Cutaneous Reactions after COVID-19 Vaccines: Analysis of the Clinical and Histopathological Spectrum—Case Series and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
3.2. Vaccine Types and Latency
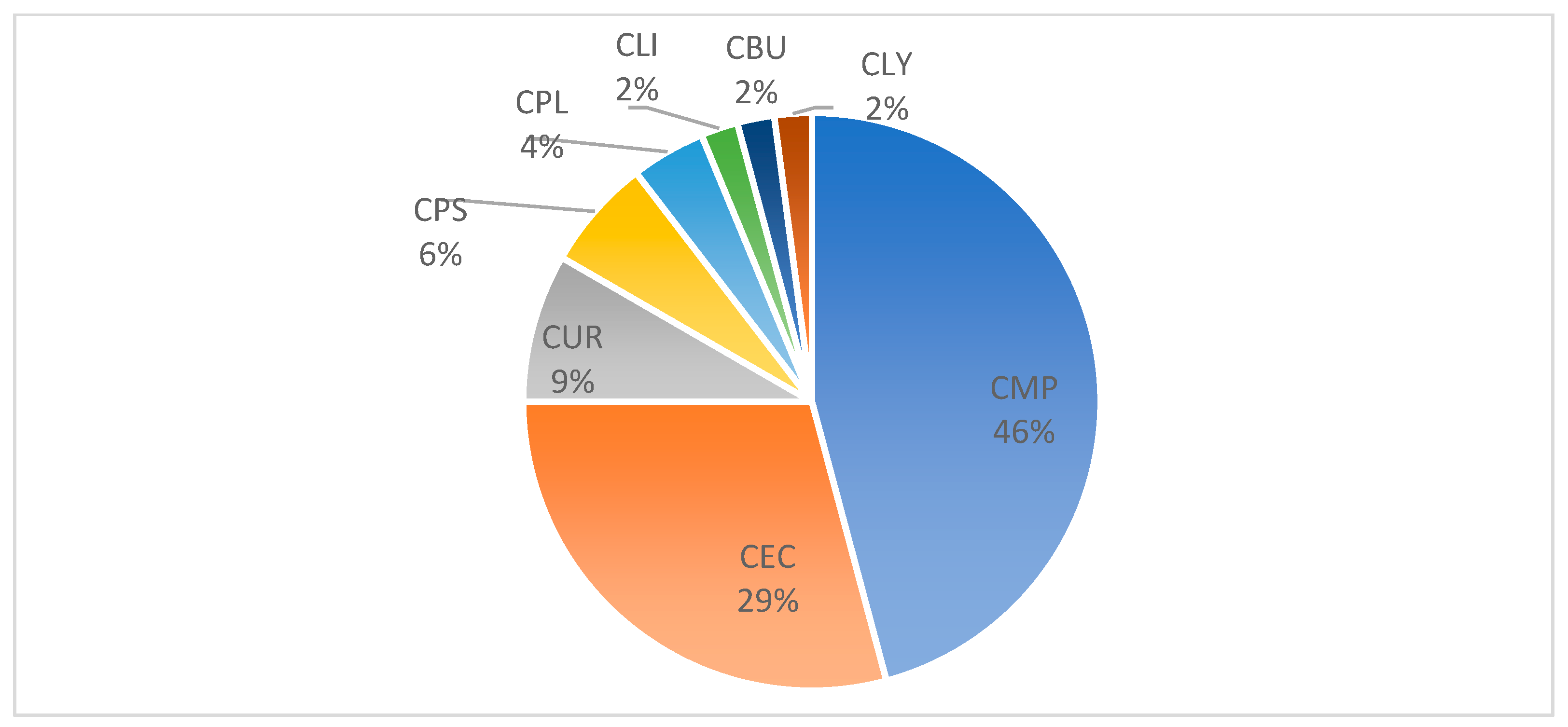
3.3. Distribution of Cutaneous Reactions
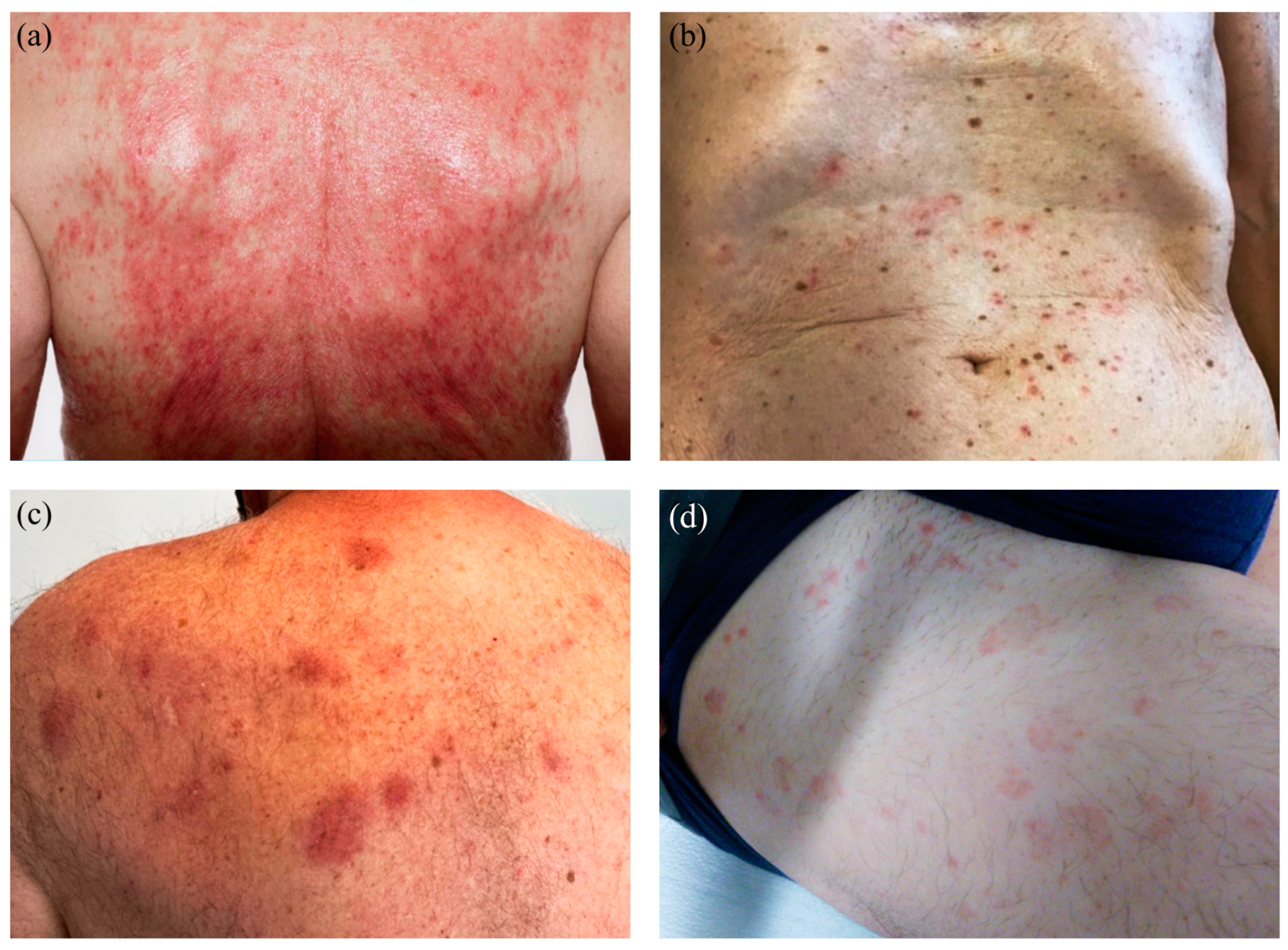
3.4. Cutaneous Reactions—Spectrum of Clinical Manifestations
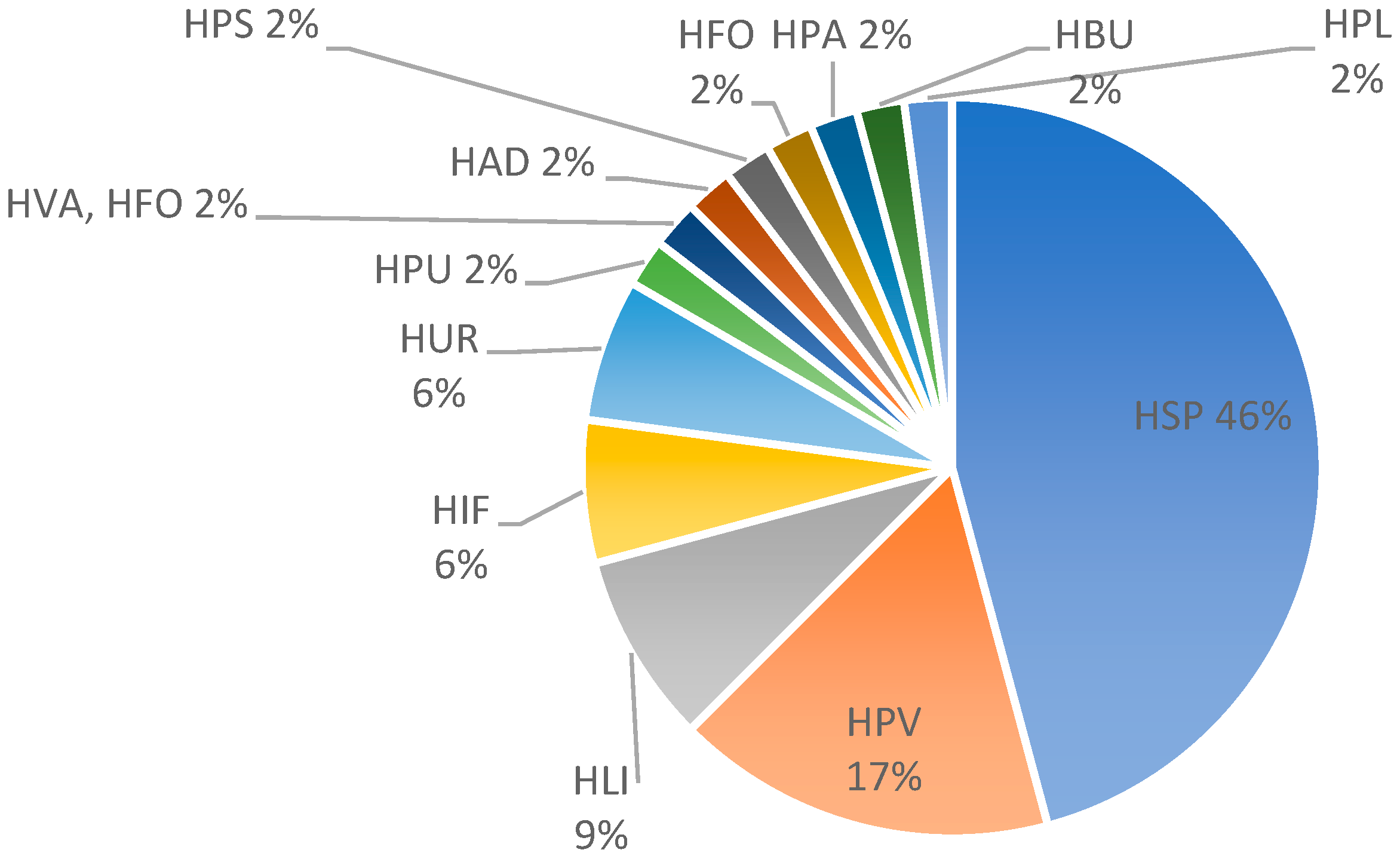
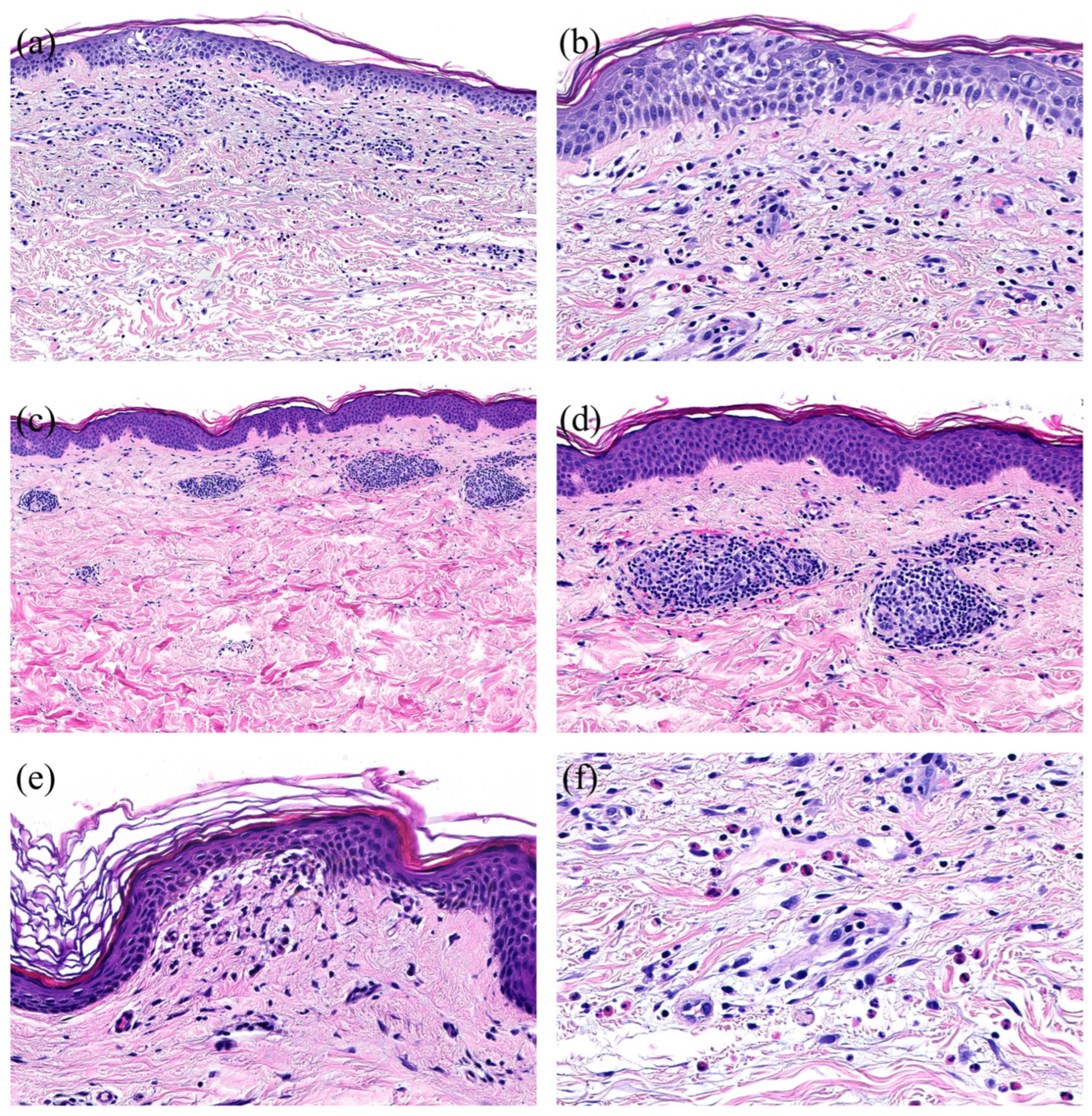
3.5. Cutaneous Reactions—Spectrum of Histopathological Features
3.6. Correlation of Histological Patterns with Clinical Presentations
3.7. New-Onset Cutaneous Reaction vs. Aggravation of Preexisting Skin Disease
3.8. Duration of Cutaneous Reactions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 1 September 2022).
- FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 2 September 2022).
- FDA Takes Additional Action in Fight against COVID-19 by Issuing Emergency Use Authorization for Second COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid (accessed on 19 April 2021).
- COVID-19 Schweiz: Informationen zur Aktuellen Lage. Available online: https://www.covid19.admin.ch/de/vaccination/persons (accessed on 5 September 2022).
- Kempf, W.; Kettelhack, N.; Kind, F.; Courvoisier, S.; Galambos, J.; Pfaltz, K. ‘COVID arm’—Histological features of a delayed-type hypersensitivity reaction to Moderna mRNA-1273 SARS-CoV2 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e730–e732. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Bellinato, F.; Maurelli, M.; Gisondi, P.; Girolomoni, G. Cutaneous Adverse Reactions Associated with SARS-CoV-2 Vaccines. J. Clin. Med. 2021, 10, 5344. [Google Scholar] [CrossRef] [PubMed]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous reactions after SARS-COV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Dermatol. 2021, 186, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Niebel, D.; Novak, N.; Wilhelmi, J.; Ziob, J.; Wilsmann-Theis, D.; Bieber, T.; Wenzel, J.; Braegelmann, C. Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective. Vaccines 2021, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.B.; Fu, X.; Hashimoto, D.; Wickner, P.; Shenoy, E.S.; Landman, A.B.; Blumenthal, K.G. Incidence of Cutaneous Reactions After Messenger RNA COVID-19 Vaccines. JAMA Dermatol. 2021, 157, 1000–1002. [Google Scholar] [CrossRef]
- Sun, Q.; Fathy, R.; McMahon, D.E.; Freeman, E.E. COVID-19 Vaccines and the Skin: The Landscape of Cutaneous Vaccine Reactions Worldwide. Dermatol. Clin. 2021, 39, 653–673. [Google Scholar] [CrossRef]
- Tihy, M.; Menzinger, S.; André, R.; Laffitte, E.; Toutous-Trellu, L.; Kaya, G. Clinicopathological features of cutaneous reactions after mRNA-based COVID-19 vaccines. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2456–2461. [Google Scholar] [CrossRef]
- Avallone, G.; Quaglino, P.; Cavallo, F.; Roccuzzo, G.; Ribero, S.; Zalaudek, I.; Conforti, C. SARS-CoV-2 vaccine-related cutaneous manifestations: A systematic review. Int. J. Dermatol. 2022, 61, 1187–1204. [Google Scholar] [CrossRef]
- Larson, V.; Seidenberg, R.; Caplan, A.; Brinster, N.K.; Meehan, S.A.; Kim, R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2022, 49, 34–41. [Google Scholar] [CrossRef]
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2022, 86, 113–121. [Google Scholar] [CrossRef]
- Kroumpouzos, G.; Paroikaki, M.E.; Yumeen, S.; Bhargava, S.; Mylonakis, E. Cutaneous Complications of mRNA and AZD1222 COVID-19 Vaccines: A Worldwide Review. Microorganisms 2022, 10, 624. [Google Scholar] [CrossRef]
- Magro, C.; Crowson, A.N.; Franks, L.; Schaffer, P.R.; Whelan, P.; Nuovo, G. The histologic and molecular correlates of COVID-19 vaccine-induced changes in the skin. Clin. Dermatol. 2021, 39, 966–984. [Google Scholar] [CrossRef]
- Askenase, P.W. Rare Skin Reactions after mRNA Vaccination, Similar to Jones-Mote Basophil Responses. N. Engl. J. Med. 2021, 385, 1720–1721. [Google Scholar] [CrossRef]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef]
- Aram, K.; Patil, A.; Goldust, M.; Rajabi, F. COVID-19 and exacerbation of dermatological diseases: A review of the available literature. Dermatol. Ther. 2021, 34, e15113. [Google Scholar] [CrossRef]
- Gambichler, T.; Boms, S.; Susok, L.; Dickel, H.; Finis, C.; Abu Rached, N.; Barras, M.; Stücker, M.; Kasakovski, D. Cutaneous findings following COVID-19 vaccination: Review of world literature and own experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Niebel, D.; Wenzel, J.; Wilsmann-Theis, D.; Ziob, J.; Wilhelmi, J.; Braegelmann, C. Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines. Dermatopathology 2021, 8, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Alelq, N.A.; Kubieniec, M.E.; French, L.E.; Prinz, J.C. Influence of Covid-19 vaccination on immune-mediated skin diseases. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e965–e968. [Google Scholar] [CrossRef] [PubMed]
- Pesqué, D.; Lopez-Trujillo, E.; Marcantonio, O.; Giménez-Arnau, A.M.; Pujol, R.M. New-onset and exacerbations of psoriasis after mRNA COVID-19 vaccines: Two sides of the same coin? J. Eur. Acad. Dermatol. Venereol. 2022, 36, e80–e81. [Google Scholar] [CrossRef]
- Juárez Guerrero, A.; Domínguez Estirado, A.; Crespo Quirós, J.; Rojas-Pérez-Esguerra, P. Delayed cutaneous reactions after the administration of mRNA vaccines against COVID-19. J. Allergy Clin. Immunol. Pract. 2021, 9, 3811–3813. [Google Scholar] [CrossRef]
- Cantisani, C.; Chello, C.; Grieco, T.; Ambrosio, L.; Kiss, N.; Tammaro, A.; Tosti, G.; Paolino, G.; Pellacani, G. Cutaneous Reactions to COVID-19 Vaccines in a Monocentric Study: A Case Series. J. Clin. Med. 2022, 11, 3811. [Google Scholar] [CrossRef] [PubMed]
- Oulee, A.; Salem, S.; Yahia, R.; Yang, K.; Garcia, D.; Holmes, A.; Furukawa, B. Cutaneous reactions due to Pfizer’s BNT162b2 mRNA and Moderna’s mRNA-1273 vaccines. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e332–e334. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Amini, Z.; Goodarzi, A. A comparative review on mucocutaneous reactions caused by COVID-19 infection versus Covid-19 vaccination. Exp. Dermatol. 2022, 31, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
| Study Number | Gender | Age | Exacerbation/New Onset | Vaccine | L (Local) | D (Distant) | Eosinophil | Neutrophil | Plasma Cells | 1. Dose, Latency | 2. Dose, Latency | 3. Dose, Latency | 4. Dose, Latency | Clinical Pattern | Histological Pattern | Comparison with McMahon et al. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 59 | new-onset | mRNA-1273 | DD | E0 | N1 | P0 | 1 d | CEC | HSP | Robust V-REPP | ||||
| 2 | m | 37 | exacerbation | mRNA-1273 | DD | E1 | N0 | P0 | 2 d | 1.5 d | CEC | HSP | Robust V-REPP | |||
| 3 | w | 67 | exacerbation | mRNA-1273 | DD | E2 | N0 | P0 | 1 d | CEC | HSP | Robust V-REPP | ||||
| 4 | w | 63 | new-onset | mRNA-1273 | DD | E2 | N0 | P0 | 14 d | CEC | HSP | Robust V-REPP | ||||
| 5 | w | 56 | new-onset | BNT162b2 | DD | E3 | N0 | P0 | 1 d | CEC | HSP | Robust V-REPP | ||||
| 6 | w | 54 | new-onset | mRNA-1273 | DD | E0 | N0 | P0 | 1 d | 1 d | 1 d | CEC | HSP | Robust V-REPP | ||
| 7 | w | 59 | new-onset | mRNA-1273 /BNT162b2 | DD | E0 | N2 | P0 | 1 d (mRNA-1273) | 1.5 d (BNT162b2) | 1 d (BNT162b2) | CEC | HSP | Robust V-REPP | ||
| 8 | m | 72 | new-onset | mRNA-1273 | DD | E1 | N1 | P0 | 9 d | CEC | HSP | Robust V-REPP | ||||
| 9 | w | 79 | exacerbation | mRNA-1273 | DD | E3 | N0 | P0 | 7 d | CEC | HSP | Robust V-REPP | ||||
| 10 | w | 32 | exacerbation | mRNA-1273 | DD | E1 | N0 | P0 | 14 d | CEC | HSP | Robust V-REPP | ||||
| 11 | w | 85 | exacerbation | BNT162b2 | DD | E0 | N2 | P0 | 2 d | 2 d | CEC | HPS | other | |||
| 12 | m | 58 | exacerbation | mRNA-1273 | DD | E2 | N0 | P0 | 10 d | CEC | HIF | mild V-REPP | ||||
| 13 | m | 62 | new-onset | BNT162b2 | DD | E0 | N0 | P0 | 14 d | CEC | HPV | dermal hypersensitivity | ||||
| 14 | m | 81 | new-onset | BNT162b2 | DD | E1 | N0 | P1 | 28 d | CMP | HSP | Robust V-REPP | ||||
| 15 | w | 82 | new-onset | mRNA-1273 | DD | E2 | N2 | P1 | 5.5 d | CMP | HSP | Robust V-REPP | ||||
| 16 | m | 38 | new-onset | mRNA-1273 | DD | E1 | N0 | P0 | 21 d | CMP | HSP | Robust V-REPP | ||||
| 17 | m | 52 | new-onset | mRNA-1273 | DD | E1 | N1 | P0 | 21 d | CMP | HSP | Robust V-REPP | ||||
| 18 | m | 70 | new-onset | mRNA-1273 | DD | E2 | N0 | P0 | 7 d | CMP | HSP | Robust V-REPP | ||||
| 19 | w | 70 | new-onset | BNT162b2 | DD | E0 | N0 | P0 | 28 d | CMP | HSP | Robust V-REPP | ||||
| 20 | w | 80 | new-onset | mRNA-1273 | DD | E2 | N2 | P0 | 5 d | CMP | HSP | Robust V-REPP | ||||
| 21 | w | 70 | new-onset | mRNA-1273 | DD | E1 | N0 | P0 | 21 d | CMP | HSP | Robust V-REPP | ||||
| 22 | m | 78 | new-onset | mRNA-1273 | DD | E1 | N2 | P1 | 3 d | CMP | HPV | dermal hypersensitivity | ||||
| 23 | w | 70 | new-onset | BNT162b2 | DD | E2 | N0 | P0 | 2 d | CMP | HPV | dermal hypersensitivity | ||||
| 24 | w | 74 | new-onset | mRNA-1273 | DD | E1 | N0 | P0 | 7 d | CMP | HPV | dermal hypersensitivity | ||||
| 25 | m | 74 | new-onset | mRNA-1273 | DD | E3 | N0 | P0 | 4 d | CMP | HPV | dermal hypersensitivity | ||||
| 26 | m | 44 | new-onset | mRNA-1273 | DD | E0 | N0 | P0 | 14 d | CMP | HPV | dermal hypersensitivity | ||||
| 27 | m | 82 | new-onset | mRNA-1273 | DD | E0 | N1 | P0 | 6 d | CMP | HPU | other | ||||
| 28 | m | 75 | new-onset | mRNA-1273 | DD | E0 | N0 | P0 | 21 d | CMP | HIF | mild V-REPP | ||||
| 29 | m | 50 | new-onset | mRNA-1273 | DL | E1 | N0 | P0 | 17 d | CMP | HFO | other | ||||
| 30 | w | 81 | new-onset | mRNA-1273 | DL | E0 | N0 | P0 | 10 d | CMP | HLI | LP-like | ||||
| 31 | w | 81 | new-onset | mRNA-1273 | DD | E3 | N2 | P0 | 14 d | CMP | HSP | Robust V-REPP | ||||
| 32 | m | 41 | new-onset | mRNA-1273 | DD | E0 | N2 | P0 | 7 d | CMP | HSP | robust V-REPP | ||||
| 33 | m | 33 | new-onset | mRNA-1273 | DD | E1 | N0 | P0 | 10 d | CUR | HIF | mild V-REPP | ||||
| 34 | w | 81 | exacerbation | mRNA-1273 | DD | E0 | N0 | P0 | 5.5 d | 6.5 d | CLI | HLI | LP-like | |||
| 35 | w | 73 | exacerbation | BNT162b2 | DD | E2 | N0 | P0 | 5 d | CUR | HUR | urticaria | ||||
| 36 | m | 39 | exacerbation | mRNA-1273 | DD | E2 | N0 | P0 | CUR | HUR | urticaria | |||||
| 37 | w | 24 | exacerbation | mRNA-1273 | DD | E2 | N2 | P0 | 3 d | CUR | HUR | urticaria | ||||
| 38 | w | 87 | new-onset | mRNA-1273 | L | E3 | N0 | P0 | 6 d | CPL | HSP | dermal hypersensitivity | ||||
| 39 | m | 77 | new-onset | BNT162b2 | L | E0 | N0 | P0 | 15 d | CEC | HSP | dermal hypersensitivity | ||||
| 40 | w | 81 | new-onset | mRNA-1273 | L | E2 | N0 | P0 | 7 d | CMP | HPV | COVID-Arm | ||||
| 41 | w | 67 | new-onset | mRNA-1273 | L | E0 | N0 | P1 | 5.5 d | 8.5 d | CMP | HPV | dermal hypersensitivity | |||
| 42 | m | 81 | new-onset | mRNA-1273 | DD | E3 | N0 | P0 | 1 d | CMP | HAD | M. Grover | ||||
| 43 | m | 71 | new-onset | BNT162b2 | DL | E1 | N0 | P0 | 6 d | CPL | HVA, HFO | vaskulitis | ||||
| 44 | m | 85 | exacerbation | mRNA-1273 | DD | E2 | N3 | P0 | 14 d | 2 d | CBU | HBU | Bullous pemphigoid-like | |||
| 45 | w | 90 | new-onset | mRNA-1273 | DD | E1 | N0 | P0 | 3.5 d | CPS | HLI | LP-like | ||||
| 46 | m | 70 | new-onset | BNT162b2 | DD | E1 | N0 | P0 | 7 d | CPS | HLI | LP-like | ||||
| 47 | w | 77 | new-onset | BNT162b2 | DD | E0 | N2 | P0 | weeks | CPS | HPA | other | ||||
| 48 | m | 69 | new-onset | BNT162b2 | DD | E0 | N0 | P0 | 16 d | CLY | HPL | other |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, U.; Galambos, J.; Pfaltz, K.; Hegyi, I.; Courvoisier, S.; Kempf, W. Cutaneous Reactions after COVID-19 Vaccines: Analysis of the Clinical and Histopathological Spectrum—Case Series and Review of the Literature. Dermatopathology 2024, 11, 130-141. https://doi.org/10.3390/dermatopathology11010013
Schmid U, Galambos J, Pfaltz K, Hegyi I, Courvoisier S, Kempf W. Cutaneous Reactions after COVID-19 Vaccines: Analysis of the Clinical and Histopathological Spectrum—Case Series and Review of the Literature. Dermatopathology. 2024; 11(1):130-141. https://doi.org/10.3390/dermatopathology11010013
Chicago/Turabian StyleSchmid, Ursina, Jörg Galambos, Katrin Pfaltz, Ivan Hegyi, Salomé Courvoisier, and Werner Kempf. 2024. "Cutaneous Reactions after COVID-19 Vaccines: Analysis of the Clinical and Histopathological Spectrum—Case Series and Review of the Literature" Dermatopathology 11, no. 1: 130-141. https://doi.org/10.3390/dermatopathology11010013
APA StyleSchmid, U., Galambos, J., Pfaltz, K., Hegyi, I., Courvoisier, S., & Kempf, W. (2024). Cutaneous Reactions after COVID-19 Vaccines: Analysis of the Clinical and Histopathological Spectrum—Case Series and Review of the Literature. Dermatopathology, 11(1), 130-141. https://doi.org/10.3390/dermatopathology11010013







