Senotherapeutic Effect of Retinaldehyde and Hyaluronate Fragments in Dermatoporosis
Abstract
1. Introduction
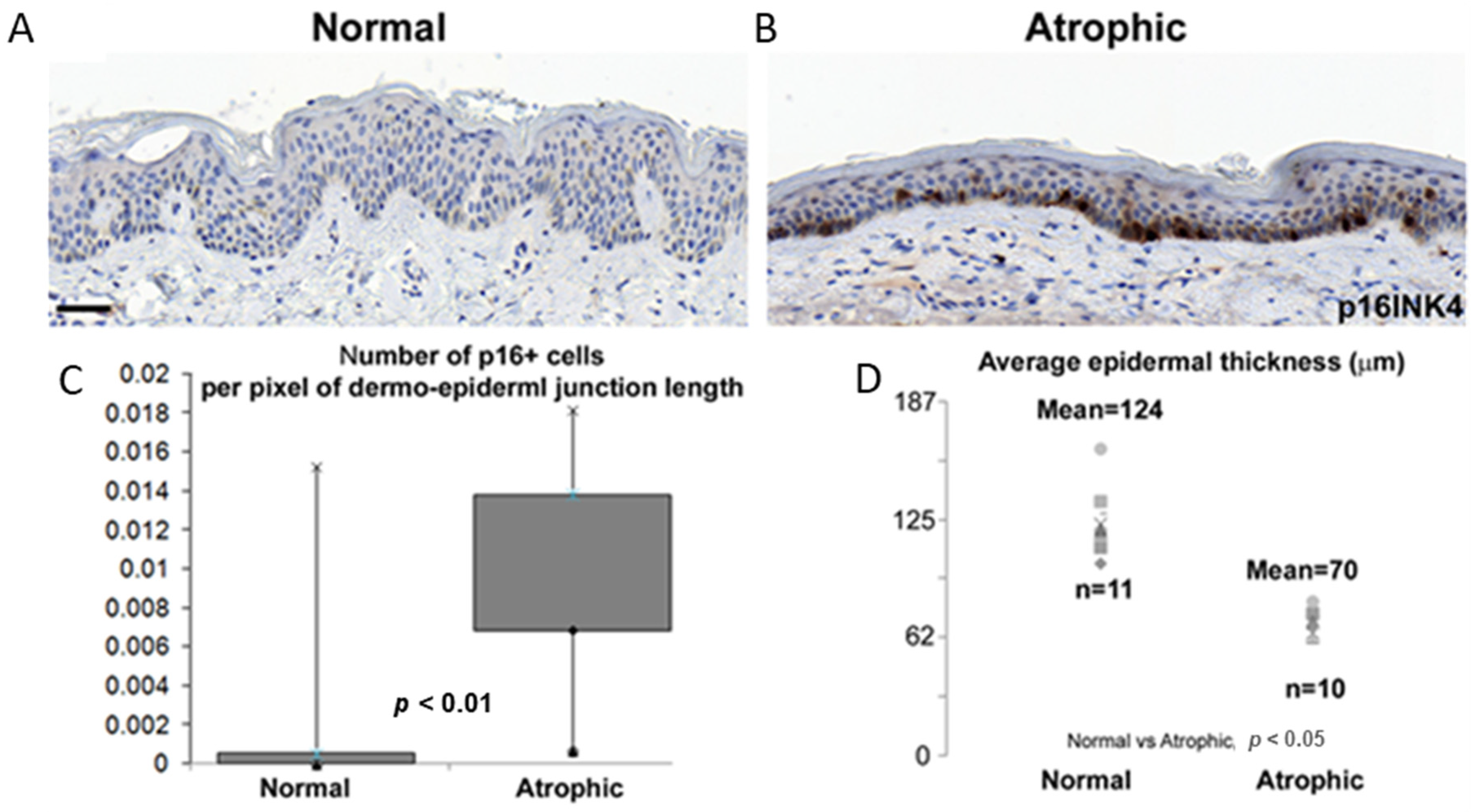
2. Results
3. Materials and Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waaijer, M.E.; Parish, W.E.; Strongitharm, B.H.; van Heemst, D.; Slagboom, P.E.; de Craen, A.J.; Sedivy, J.M.; Westendorp, R.G.; Gunn, D.A.; Maier, A.B. The number of p16Ink4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.M.; Baker, D.J.; van Deursen, J.M. Senescent Cells: A Novel Therapeutic Target for Aging and Age-Related Diseases. Clin. Pharmacol. Ther. 2013, 93, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Kaya, G.; Saurat, J.H. Dermatoporosis: A chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology 2007, 215, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Saurat, J.H.; Kaya, G. Senescent Atrophic Epidermis Retains Lrig1+ Stem Cells and Loses Wnt Signaling, a Phenotype Shared with CD44KO Mice. PLoS ONE 2017, 12, e0169452. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Tran, C.; Sorg, O.; Hotz, R.; Grand, D.; Carraux, P.; Didierjean, L.; Stamenkovic, I.; Saurat, J.H.; Kaya, G. Synergistic effect of hyaluronate fragments in retinaldehyde-induced skin hyperplasia which is a CD44-dependent phenomenon. PLoS ONE 2010, 5, e14372. [Google Scholar] [CrossRef] [PubMed]
- Kaya, G.; Tran, C.; Sorg, O.; Hotz, R.; Grand, D.; Carraux, P.; Didierjean, L.; Stamenkovic, I.; Saurat, J.H. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006, 3, e493. [Google Scholar] [CrossRef] [PubMed]
- 8 Kaya, G.; Rodriguez, I.; Jorcano, J.L.; Vassalli, P.; Stamenkovic, I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997, 11, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- 9 Nikolic, D.S.; Ziori, C.; Kostaki, M.; Fontao, L.; Saurat, J.H.; Kaya, G. Hyalurosome gene regulation and dose-dependent restoration of skin atrophy by retinaldehyde and defined-size hyaluronate fragments in dermatoporosis. Dermatology 2014, 229, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Martyanov, V.; Whitfield, M.L.; Varga, J. Senescence Signature in Skin Biopsies From Systemic Sclerosis Patients Treated with Senolytic Therapy: Potential Predictor of Clinical Response? Arthritis Rheumatol. 2019, 71, 1766–1767. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Wyles, S.P.; Tchkonia, T.; Kirkland, J.L. Targeting Cellular Senescence for Age-Related Diseases: Path to Clinical Translation. Plast. Reconstr. Surg. 2022, 150, 20S–26S. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, A.; Saurat, J.-H.; Kaya, G. Senotherapeutic Effect of Retinaldehyde and Hyaluronate Fragments in Dermatoporosis. Dermatopathology 2023, 10, 168-172. https://doi.org/10.3390/dermatopathology10020024
Kaya A, Saurat J-H, Kaya G. Senotherapeutic Effect of Retinaldehyde and Hyaluronate Fragments in Dermatoporosis. Dermatopathology. 2023; 10(2):168-172. https://doi.org/10.3390/dermatopathology10020024
Chicago/Turabian StyleKaya, Aysin, Jean-Hilaire Saurat, and Gürkan Kaya. 2023. "Senotherapeutic Effect of Retinaldehyde and Hyaluronate Fragments in Dermatoporosis" Dermatopathology 10, no. 2: 168-172. https://doi.org/10.3390/dermatopathology10020024
APA StyleKaya, A., Saurat, J.-H., & Kaya, G. (2023). Senotherapeutic Effect of Retinaldehyde and Hyaluronate Fragments in Dermatoporosis. Dermatopathology, 10(2), 168-172. https://doi.org/10.3390/dermatopathology10020024







