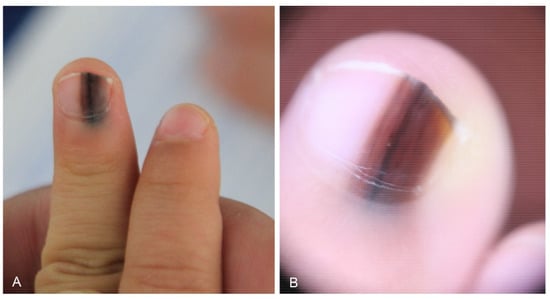
Figure 3.
Nail matrix nevus in a 2-year-old girl. (A) Wide melanonychia on the 3rd fingernail. (B) Dermoscopy showing irregular multicolored lines.
1.4. Histopathological Features
Acral melanocytic nevus presents as a junctional or compound melanocytic nevus, characterized by relatively small, symmetric, and well-circumscribed lentiginous and nested melanocytic proliferation along the dermal–epidermal junction (Figure 4). Nevus nests are variable in size and often vertically oriented. Pagetoid scatter, bridging between rete ridges, and fibroplasia are not uncommon, but these features tend to be confined to the center of the lesion [13,24]. When dermal components are present, maturation with depth and lack of mitotic activity on deeper portions are usually observed. Nevus cells occasionally show mild cytological atypia.
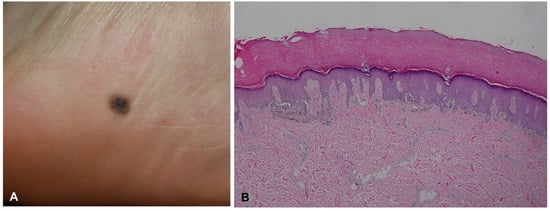
Figure 4.
Acral melanocytic nevus in a 23-year-old woman. (A) Small round macule on the inner heel. (B) Histopathological analysis revealed bridging formation of rete ridges with junctional melanocytic nests.
Nail matrix nevus is usually junctional, and compound nevus is rare [25,26,27]. Nuclear atypia, confluence of melanocytes, focal pagetoid spread, and periungual involvement are common in children, which can lead to difficulties in the interpretation of histological specimens [24,28].
1.5. Management of Acral Melanocytic Nevus
The lesions are benign, and progression to melanoma is rare. Most acral melanomas arise de novo, not in association with a preexisting acral nevus [12,29]. Acral melanocytic nevi which do not show typical benign dermoscopic patterns may require clinical and dermoscopic surveillance, and once or twice a year is enough [30]. Biopsies can be considered if the lesion enlarges to more than 7 mm, or changes in size and shape on regular follow-up. Pediatric patients with longitudinal melanonychia can be followed up without intervention for several years even if lesions grow darker or wider, whereas only 5% completely regress within 4.5 years [31]. Decisions should be based on clinicodermoscopic and pathologic correlations.
2. Acral Melanoma
Acral melanoma is a subtype of cutaneous melanoma occurring in glabrous acral skin, such as the palms, soles, and nail units, especially the nail matrix [32]. The most common clinicohistological type is acral lentiginous melanoma based on the lentiginous pattern on histology, and was first discussed by Reed in 1976. Subungual melanoma, which was first described as melanotic whitlow by Hutchinson in 1886 [33], is an acral melanoma occurring in the nail matrix. The recent WHO Classification of Skin Tumours presents nine pathways of melanoma; acral melanoma is pathway V [34].
2.1. Epidemiology
Acral melanoma is rare in Caucasians, who have a high overall incidence of melanoma. In the USA, acral melanoma accounts for only 2–3% of all malignant melanomas [35], whereas it accounts for a high proportion of melanomas in Asian and African populations. In Korea, approximately 50% of melanoma patients have acral melanoma [36,37,38,39] which is similar to the proportions in other Asian populations, including Japan [40], Singapore [41], China [42], and Taiwan [43]. Although the relative proportion of acral melanoma varies, its absolute incidence is similar in all races [44]. Subungual melanomas affect the fingernails more frequently than the toenails, and the fingernails of the right hand are more susceptible than those of the left hand. The thumbnail and great toenail are more common sites than other digits [34]. We speculate that trauma or external factors might be associated with this location preference.
2.2. Etiology and Genetics
The precise etiology of acral melanoma has not been determined. In a study in White populations, acral melanoma was strongly associated with high total body nevus count and nevi on the soles of the feet [45]. However, in a Japanese study, although a high number of acquired nevi was a risk factor for nonacral melanoma development, acquired nevi on the soles, palms, and nail apparatus did not seem to be a risk factor for the development of acral melanoma [46]. In the abovementioned study of a White population, there were also significant associations with penetrative injury of the feet or hands and heavy exposure to agricultural chemicals [45]. Several recent studies, including ones conducted in our department, revealed an association of acral melanoma on the plantar or palmar surfaces with mechanical or physical stress through anatomical mapping [15,47,48]. Analysis in 313 Korean acral melanoma patients revealed that 26.8% reported traumatic events, and 29.1% experienced physical stress. The most common occupations were farmers and fishermen [49]. Physical stress, pressure, friction, maceration, irritation, and trauma may play roles in the pathogenesis of acral melanoma, and further studies are required to precisely determine the underlying molecular mechanism.
Genetic analysis showed that acral melanoma is characterized by a relatively low mutation burden and a relatively high frequency of CNVs [11,50,51]. In addition to oncogenic mutations of BRAF (10–35%) and NRAS (9–22%), which are mutually exclusive, as well as NF1 (11–23%), triple-wild-type driver mutations (45–58%) were observed [50] Bastian et al. [52] reported that genetic aberrations of KIT frequently occur in acral melanoma. In Koreans, KIT mutations and increased KIT copy numbers are commonly observed in acral melanoma, and KIT mutations were found to be independent risk factors for a poor prognosis [38]. Identified CNVs in acral melanoma have implicated several genes, including CCND1, CDK4, hTERT, PAK1, GAB2, EP300, YAP1, and MDM2 [32,50,51,53]. Deletions in PTEN and CDKN2A are seen in up to 25% of cases [51]. The earliest genetic alteration in acral melanoma is the amplification of CCND1, which is detected in the very early stages of acral melanoma in situ [11,49]. In Taiwan, NRAS/KRAS mutations, cell cycle aberrations, increased copy numbers in antiapoptotic genes BIRC2, BIRC3, and BIRC5, and the amplification of receptor tyrosine kinase genes were significantly enriched in acral melanoma [54]. Further genetic profiling is needed to understand the pathogenesis of acral melanoma and identify suitable agents for targeted therapy.
2.3. Clinical Features
Acral melanoma begins as an asymmetrical, black-to-brown macule or small irregular patch. At the time of biopsy, the lesion is typically large in size after a long radial growth phase (RGP), and elevated nodules and ulceration associated with the vertical growth phase (VGP) may occur [34]. Acral melanoma on the sole primarily occurs on the weight-bearing portions, including the heel and forefoot areas [47,48]. One study showed that acral melanoma on the palms tends to occur in the finger pulp and distal area of the distal transverse crease of the palm, and suggested that mechanical stress may also affect the occurrence of palmar melanoma [15]. Due to their long RGP and clinical similarity with acral melanocytic nevus, differential diagnoses can sometimes be difficult based only on clinical findings. Dermoscopy can increase the sensitivity and specificity of diagnosis of early acral melanoma [19]. The parallel ridge pattern, consisting of brown-to-black band-like pigmentation on the ridges of the skin on dermoscopy, was correlated with histopathological findings (Figure 5). In an early evolving acral melanoma lesion, solitary arranged melanocytes preferentially proliferated in the crista profunda intermedia, which is an epidermal rete ridge underlying the surface ridge. This can be seen in tissue sections cut perpendicular to the surface skin markings [19]. Acral melanoma sometimes manifests as nodular melanoma, i.e., as a large nodule without a surrounding pigmented patch. It may be due to the very short RGP, but the precise mechanism remains unclear. Rarely, amelanotic acral melanomas, which show little or no brown-to-black pigmentation, can occur and can easily be misdiagnosed as benign conditions [55].
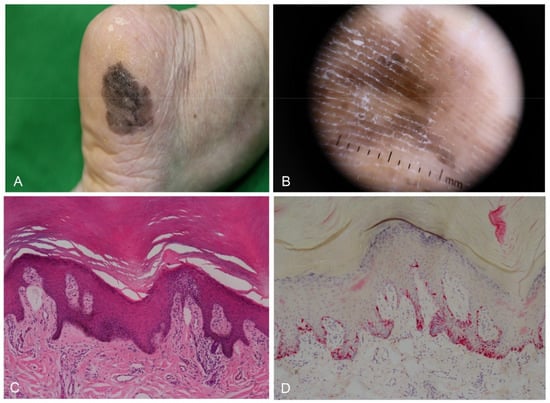
Figure 5.
Acral melanoma in situ in a 68-year-old woman. (A) Large irregular black patch on the outer area of the heel. (B) Dermoscopy showing parallel ridge pattern. (C) Skin biopsy showing a slight increase in basal pigmentation with mild lymphocytic infiltrate (H&E staining, original magnification, ×100). (D) Melan-A immunostaining highlights lentiginous proliferation of atypical melanocytes with prominent dendrites (×100).
Subungual melanoma often starts as longitudinal melanonychia, and a pigmented patch then spreads over the entire nail plate and into the skin beyond the nailfolds and hyponychium, which is called Hutchinson’s sign [56]. Over time, nodules, ulcers, bleeding, and nail destruction can occur. Dermoscopy is also useful for differential diagnoses of nail matrix nevus and subungual melanoma [57]. The dermoscopic features mostly indicative of early subungual melanoma are multiple irregular longitudinal brown-to-black lines on a brown background, micro-Hutchinson’s sign, a wide pigmented band, and triangular pigmentation on the nail plate (Figure 6). The sensitivity and specificity of micro-Hutchinson’s sign on dermoscopy in subungual melanoma in situ were reported to be 0.42 and 0.96, respectively [58].
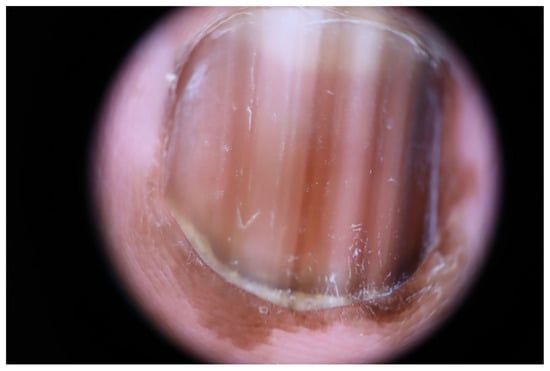
Figure 6.
Subungual melanoma in situ in a 63-year-old woman. Dermoscopy showing irregular, multicolored lines, and Hutchinson’s sign.
2.4. Histopathological Features
The most common histopathological subtype of acral melanoma is acral lentiginous melanoma, but nodular melanoma and superficial spreading melanoma can be seen in acral sites. The histopathological and molecular features of superficial spreading melanoma and nodular melanoma on acral sites are similar to those of examples at other body sites. Superficial spreading melanoma occurs mostly in the dorsal surface of acral sites, but rarely on volar surfaces [59]. Nodular melanoma on acral sites represent VGP of acral melanoma without an RGP. In the very early stages of acral lentiginous melanoma, there are only scattered lentiginous atypical melanocytes with enlarged hyperchromatic nuclei and prominent dendrites in the epidermal basal layer [60]. Over time, the continuous lentiginous proliferation of atypical melanocytes is observed, and after a long period of RGP, VGP occurs and the dermal proliferation of atypical melanocytes with large numbers of mitotic figures may be seen. The dominant cell morphology is variable in advanced acral melanoma (Figure 7). One author (S.J.Y.) and coworkers found correlations of the cytomorphological features and mutation status of acral melanomas. Round epithelioid cells are common in cases with BRAF mutation, bizarre giant cells are associated with NRAS mutation, and spindle cells with prominent dendrites are frequently observed in cases with NF1 and GNAQ mutations [10]. Histopathological features may be desmoplastic, neurotropic, or syringotropic. Immunohistochemical staining for HMB45 and Melan-A highlights prominent dendritic processes of melanoma cells in the epidermis of acral lentiginous melanoma, whereas HMB45 is sometimes focally positive or negative in amelanotic melanoma [55]. Recent studies have evaluated the role of preferentially expressed antigen in melanoma (PRAME) and p16 immunohistochemical staining in acral melanocytic neoplasm [61]. There are strong expressions of PRAME in acral melanomas, compared with weak or negative expression in acral melanocytic nevi. PRAME expressions are strong in both subungual and non-subungual acral melanomas [62]. Loss of p16 is also helpful to the diagnosis of acral melanoma.
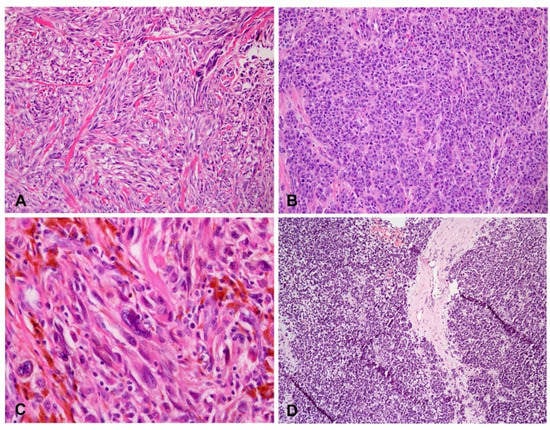
Figure 7.
Variable dominant cell morphology in advanced acral melanoma. (A) Spindle cells (H&E staining, original magnification, ×200). (B) Epithelioid cells (H&E staining, original magnification, ×200). (C) Bizarre giant cells (H&E staining, original magnification, ×400). (D) Lymphoid cells (H&E staining, original magnification, ×100).
2.5. Staging and Treatment
Similar to other cutaneous melanomas, acral melanoma is staged according to the American Joint Committee on Cancer (AJCC) TNM (tumor, node, metastasis) criteria [63]. However, compared with similar T stages of non-acral melanomas, acral melanomas have higher locoregional recurrence rates and poorer survival rates [64]. Previously, the poor outcomes of acral melanoma were explained by delays in diagnoses, but several recent studies have shown that acral melanoma has a high rate of locoregional metastasis and poorer survival outcomes than nonacral melanoma, even after controlling for melanoma stage.
The National Comprehensive Cancer Network recommends sentinel lymph node biopsy (SLNB) for primary melanomas with AJCC stage T1b or greater [65]. SLNB may be particularly important for acral melanoma because of its high locoregional recurrence rate. Patients with a positive SLNB are at higher risk of recurrence and should be followed via nodal basin ultrasound surveillance without complete lymph node dissection, given the lack of any improvement in melanoma-specific survival in two randomized controlled trials comparing the merits of complete lymph node dissection following a positive SLNB versus clinical observation with nodal ultrasound [66]. Recently, one author (S.J.Y.) and coworkers reported that the degree of acral melanoma pigmentation was a key predictor of metastasis. Amelanotic acral melanomas, and those with mild pigmentation, were associated with a first lung metastasis, whereas heavy pigmentation was associated with first lymph node metastasis [67,68]. These observations may be relevant to the initial workup, i.e., in cases of melanoma with low pigmentation, full-body imaging examination may be necessary at an early stage.
Wide radical excision is the standard treatment for primary acral melanoma, similar to other cutaneous melanomas. Current recommendations regarding the clinical margins differ according to the Breslow thickness of the primary lesion, and are based on several large randomized trials comparing margins of different size: margins of 0.5–1 cm are recommended for melanoma in situ, whereas 1 cm is recommended for melanoma with Breslow depth < 1 mm, 1–2 cm for melanoma 1–2 mm thick, and 2 cm for melanoma ≥ 2 mm thick [69]. However, acral melanoma was not included in the randomized trials on which the guidelines are based; thus, questions remain regarding whether the current guidelines should continue to be applied to acral melanoma. In addition, the management of acral lentiginous melanoma presents surgical challenges because of the subclinical extension of tumor cells and anatomically constrained locations. Therefore, Mohs micrographic surgery with complete circumferential peripheral and deep margin assessment has been examined as a means to improve histological clearance and reduce the chance of local recurrence in comparison with conventional wide excision [66].
BRAF mutation is an important driver of mutation in melanoma; therefore, BRAF inhibitors have been used to treat metastatic melanoma and recurrent melanoma with BRAF mutation. However, because BRAF mutation is less common in acral melanoma, BRAF inhibitors are not generally useful for their treatment. Positive responses to KIT inhibitor treatment have been reported in cases of acral melanoma with KIT mutation [70]. The introduction of immune checkpoint inhibitors has changed the management of metastatic melanoma. However, lower response rates to immune checkpoint blockades have been observed in acral melanoma and nail unit melanoma, which may be due to smaller numbers of tumor-infiltrating lymphocytes, lower PD-L1 expression, and a lower mutational burden in acral melanoma compared with other subtypes of melanoma [71].
3. Conclusions
Acral melanocytic nevus and acral melanoma are frequent melanocytic neoplasms in Asian populations; therefore, precise diagnoses and a detailed understandings of treatment options are essential.
Author Contributions
S.P. and S.-J.Y. contributed to Conceptualization, Methodology, and Writing—Original Draft Preparation. S.-J.Y. contributed to Resources, Funding Acquisition, Supervision and Project Administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1D1A3A03103808).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the research involving no more than minimal risk to the participants, and the waiver not adversely affecting the rights and welfare of the participants.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palicka, G.A.; Rhodes, A.R. Acral melanocytic nevi: Prevalence and distribution of gross morphologic features in white and black adults. Arch. Dermatol. 2010, 146, 1085–1094. [Google Scholar] [CrossRef]
- Madankumar, R.; Gumaste, P.V.; Martires, K.; Schaffer, P.R.; Choudhary, S.; Falto-Aizpurua, L.; Arora, H.; Kallis, P.J.; Patel, S.; Damanpour, S.; et al. Acral melanocytic lesions in the United States: Prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J. Am. Acad. Dermatol. 2016, 74, 724–730.e721. [Google Scholar] [CrossRef]
- Tuma, B.; Yamada, S.; Atallah, A.N.; Araujo, F.M.; Hirata, S.H. Dermoscopy of black skin: A cross-sectional study of clinical and dermoscopic features of melanocytic lesions in individuals with type V/VI skin compared to those with type I/II skin. J. Am. Acad. Dermatol. 2015, 73, 114–119. [Google Scholar] [CrossRef]
- Watanabe, S.; Sawada, M.; Ishizaki, S.; Kobayashi, K.; Tanaka, M. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol. Pract. Concept. 2014, 4, 47–50. [Google Scholar] [CrossRef]
- Kogushi-Nishi, H.; Kawasaki, J.; Kageshita, T.; Ishihara, T.; Ihn, H. The prevalence of melanocytic nevi on the soles in the Japanese population. J. Am. Acad. Dermatol. 2009, 60, 767–771. [Google Scholar] [CrossRef]
- Jin, H.J.; Kim, G.W.; Kim, W.J.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B.; Mun, J.H. A Study of the prevalence, distribution and dermoscopic patterns of acral melanocytic nevi in a Korean population. Korean J. Dermatol. 2015, 53, 612–616. [Google Scholar]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef]
- Colebatch, A.J.; Ferguson, P.; Newell, F.; Kazakoff, S.H.; Witkowski, T.; Dobrovic, A.; Johansson, P.A.; Saw, R.P.M.; Stretch, J.R.; McArthur, G.A.; et al. Molecular genomic profiling of melanocytic nevi. J. Investig. Dermatol. 2019, 139, 1762–1768. [Google Scholar] [CrossRef]
- Stark, M.S.; Tan, J.M.; Tom, L.; Jagirdar, K.; Lambie, D.; Schaider, H.; Soyer, H.P.; Sturm, R.A. Whole-exome sequencing of acquired nevi identifies mechanisms for development and maintenance of benign neoplasms. J. Investig. Dermatol. 2018, 138, 1636–1644. [Google Scholar] [CrossRef]
- Moon, K.R.; Choi, Y.D.; Kim, J.M.; Jin, S.; Shin, M.H.; Shim, H.J.; Lee, J.B.; Yun, S.J. Genetic alterations in primary acral melanoma and acral melanocytic nevus in Korea: Common mutated genes show distinct cytomorphological features. J. Investig. Dermatol. 2018, 138, 933–945. [Google Scholar] [CrossRef]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.H.; Aiba, S.; Brocker, E.B.; LeBoit, P.E.; et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef]
- Smalley, K.S.M.; Teer, J.K.; Chen, Y.A.; Wu, J.Y.; Yao, J.; Koomen, J.M.; Chen, W.S.; Rodriguez-Waitkus, P.; Karreth, F.A.; Messina, J.L. A mutational survey of acral nevi. JAMA Dermatol. 2021, 157, 831–835. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, Y.D.; Seon, H.J.; Lee, J.B.; Yun, S.J. Anatomic mapping and clinicopathologic analysis of benign acral melanocytic neoplasms: A comparison between adults and children. J. Am. Acad. Dermatol. 2017, 77, 735–745. [Google Scholar] [CrossRef]
- Ghanavatian, S.; Costello, C.M.; Buras, M.R.; Cumsky, H.J.L.; Pittelkow, M.R.; Swanson, D.L.; Mangold, A.R. Density and distribution of acral melanocytic nevi and acral melanomas on the plantar surface of the foot. J. Am. Acad. Dermatol. 2019, 80, 790–792.e792. [Google Scholar] [CrossRef]
- Nishiguchi, M.; Yamamoto, Y.; Hara, T.; Okuhira, H.; Inaba, Y.; Kunimoto, K.; Mikita, N.; Kaminaka, C.; Kanazawa, N.; Jinnin, M. Difference in distribution of malignant melanoma and melanocytic nevus in the palm and finger. Biosci. Trends 2019, 13, 361–363. [Google Scholar] [CrossRef]
- Saida, T.; Koga, H. Dermoscopic patterns of acral melanocytic nevi: Their variations, changes, and significance. Arch. Dermatol. 2007, 143, 1423–1426. [Google Scholar] [CrossRef]
- Altamura, D.; Altobelli, E.; Micantonio, T.; Piccolo, D.; Fargnoli, M.C.; Peris, K. Dermoscopic patterns of acral melanocytic nevi and melanomas in a white population in central Italy. Arch. Dermatol. 2006, 142, 1123–1128. [Google Scholar] [CrossRef]
- Wawrzynkiewicz, M.; Pastuszczak, M.; Chlebicki, M.; Wojas-Pelc, A. Acral melanocytic nevi in the Polish population: Prevalence, clinical characteristics and dermoscopic patterns. Postepy Dermatol. Alergol. 2019, 36, 581–588. [Google Scholar] [CrossRef]
- Saida, T.; Koga, H.; Uhara, H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J. Dermatol. 2011, 38, 25–34. [Google Scholar] [CrossRef]
- Miyazaki, A.; Saida, T.; Koga, H.; Oguchi, S.; Suzuki, T.; Tsuchida, T. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: A retrospective study. J. Am. Acad. Dermatol. 2005, 53, 230–236. [Google Scholar] [CrossRef]
- Ohn, J.; Choe, Y.S.; Mun, J.H. Dermoscopic features of nail matrix nevus (NMN) in adults and children: A comparative analysis. J. Am. Acad. Dermatol. 2016, 75, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Soura, E.; Sgouros, D.; Leotsakos, G.; Rigopoulos, D.; Stratigos, A.; Kontochrostopoulos, G. Nail matrix naevi in children: A prospective study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e203–e205. [Google Scholar] [CrossRef]
- Ronge, R.S.; Touzet, S.; Ligeron, C.; Balme, B.; Barrut, D.; Colin, C.; Thomas, L. Dermoscopic examination of nail pigmentation. Arch. Dermatol. 2002, 138, 1327–1333. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, Y.; Park, J.H.; Lee, J.H.; Jang, K.T.; Kwon, E.J.; Lee, D.Y. Clinicopathologic features of 28 cases of nail matrix nevi (NMNs) in Asians: Comparison between children and adults. J. Am. Acad. Dermatol. 2018, 78, 479–489. [Google Scholar] [CrossRef]
- Gunes, P.; Goktay, F.; Haneke, E. A case of adult-onset longitudinal melanonychia due to nail matrix compound nevus. J. Cutan. Pathol. 2020, 47, 1159–1163. [Google Scholar] [CrossRef]
- Tosti, A.; Baran, R.; Piraccini, B.M.; Cameli, N.; Fanti, P.A. Nail matrix nevi: A clinical and histopathologic study of twenty-two patients. J. Am. Acad. Dermatol. 1996, 4, 765–771. [Google Scholar] [CrossRef]
- Haneke, E. Histopathology of the Nail: Onychopathology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ren, J.; Ren, M.; Kong, Y.Y.; Lv, J.J.; Cai, X.; Kong, J.C. Clinicopathological diversity and outcome of longitudinal melanonychia in children and adolescents: Analysis of 35 cases identified by excision specimens. Histopathology 2020, 77, 380–390. [Google Scholar] [CrossRef]
- Saida, T. Lessons learned from studies of the development of early melanoma. Int. J. Clin. Oncol. 2005, 10, 371–374. [Google Scholar] [CrossRef]
- Hiroshi, K.; Toshiaki, S. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch. Dermatol. 2011, 147, 741–743. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.; Kim, D.H.; Lee, J.W.; Mun, J.H.; Lee, S.H. Clinical features and natural course of pediatric longitudinal melanonychia: A retrospective cohort study in Korea. J. Am. Acad. Dermatol. 2022, 87, 366–372. [Google Scholar] [CrossRef]
- Bastian, B.C. The molecular pathology of melanoma: An integrated taxonomy of melanocytic neoplasia. Annu. Rev. Pathol. 2014, 9, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J. Clinical Memoranda, Melanosis often not black: Melanotic whitlow. Br. Med. J. 1886, 1, 491–4933. [Google Scholar]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumours, 4th ed.; WHO: Lyon, France, 2018; ISBN 978-92-832-2440-2.
- Ossio, R.; Roldan-Marin, R.; Martinez-Said, H.; Adams, D.J.; Robles-Espinoza, C.D. Melanoma: A global perspective. Nat. Rev. Cancer 2017, 17, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kim, J.H.; Park, K.H.; Lee, J.S.; Bae, J.M.; Oh, B.H.; Rha, S.Y.; Roh, M.R.; Chung, K.Y. Comparison of melanoma subtypes among Korean patients by morphologic features and ultraviolet exposure. Ann. Dermatol. 2014, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Chung, B.Y.; Sim, C.Y.; Park, A.Y.; Lee, J.S.; Whang, K.U.; Park, Y.L.; Kim, H.O.; Park, C.W.; Lee, S.Y. Clinicopathologic features and prognostic factors of primary cutaneous melanoma: A multicenter study in Korea. J. Korean Med. Sci. 2019, 34, e126. [Google Scholar] [CrossRef]
- Jin, S.A.; Chun, S.M.; Choi, Y.D.; Kweon, S.S.; Jung, S.T.; Shim, H.J.; Yun, S.J. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J. Investig. Dermatol. 2013, 133, 579–582. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.H.; Lee, D.Y. Site distribution of cutaneous melanoma in South Korea: A retrospective study at a single tertiary institution. Int. J. Dermatol. 2015, 54, e38–e39. [Google Scholar] [CrossRef]
- Ishihara, K.; Saida, T.; Otsuka, F.; Yamazaki, N.; Prognosis & Statistical Investigation Committee of the Japanese Skin Cancer Society. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int. J. Clin. Oncol. 2008, 13, 33–41. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chay, W.Y.; Tang, M.B.; Chio, M.T.; Tan, S.H. Melanoma: Differences between Asian and Caucasian patients. Ann. Acad. Med. Singap. 2012, 41, 17–20. [Google Scholar] [PubMed]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef]
- Chang, J.W.; Yeh, K.Y.; Wang, C.H.; Yang, T.S.; Chiang, H.F.; Wei, F.C.; Kuo, T.T.; Yang, C.H. Malignant melanoma in Taiwan: A prognostic study of 181 cases. Melanoma Res. 2004, 14, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ma, S. Racial differences in six major subtypes of melanoma: Descriptive epidemiology. BMC Cancer 2016, 16, 691. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; McCredie, M.; MacKie, R.; Giles, G.; Young, P.; Morton, C.; Jackman, L.; Thursfield, V. A case-control study of melanomas of the soles and palms (Australia and Scotland). Cancer Causes Control 1999, 10, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Rokuhara, S.; Saida, T.; Oguchi, M.; Matsumoto, K.; Murase, S.; Oguchi, S. Number of acquired melanocytic nevi in patients with melanoma and control subjects in Japan: Nevus count is a significant risk factor for nonacral melanoma but not for acral melanoma. J. Am. Acad. Dermatol. 2004, 50, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kweon, S.S.; Lee, J.B.; Lee, S.C.; Yun, S.J. A clinicopathologic analysis of 177 acral melanomas in Koreans: Relevance of spreading pattern and physical stress. JAMA Dermatol. 2013, 149, 1281–1288. [Google Scholar] [CrossRef]
- Minagawa, A.; Omodaka, T.; Okuyama, R. Melanomas and mechanical stress points on the plantar surface of the foot. N. Engl. J. Med. 2016, 374, 2404–2406. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, Y.D.; Hwang, J.H.; Shin, M.H.; Yun, S.J. Frequency of trauma, physical stress, and occupation in acral melanoma: Analysis of 313 acral melanoma patients in Korea. Ann. Dermatol. 2021, 33, 228–236. [Google Scholar] [CrossRef]
- Tod, B.M.; Schneider, J.W.; Bowcock, A.M.; Visser, W.I.; Kotze, M.J. The tumor genetics of acral melanoma: What should a dermatologist know? JAAD Int. 2020, 1, 135–147. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Yeh, I.; Jorgenson, E.; Shen, L.; Xu, M.; North, J.P.; Shain, A.H.; Reuss, D.; Wu, H.; Robinson, W.A.; Olshen, A.; et al. Targeted genomic profiling of acral melanoma. J. Natl. Cancer Inst. 2019, 111, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.S.; Tan, K.T.; Tse, K.P.; Liao, Y.H.; Lin, M.H.; Chen, J.S.; Liau, J.Y.; Tseng, Y.J.; Lee, C.H.; Hong, C.H.; et al. Genetic alterations in primary melanoma in Taiwan. Br. J. Dermatol. 2020, 182, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.D.; Chun, S.M.; Jin, S.A.; Lee, J.B.; Yun, S.J. Amelanotic acral melanomas: Clinicopathological, BRAF mutation, and KIT aberration analyses. J. Am. Acad. Dermatol. 2013, 69, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Kim, S.J. Images in clinical medicine. Hutchinson’s nail sign. N. Engl. J. Med. 2011, 364, e38. [Google Scholar] [CrossRef] [PubMed]
- Koga, H. Dermoscopic evaluation of melanonychia. J. Dermatol. 2017, 44, 515–517. [Google Scholar] [CrossRef]
- Ohn, J.; Jo, G.; Cho, Y.; Sheu, S.L.; Cho, K.H.; Mun, J.H. Assessment of a predictive scoring model for dermoscopy of subungual melanoma in situ. JAMA Dermatol. 2018, 154, 890–896. [Google Scholar] [CrossRef]
- Haugh, A.M.; Zhang, B.; Quan, V.L.; Garfield, E.M.; Bubley, J.A.; Kudalkar, E.; Verzi, A.E.; Walton, K.; VandenBoom, T.; Merkel, E.A.; et al. Distinct patterns of acral melanoma based on site and relative sun exposure. J. Investig. Dermatol. 2018, 138, 384–393. [Google Scholar] [CrossRef]
- Desai, A.; Ugorji, R.; Khachemoune, A. Acral melanoma foot lesions. Part 2: Clinical presentation, diagnosis, and management. Clin. Exp. Dermatol. 2018, 43, 117–123. [Google Scholar] [CrossRef]
- McBride, J.D.; McAfee, J.L.; Piliang, M.; Bergfeld, W.F.; Fernandez, A.P.; Ronen, S.; Billings, S.D.; Ko, J.S. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J. Cutan. Pathol. 2022, 49, 220–230. [Google Scholar] [CrossRef]
- Rothrock, A.T.; Torres-Cabala, C.A.; Milton, D.R.; Cho, W.C.; Nagarajan, P.; Vanderbeck, K.; Curry, J.L.; Ivan, D.; Prieto, V.G.; Aung, P.P. Diagnostic utility of PRAME expression by immunohistochemistry in subungual and non-subungual acral melanocytic lesions. J. Cutan. Pathol. 2022. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Gumaste, P.V.; Fleming, N.H.; Silva, I.; Shapiro, R.L.; Berman, R.S.; Zhong, J.; Osman, I.; Stein, J.A. Analysis of recurrence patterns in acral versus nonacral melanoma: Should histologic subtype influence treatment guidelines? J. Natl. Compr. Cancer Netw. 2014, 12, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Coit, D.G.; Thompson, J.A.; Albertini, M.R.; Barker, C.; Carson, W.E.; Contreras, C.; Daniels, G.A.; DiMaio, D.; Fields, R.C.; Fleming, M.D.; et al. Cutaneous melanoma, Version 2.2019, NCCN Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Thompson, J.A.; Albertini, M.R.; Barker, C.A.; Baumgartner, J.; Boland, G.; Chmielowski, B.; DiMaio, D.; Durham, A.; Fields, R.C.; et al. NCCN Guidelines(R) Insights: Melanoma: Cutaneous, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 364–376. [Google Scholar] [CrossRef]
- Ryu, G.W.; Choi, Y.D.; Jin, S.; Chung, I.J.; Shin, M.H.; Yun, S.J. Volar location and degree of pigmentation are associated with poor survival and first metastasis pattern in acral melanoma. Pigment. Cell Melanoma Res. 2021, 34, 1094–1104. [Google Scholar] [CrossRef]
- Ryu, G.W.; Choi, Y.D.; Ryu, Y.J.; Lee, J.B.; Shin, M.H.; Yun, S.J. Risk factors affecting the first metastasis of acral melanoma: Low- pigmentation independently predicts a first lung metastasis. J. Am. Acad. Dermatol. 2021, 84, 1739–1742. [Google Scholar] [CrossRef]
- Sladden, M.J.; Balch, C.; Barzilai, D.A.; Berg, D.; Freiman, A.; Handiside, T.; Hollis, S.; Lens, M.B.; Thompson, J.F. Surgical excision margins for primary cutaneous melanoma. Cochrane Database Syst. Rev. 2009, 4, CD004835. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, S.J.; McMurray, S.L.; Miller, C.J. Management of nail unit melanoma. Dermatol. Clin. 2021, 39, 269–280. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).