Histopathology of Hidradenitis Suppurativa: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Search Strategies
2.2. Inclusion Criteria
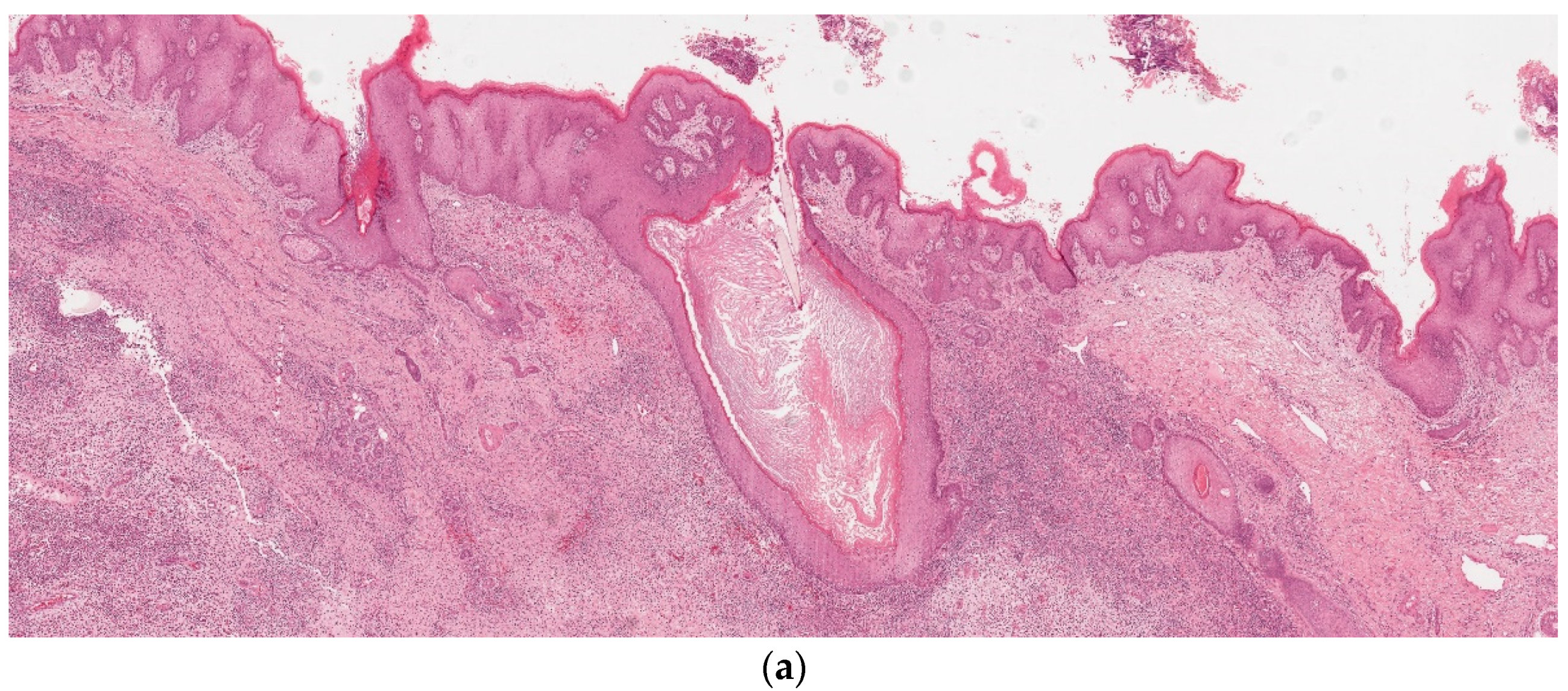
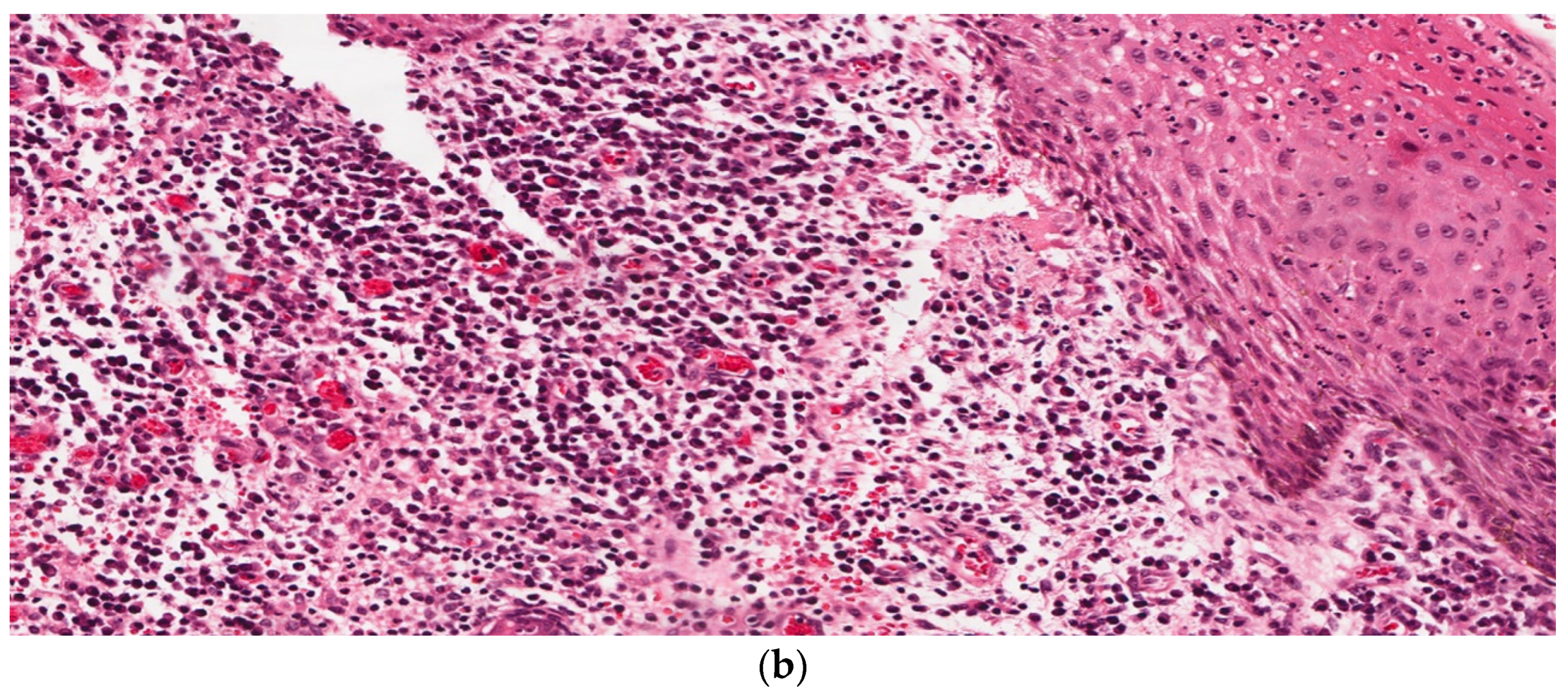
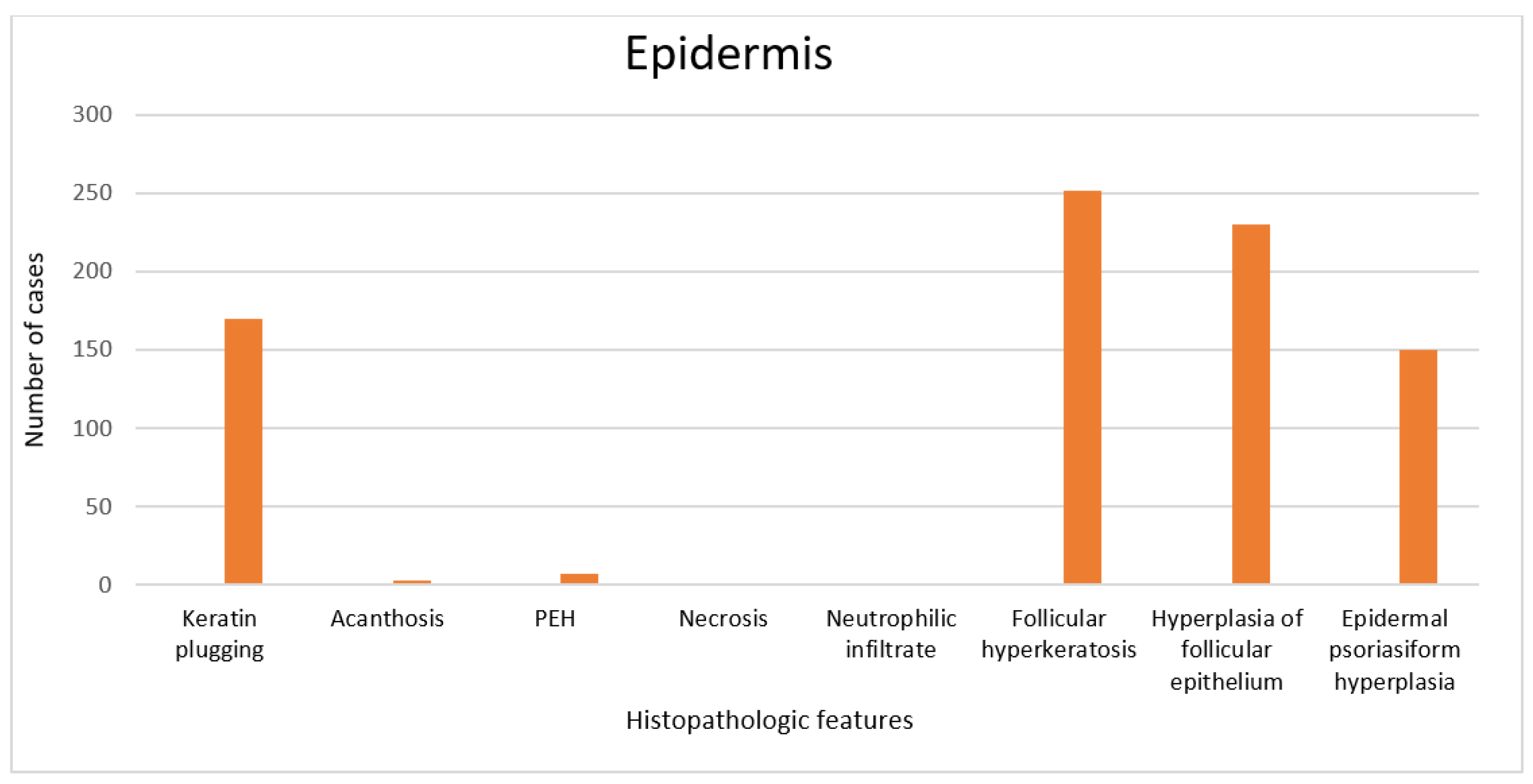
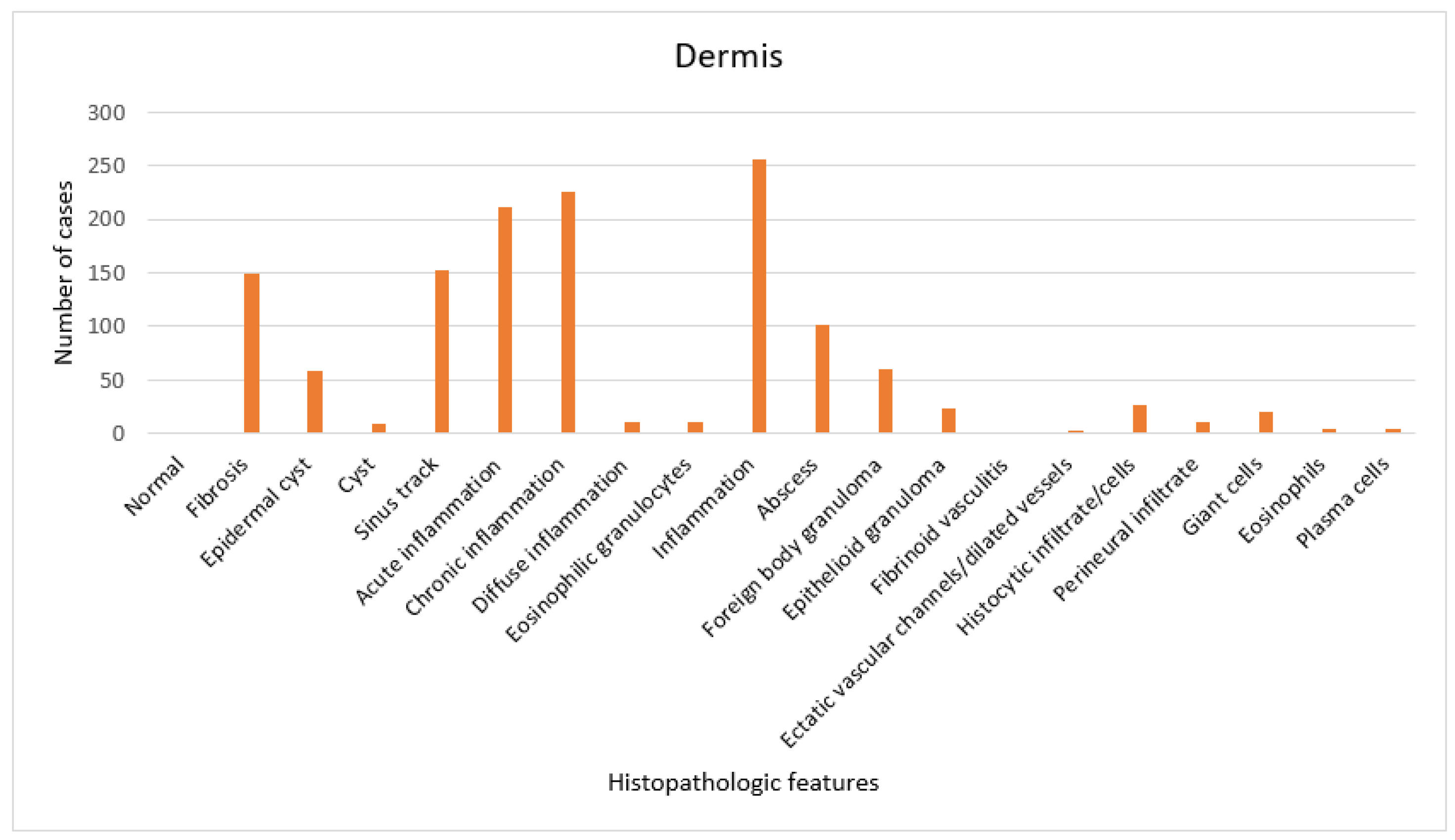
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunsting, H. Hidradenitis suppurativa; abscess of apocrine sweat glands—A study of clinical and pathological features with a report of 22 cases and a review of the literature. Arch. Dermatol. Syphilol. 1939, 39, 108–120. [Google Scholar] [CrossRef]
- Verneuil, A. Etudes sur les tumeurs de la peau de quelques maladies des glandes sudoripares. Arch. Gen. Med. 1854, 4, 447–468. [Google Scholar]
- Garg, A.; Lavian, J.; Lin, G.; Strunk, A.; Alloo, A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J. Am. Acad. Derm. 2017, 77, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Benhadou, F.; Byrd, A.S.; Chandran, N.S.; Giamarellos-Bourboulis, E.J.; Fabbrocini, G.; Frew, J.W.; Fujita, H.; González-López, M.A.; Guillem, P.; et al. What causes hidradenitis suppurativa?—15 years after. Exp. Dermatol. 2020, 29, 1154–1170. [Google Scholar] [CrossRef]
- Gao, M.; Wang, P.G.; Cui, Y.; Yang, S.; Zhang, Y.H.; Lin, D.; Zhang, K.-Y.; Liang, Y.-H.; Sun, L.-D.; Yan, K.-L.; et al. Inversa acne (hidradenitis suppurativa): A case report and identification of the locus at chromosome 1p21.1–1q25.3. J. Investig. Dermatol. 2006, 126, 1302–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Yang, W.; Wen, W.; Sun, J.; Su, B.; Liu, B.; Ma, D.; Lv, D.; Wen, Y.; Qu, T.; et al. Secretase gene mutations in familial acne inversa. Science 2010, 330, 1065. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K.; Krahl, D. “Hidradenitis suppurativa” is acne inversa! An appeal to (Finally) abandon a misnomer. Int. J. Dermatol. 2005, 44, 535–540. [Google Scholar] [CrossRef]
- von Laffert, M.; Stadie, V.; Wohlrab, J.; Marsch, W.C. Hidradenitis suppurativa/acne inversa: Bilocated epithelial hyperplasia with very different sequelae: HS with bilocated epithelial hyperplasia. Br. J. Dermatol. 2011, 164, 367–371. [Google Scholar] [CrossRef]
- Thomas, R.; Barnhill, D.; Bibro, M.; Hoskins, W. Hidradenitis suppurativa: A case presentation and review of the literature. Obstet. Gynecol. 1985, 66, 592–595. [Google Scholar]
- Yu, C.; Cook, M. Hidradenitis suppurativa: A disease of follicular epithelium, rather than apocrine glands. Br. J. Dermatol. 1990, 122, 763–769. [Google Scholar] [CrossRef]
- von Laffert, M.; Helmbold, P.; Wohlrab, J.; Fiedler, E.; Stadie, V.; Marsch, W.C. Hidradenitis suppurativa (acne inversa): Early inflammatory events at terminal follicles and at interfollicular epidermis. Exp. Dermatol. 2010, 19, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T. Squamous cell carcinoma: Complication of chronic skin disorders in black patients. J. Natl. Med. Assoc. 1986, 78, 1203–1205. [Google Scholar] [PubMed]
- Romero-Pérez, D.; Encabo-Durán, B.; de Jaime, M.N.; Peiro-Cabrera, G.; Pascual, J.C. Crystal Deposition in Hidradenitis Suppurativa. Am. J. Dermatopathol. 2019, 41, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.S.; Haefner, H.K.; Hameed, M.; Lieberman, R. Vulvar hidradenitis suppurativa. Immunohistochemical evaluation of apocrine and eccrine involvement. J. Reprod. Med. 2002, 47, 695–700. [Google Scholar]
- Lin, M.; Breiner, M.; Fredricks, S. Marjolin’s ulcer occurring in hidradenitis suppurativa. Plast. Reconstr. Surg. 1999, 103, 1541–1543. [Google Scholar]
- McCarthy, S.; Foley, C.C.; Dvorakova, V.; Quinlan, C.; Murphy, M.; Maher, M. PASH syndrome with bony destruction. Clin. Exp. Dermatol. 2019, 44, 918–920. [Google Scholar] [CrossRef]
- Jemec, G.; Thomsen, B.; Hansen, U. The homogeneity of hidradenitis suppurativa lesions. A histological study of intra-individual variation. APMIS 1997, 105, 378–383. [Google Scholar] [CrossRef]
- Ward, R.A.; Udechukwu, N.S.; Selim, M.A.; Jaleel, T. Vulvar and perineal verrucous changes complicating hidradenitis suppurativa after wide excision: A case and literature review. Dermatol. Online J. 2020, 26, 13030. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; Schoenmakers, A.; van Straalen, K.; Prens, E.P.; Van Der Zee, H.H. Assessing pruritus in hidradenitis suppurativa: A cross-sectional study. Am. J. Clin. Dermatol. 2017, 18, 687–695. [Google Scholar]
- Boer, J.; Weltevreden, E. Hidradenitis suppurativa or acne inversa. A clinicopathological study of early lesions. Br. J. Dermatol. 1996, 135, 721–725. [Google Scholar] [CrossRef]
- Attanoos, R.; Appleton, M.; Hughes, L.; Ansell, I.; Douglas-Jones, A.; Williams, G. Granulomatous hidradenitis suppurativa and cutaneous Crohn’s disease. Histopathology 1993, 23, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Attanoos, R.; Appleton, M.; Douglas-Jones, A. The pathogenesis of hidradenitis suppurativa: A closer look at apocrine and apoeccrine glands. Br. J. Dermatol. 1995, 133, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Gosnell, H.L.; Sharghi, K.; Pickard, C.; Grider, D.J. Hidradenitis suppurativa at the knees. Dermatol. Online J. 2020, 26, 13030. [Google Scholar] [CrossRef] [PubMed]
- Jemec, G.; Hansen, U. Histology of hidradenitis suppurativa. J. Am. Acad. Derm. 1996, 34, 994–999. [Google Scholar] [CrossRef]
- Chu, E.; Kovarik, C.; Lee, R. Lymphedematous verrucous changes simulating squamous cell carcinoma in long-standing hidradenitis suppurativa. Int. J. Dermatol. 2013, 52, 808–812. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Horvath, B.; Jemec, G.B.; Prens, E.P. The association between hidradenitis suppurativa and Crohn’s disease: In search of the missing pathogenic link. J. Investig. Dermatol. 2016, 136, 1747–1748. [Google Scholar] [CrossRef] [Green Version]
- Kalen, J.E.; Shokeen, D.; Mislankar, M.; Wangia, M.; Motaparthi, K. Langerhans Cell Histiocytosis with Clinical and Histologic Features of Hidradenitis Suppurativa: Brief Report and Review. Am. J. Dermatopathol. 2018, 40, 502–505. [Google Scholar] [CrossRef]
- Shamim, H.; Riemer, C.; Weenig, R.; Sokumbi, O.; Sciallis, G.; McEvoy, M.; Mischke, D.; Comfere, N. Acneiform Presentations of Folliculotropic Mycosis Fungoides. Am. J. Dermatopathol. 2021, 43, 85–92. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.D.B.; Okoye, G.A.; Sokumbi, O. Histopathology of Hidradenitis Suppurativa: A Systematic Review. Dermatopathology 2022, 9, 251-257. https://doi.org/10.3390/dermatopathology9030029
Smith SDB, Okoye GA, Sokumbi O. Histopathology of Hidradenitis Suppurativa: A Systematic Review. Dermatopathology. 2022; 9(3):251-257. https://doi.org/10.3390/dermatopathology9030029
Chicago/Turabian StyleSmith, Shane David Basil, Ginette A. Okoye, and Olayemi Sokumbi. 2022. "Histopathology of Hidradenitis Suppurativa: A Systematic Review" Dermatopathology 9, no. 3: 251-257. https://doi.org/10.3390/dermatopathology9030029
APA StyleSmith, S. D. B., Okoye, G. A., & Sokumbi, O. (2022). Histopathology of Hidradenitis Suppurativa: A Systematic Review. Dermatopathology, 9(3), 251-257. https://doi.org/10.3390/dermatopathology9030029





