TRPS1: A Marker of Follicular Differentiation
Abstract
1. Introduction
2. Materials and Methods
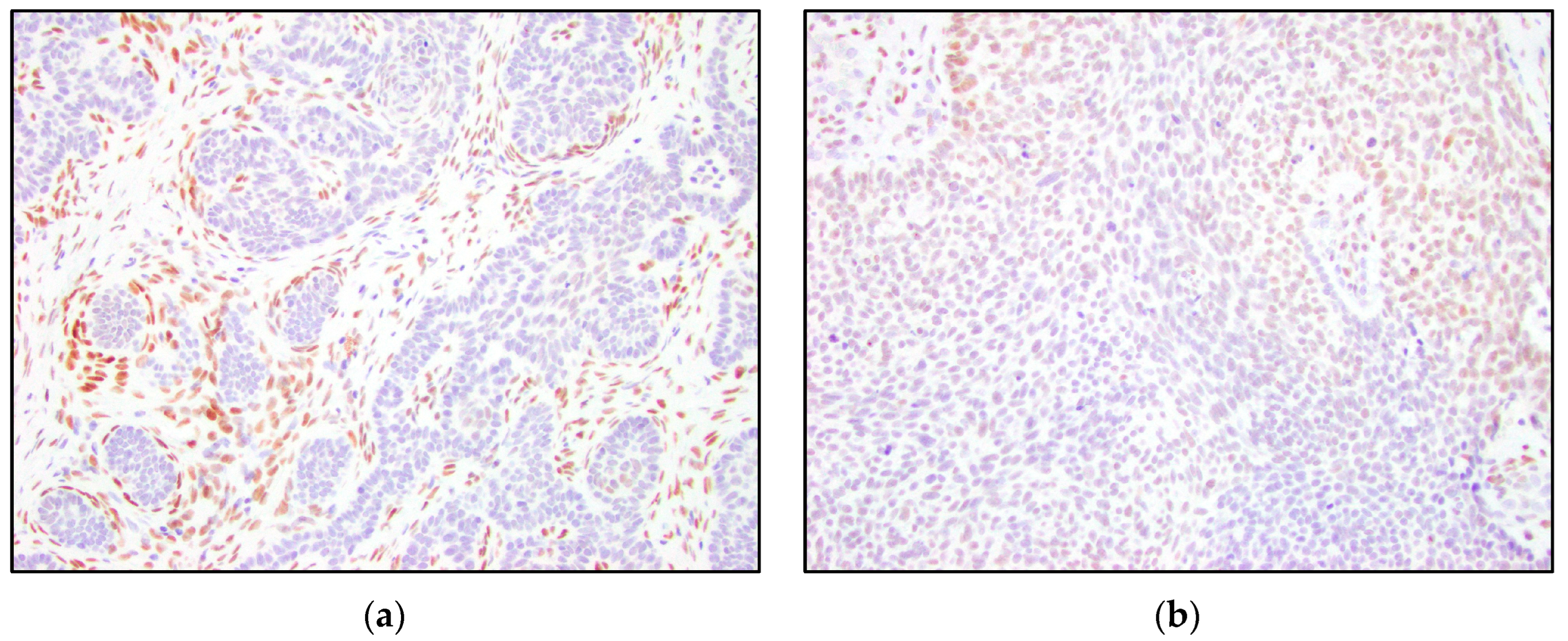
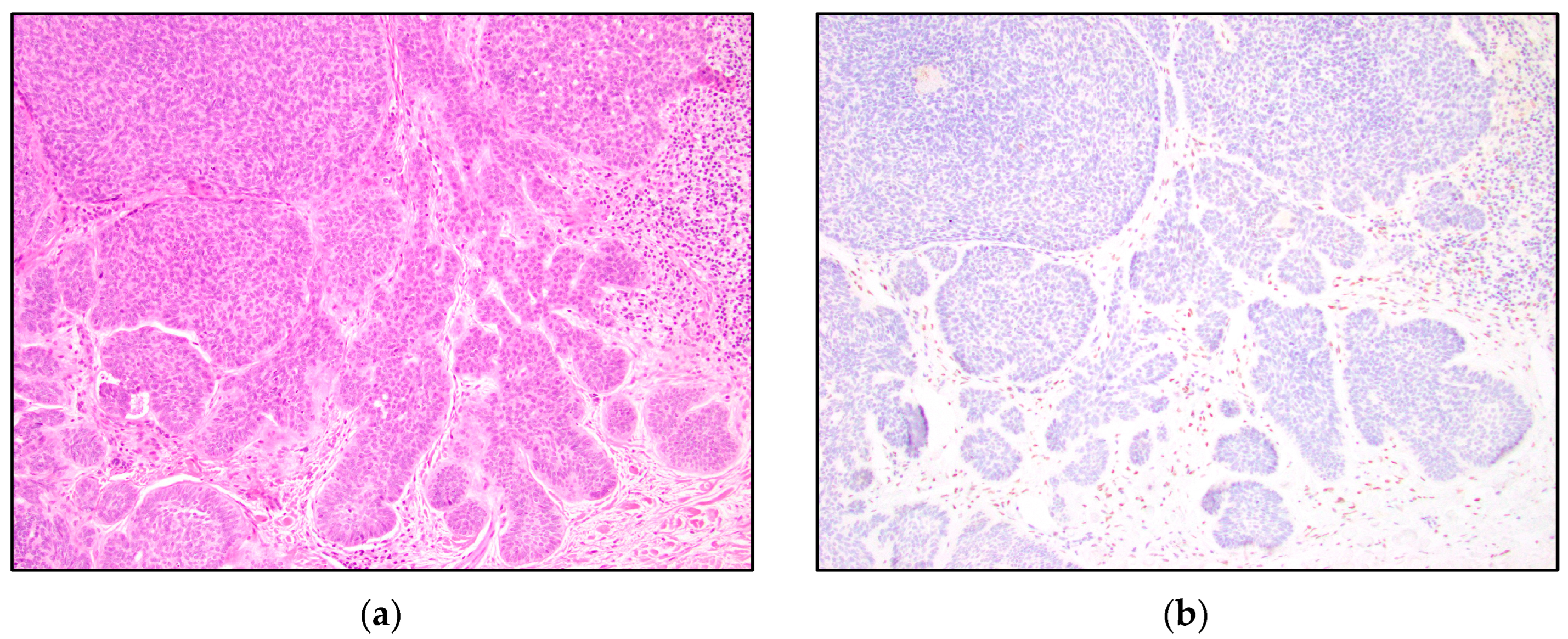
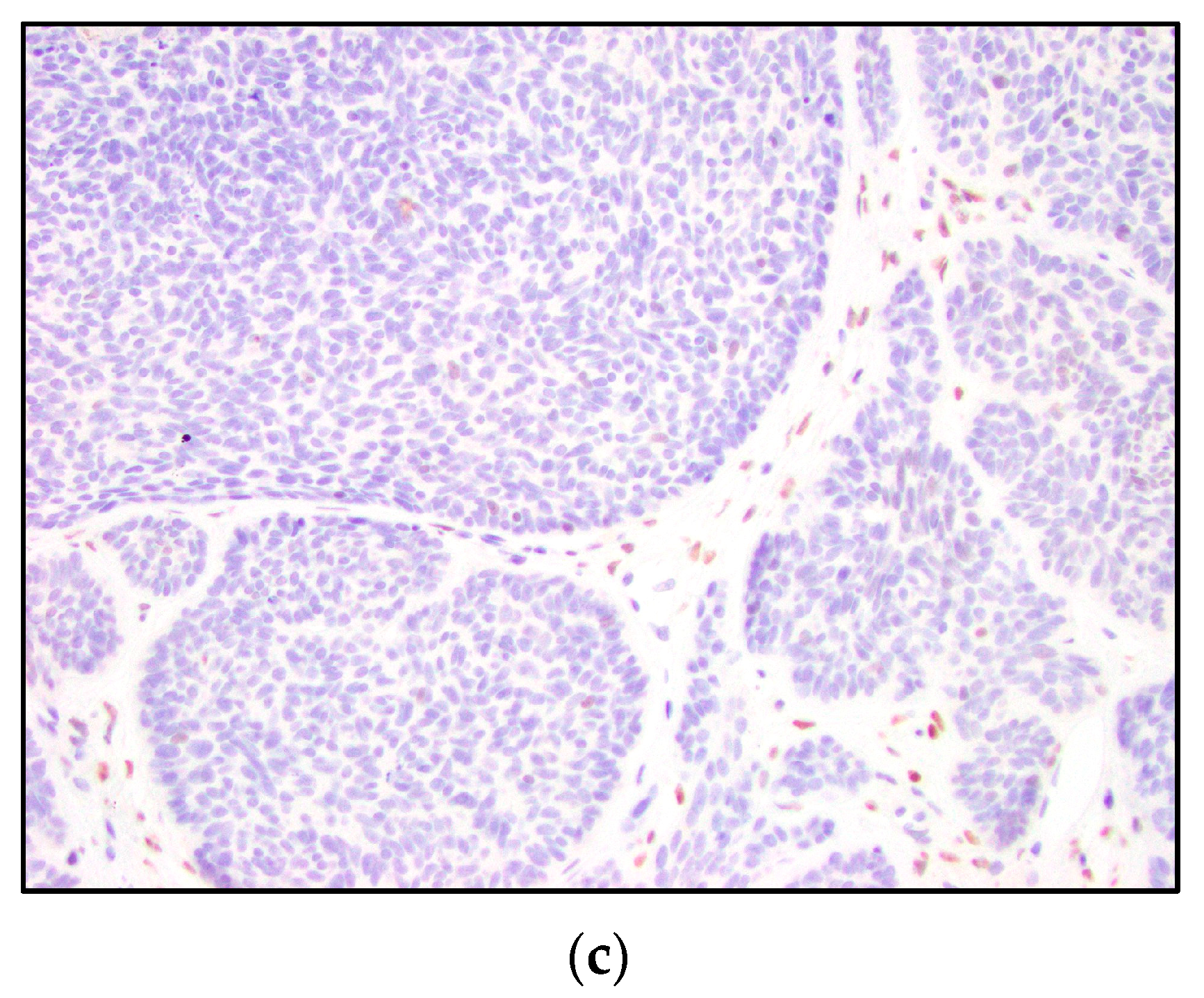
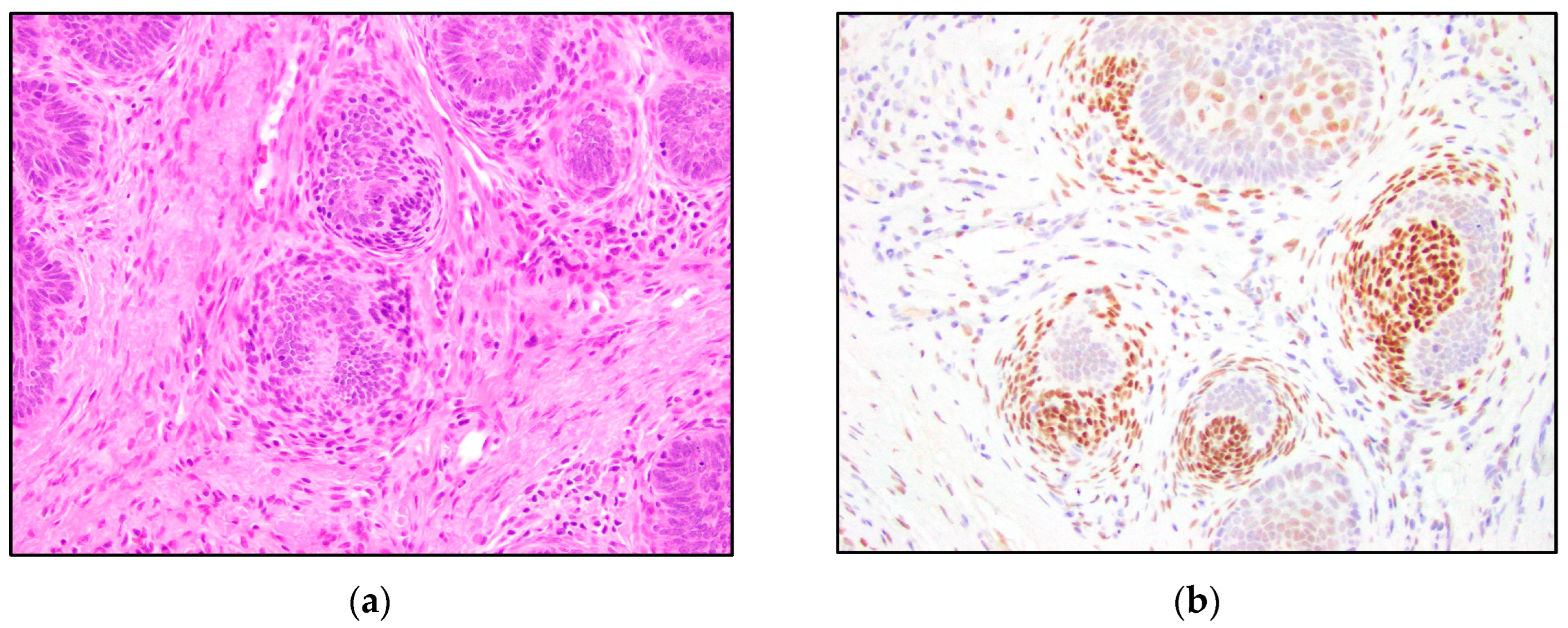
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ai, D.; Yao, J.; Yang, F.; Huo, L.; Chen, H.; Lu, W.; Soto, L.M.S.; Jiang, M.; Raso, M.G.; Wang, S.; et al. TRPS1: A highly sensitive and specific marker for breast carcinoma, especially for triple-negative breast cancer. Mod. Pathol. 2021, 34, 710–719. [Google Scholar] [CrossRef]
- Maas, S.M.; Shaw, A.C.; Bikker, H.; Lüdecke, H.-J.; van der Tuin, K.; Badura-Stronka, M.; Belligni, E.; Biamino, E.; Bonati, M.T.; Carvalho, D.R.; et al. Phenotype and genotype in 103 patients with tricho-rhino-phalangeal syndrome. Eur. J. Med. Genet. 2015, 58, 279–292. [Google Scholar] [CrossRef]
- Momeni, P.; Glöckner, G.; Schmidt, O.; von Holtum, D.; Albrecht, B.; Gillessen-Kaesbach, G.; Hennekam, R.; Meinecke, P.; Zabel, B.; Rosenthal, A.; et al. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat. Genet. 2000, 24, 71–74. [Google Scholar] [CrossRef]
- Gai, Z.; Gui, T.; Muragaki, Y. The function of TRPS1 in the development and differentiation of bone, kidney, and hair follicles. Histol. Histopathol. 2011, 26, 915–921. [Google Scholar]
- Fantauzzo, K.A.; Bazzi, H.; Jahoda, C.A.; Christiano, A.M. expression of the zinc-finger transcription factor Trps1 during hair follicle morphogenesis and cycling. Gene Expr. Patterns 2008, 8, 51–57. [Google Scholar] [CrossRef]
- Fantauzzo, K.A.; Kurban, M.; Levy, B.; Christiano, A.M. Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis. PLoS Genet. 2012, 8, e1003002. [Google Scholar] [CrossRef]
- Zengin, H.B.; Bui, C.M.; Rybski, K.; Pukhalskaya, T.; Yildiz, B.; Smoller, B.R. TRPS1 Is Differentially Expressed in a Variety of Malignant and Benign Cutaneous Sweat Gland Neop.plasms. Dermatopathology 2023, 10, 75–85. [Google Scholar] [CrossRef]
- Bourlond, F.; Battistella, M.; Amici, J.M.; Dousset, L.; Vergier, B.; Beylot-Barry, M.; Cribier, B. Clinicopathologic analysis of trichoblastoma and comparison with nodular basal cell carcinoma. Ann. Dermatol. Venereol. 2021, 148, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Nawrocki, S.; Hinther, K.; Khachemoune, A. Trichoblastomas Mimicking Basal Cell Carcinoma: The Importance of Identification and Differentiation. Cureus 2020, 12, e8272. [Google Scholar] [CrossRef] [PubMed]
- Stanoszek, L.M.; Wang, G.Y.; Harms, P.W. Histologic Mimics of Basal Cell Carcinoma. Arch. Pathol. Lab. Med. 2017, 141, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.D.; Fitzpatrick, J.E.; Golitz, L.E. Papillary mesenchymal bodies: A histologic finding useful in differentiating trichoepitheliomas from basal cell carcinomas. J. Am. Acad. Dermatol. 1989, 21 Pt 1, 523–528. [Google Scholar] [CrossRef]
- Smoller, B.R.; Van de Rijn, M.; Lebrun, D.; Warnke, R.A. bcl-2 expression reliably distinguishes trichoepitheliomas from basal cell carcinomas. Br. J. Dermatol. 1994, 131, 28–31. [Google Scholar] [CrossRef]
- Carrasquillo, O.Y.; Cruzval-O’Reilly, E.; Sánchez, J.E.; Valentín-Nogueras, S.M. Differentiation of Basal Cell Carcinoma and Trichoepithelioma: An Immunohistochemical Study. Am. J. Dermatopathol. 2021, 43, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kirchmann, T.T.; Prieto, V.G.; Smoller, B.R. CD34 staining pattern distinguishes basal cell carcinoma from trichoepithelioma. Arch. Dermatol. 1994, 130, 589–592. [Google Scholar] [CrossRef]
- Illueca, C.; Monteagudo, C.; Revert, A.; Llombart-Bosch, A. Diagnostic value of CD34 immunostaining in desmoplastic trichilemmoma. J. Cutan. Pathol. 1998, 25, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Basarab, T.; Orchard, G.; Russell-Jones, R. The use of immunostaining for bcl-2 and CD34 and the lectin peanut agglutinin in differentiating between basal cell carcinomas and trichoepitheliomas. Am. J. Dermatopathol. 1998, 20, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O.; Asahi, M. Cytokeratin expression in trichoblastic fibroma (small nodular type trichoblastoma), trichoepithelioma and basal cell carcinoma. Br. J. Dermatol. 1999, 140, 8–16. [Google Scholar] [CrossRef]
- Pham, T.T.; Selim, M.A.; Burchette, J.L., Jr.; Madden, J.; Turner, J.; Herman, C. CD10 expression in trichoepithelioma and basal cell carcinoma. J. Cutan. Pathol. 2006, 33, 123–128. [Google Scholar] [CrossRef]
- Plaza, J.A.; Ortega, P.F.; Bengana, C.; Stockman, D.L.; Suster, S. Immunolabeling pattern of podoplanin (d2-40) may distinguish basal cell carcinomas from trichoepitheliomas: A clinicopathologic and immunohistochemical study of 49 cases. Am. J. Dermatopathol. 2010, 32, 683–687. [Google Scholar] [CrossRef]
- Poniecka, A.W.; Alexis, J.B. An immunohistochemical study of basal cell carcinoma and trichoepithelioma. Am. J. Dermatopathol. 1999, 21, 332–336. [Google Scholar] [CrossRef]
- Abdelsayed, R.A.; Guijarro-Rojas, M.; Ibrahim, N.A.; Sangueza, O.P. Immunohistochemical evaluation of basal cell carcinoma and trichepithelioma using Bcl-2, Ki67, PCNA and P53. J. Cutan. Pathol. 2000, 27, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Izikson, L.; Bhan, A.; Zembowicz, A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am. J. Dermatopathol. 2005, 27, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tebcherani, A.J.; de Andrade, H.F., Jr.; Sotto, M.N. Diagnostic utility of immunohistochemistry in distinguishing trichoepithelioma and basal cell carcinoma: Evaluation using tissue microarray samples. Mod. Pathol. 2012, 25, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Katona, T.M.; Perkins, S.M.; Billings, S.D. Does the panel of cytokeratin 20 and androgen receptor antibodies differentiate desmoplastic trichoepithelioma from morpheaform/infiltrative basal cell carcinoma? J. Cutan. Pathol. 2008, 35, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Hartschuh, W.; Schulz, T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: An immunohistochemical and electron microscopic study. J. Cutan. Pathol. 1995, 22, 413–421. [Google Scholar] [CrossRef]
- Abesamis-Cubillan, E.; El-Shabrawi-Caelen, L.; LeBoit, P.E. Merkel cells and sclerosing epithelial neoplasms. Am. J. Dermatopathol. 2000, 22, 311–315. [Google Scholar] [CrossRef]
- Sellheyer, K.; Nelson, P. Follicular stem cell marker PHLDA1 (TDAG51) is superior to cytokeratin-20 in differentiating between trichoepithelioma and basal cell carcinoma in small biopsy specimens. J. Cutan Pathol. 2011, 38, 542–550. [Google Scholar] [CrossRef]
- Yeh, I.; McCalmont, T.H.; LeBoit, P.E. Differential expression of PHLDA1 (TDAG51) in basal cell carcinoma and trichoepithelioma. Br. J. Dermatol. 2012, 167, 1106–1110. [Google Scholar] [CrossRef]
- Hardy, M.H. The secret life of the hair follicle. Trends Genet. 1992, 8, 55–61. [Google Scholar] [CrossRef]
- Tan, S.T.; Ghaznawie, M.; Heenan, P.J.; Dosan, R. Basal Cell Carcinoma Arises from Interfollicular Layer of Epidermis. J. Oncol. 2018, 2018, 3098940. [Google Scholar] [CrossRef]
- Youssef, K.K.; Van Keymeulen, A.; Lapouge, G.; Beck, B.H.; Michaux, C.; Achouri, Y.; Sotiropoulou, P.A.; Blanpain, C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 2010, 12, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Blume-Peytavi, U.; Orfanos, C.E. Basal cell carcinoma possibly originates from the outer root sheath and/or the bulge region of the vellus hair follicle. Arch. Dermatol. Res. 1999, 291, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kurzen, H.; Esposito, L.; Langbein, L.; Hartschuh, W. Cytokeratins as Markers of Follicular Differentiation. Am. J. Dermatopathol. 2001, 23, 501–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Nakamura, T.; Furukawa, F.; Muragaki, Y. Trps1-deficient transplanted skin gave rise to a substantial amount of hair: Trps1 is unnecessary for hair development. Dermatol. Rep. 2019, 11, 7853. [Google Scholar] [CrossRef]


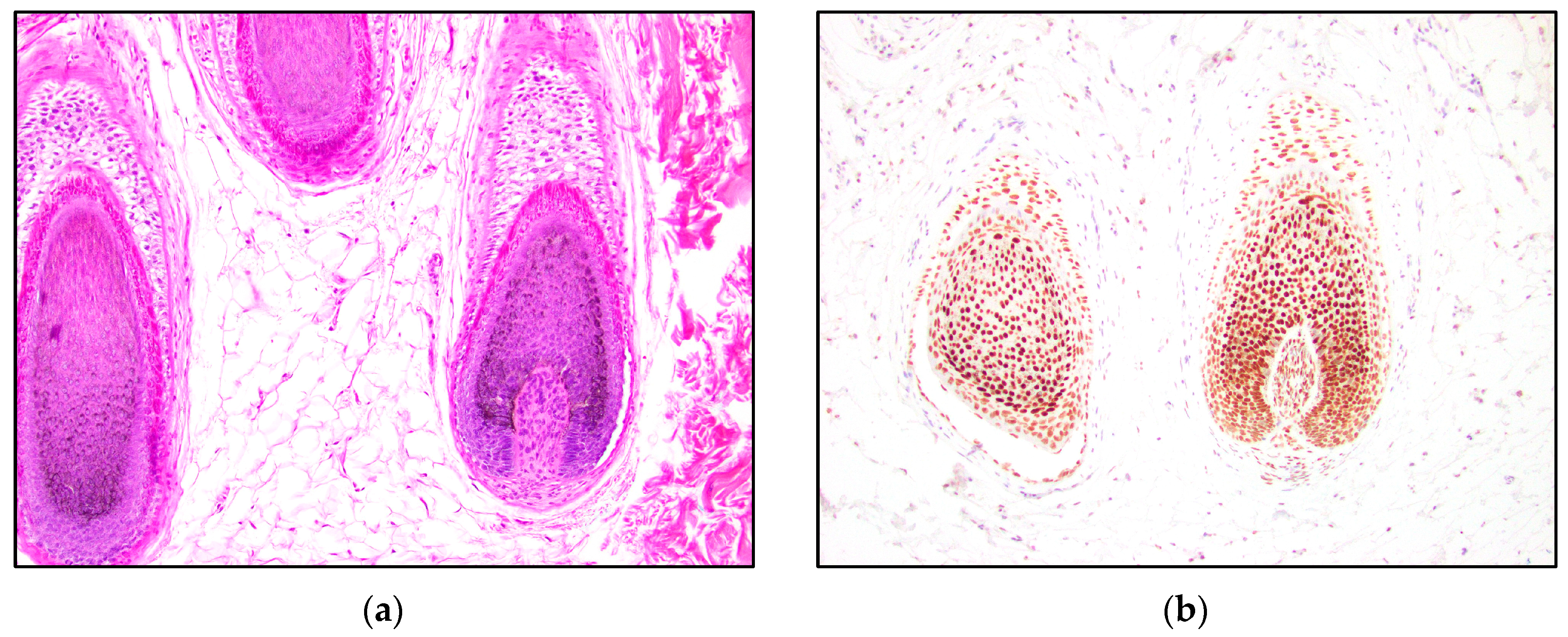
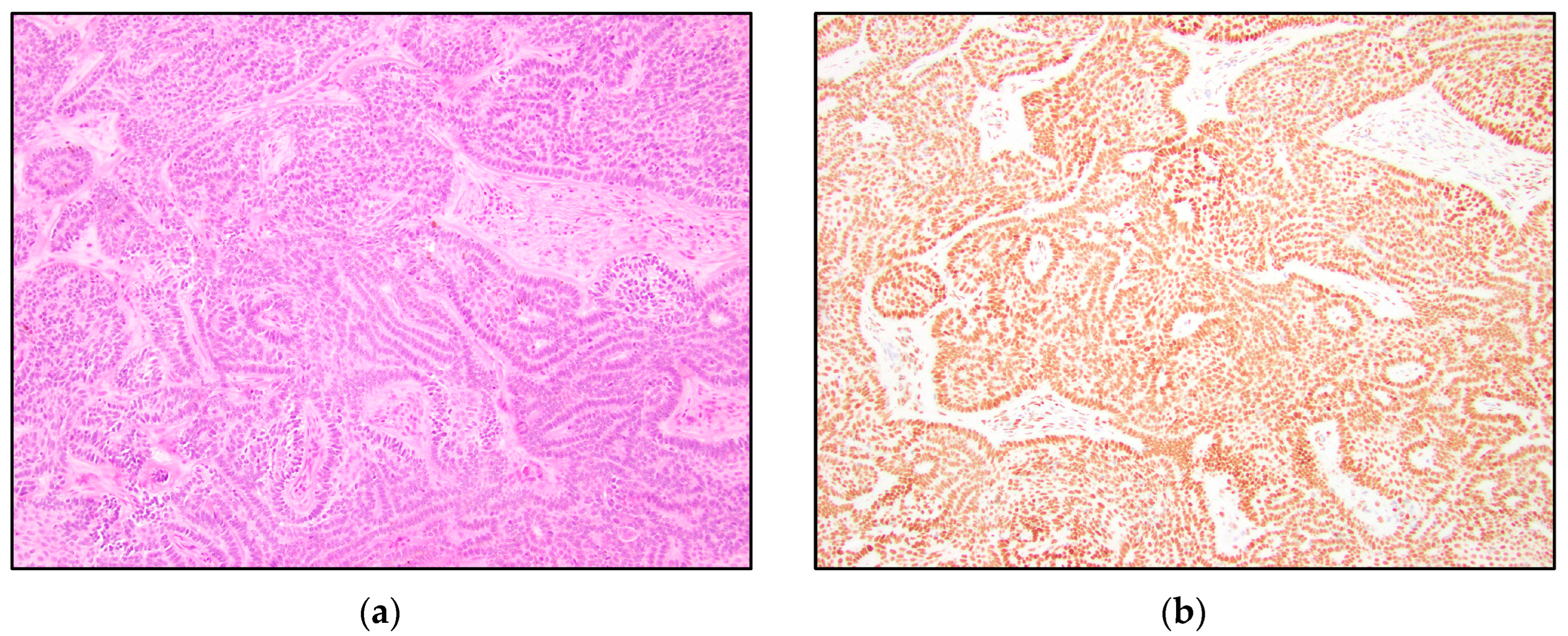
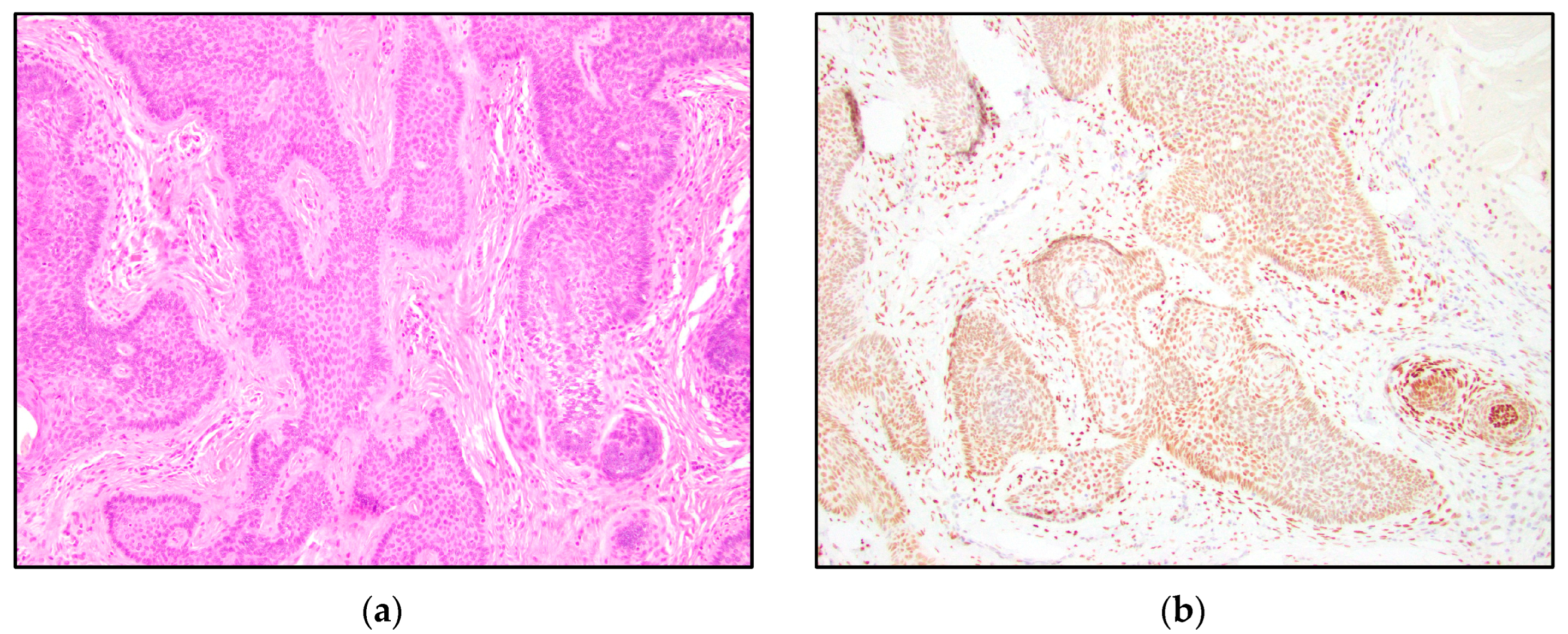
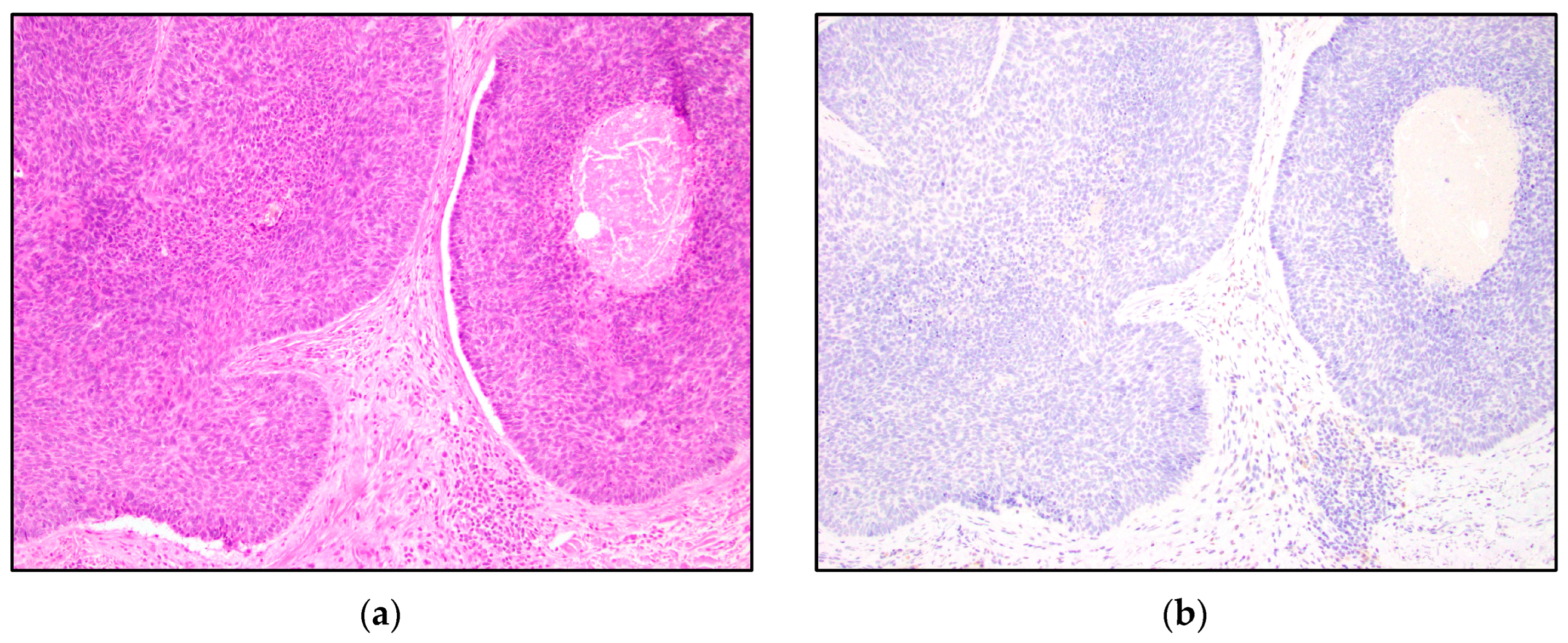
| TRPS1 Expression | Trichoblastoma N = 13 | Trichoepithelioma N = 15 | Basal Cell Carcinoma N = 15 |
|---|---|---|---|
| Negative n (%) | 7 (54%) | 6 (40%) | 10 (67%) |
| Low Positive n (%) | 1 (8%) | 6 (40%) | 5 (33%) |
| Intermediate Positive n (%) | 2 (15%) | 1 (7%) | 0 (0%) |
| High Positive n (%) | 3 (23%) | 2 (13%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybski, K.J.; Zengin, H.B.; Smoller, B.R. TRPS1: A Marker of Follicular Differentiation. Dermatopathology 2023, 10, 173-183. https://doi.org/10.3390/dermatopathology10020025
Rybski KJ, Zengin HB, Smoller BR. TRPS1: A Marker of Follicular Differentiation. Dermatopathology. 2023; 10(2):173-183. https://doi.org/10.3390/dermatopathology10020025
Chicago/Turabian StyleRybski, Kristin J., Hatice B. Zengin, and Bruce R. Smoller. 2023. "TRPS1: A Marker of Follicular Differentiation" Dermatopathology 10, no. 2: 173-183. https://doi.org/10.3390/dermatopathology10020025
APA StyleRybski, K. J., Zengin, H. B., & Smoller, B. R. (2023). TRPS1: A Marker of Follicular Differentiation. Dermatopathology, 10(2), 173-183. https://doi.org/10.3390/dermatopathology10020025







