Spiny Keratoderma in Association with Melanoma
Abstract
1. Introduction
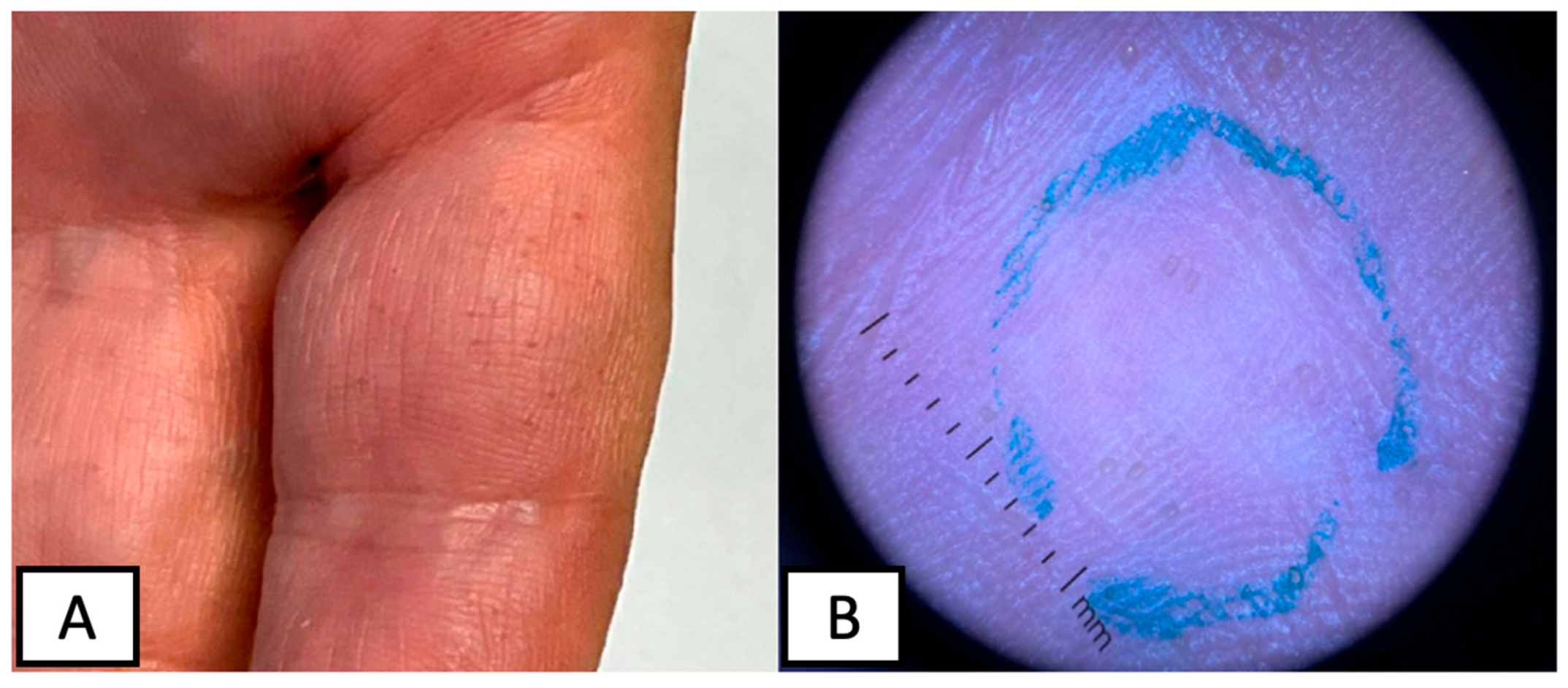
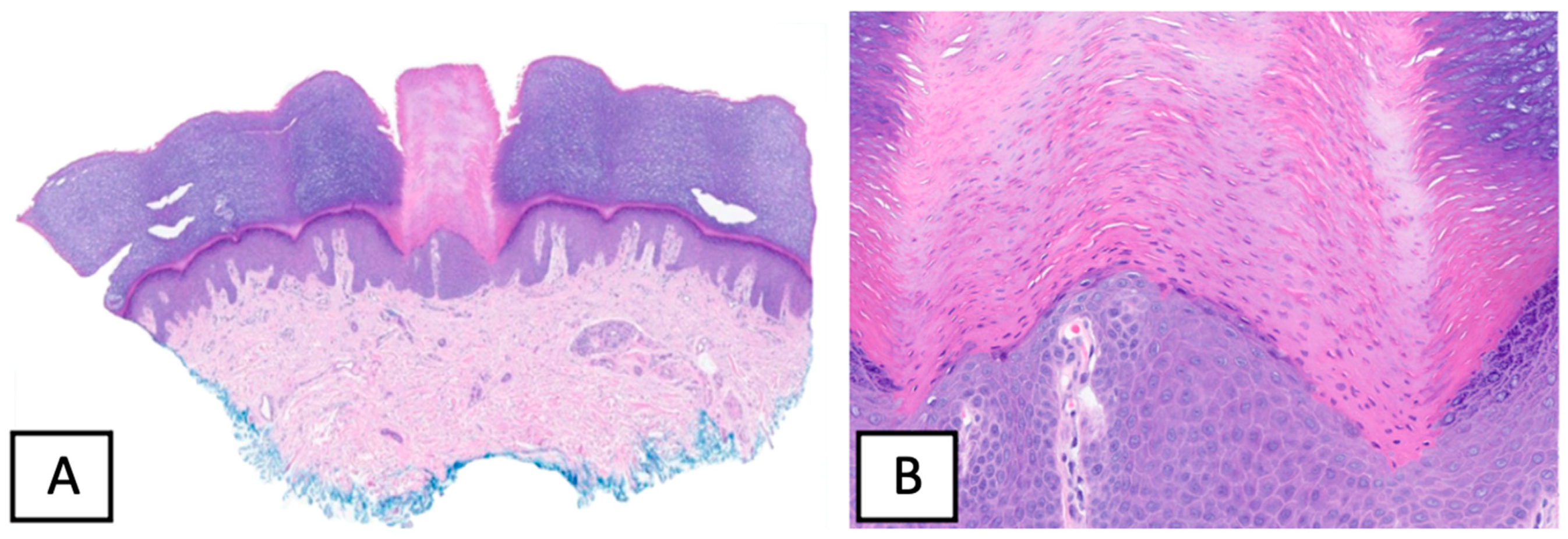
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, F.C. Punctate keratoderma. Arch. Dermatol. 1971, 104, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Osman, Y.; Daly, T.J.; Don, P.C. Spiny keratoderma of the palms and soles. J. Am. Acad. Dermatol. 1992, 26, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Sakas, E.L.; Gentry, L.R.H. Porokeratosis punctata palmaris et plantaris (punctate porokeratosis): Case report and literature review. J. Am. Acad. Dermatol. 1985, 13, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.C.; DeVillez, R.L. Congenital unilateral punctate porokeratosis. Am. J. Dermatopathol. 1984, 6, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.K.; Mallett, R.B.; Green, C.; Rytina, E. Palmar filiform hyperkeratosis (FH) associated with underlying pathology? Clin. Exp. Dermatol. 2002, 27, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Poppe, H.; Kneitz, H.; Bröcker, E.-B.; Hamm, H. In situ melanoma and palmar spiny hyperkeratoses: Coincidence or paraneoplasia? Eur. J. Dermatol. 2012, 22, 281–282. [Google Scholar] [CrossRef]
- Kaddu, S.; Soyer, H.P.; Kerl, H. Palmar filiform hyperkeratosis: A new paraneoplastic syndrome? J. Am. Acad. Dermatol. 1995, 33, 337–340. [Google Scholar] [CrossRef]
- Caccetta, T.P.; Dessauvagie, B.; McCallum, D.; Kumarasinghe, S.P. Multiple minute digitate hyperkeratosis: A proposed algorithm for the digitate keratoses. J. Am. Acad. Dermatol. 2012, 67, e49–e55. [Google Scholar] [CrossRef]
- Nakamura, Y.; Muto, M. Spiny keratoderma of the palms in an insulin-treated diabetic patient. Int. J. Dermatol. 2013, 52, 1460–1461. [Google Scholar] [CrossRef]
- Handa, Y.; Sakakibara, A.; Araki, M.; Yamanaka, N. Spiny keratoderma of the palms and soles-report of two cases. Eur. J. Dermatol. 2000, 10, 542–545. [Google Scholar]
- Urbani, C.E.; Moneghini, L. Palmar spiny keratoderma associated with type IV hyperlipoproteinemia. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Cohen, D.E.; Lee, H.S.; Thellman, C. Spiny keratoderma in association with autosomal dominant polycystic kidney disease with liver cysts. J. Am. Acad. Dermatol. 1996, 34, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Ehr, V.; Grosieux, C.; Derancourt, C.; Durlach, A.; Kalis, B.; Bernard, P. Palmoplantar filiform hyperkeratosis with Darier’s disease. Association or coincidence? Eur. J. Dermatol. 1998, 8, 519–520. [Google Scholar] [PubMed]
- Valverde, R.; Sánchez-Caminero, M.P.; Calzado, L.; de Frutos, F.J.O.; Rodríguez-Peralto, J.L.; Vanaclocha, F. Der-matomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma. Actas Dermosifiliogr. 2007, 98, 358–360. [Google Scholar] [CrossRef]
- Herman, P. Punctate Porokeratotic Keratoderma. Dermatology 1973, 147, 206–213. [Google Scholar] [CrossRef]
- Paul, C.; Fermand, J.-P.; Flageul, B.; Caux, F.; Duterque, M.; Dubertret, L.; Aractingi, S. Hyperkeratotic spicules and monoclonal gammopathy. J. Am. Acad. Dermatol. 1995, 33, 346–351. [Google Scholar] [CrossRef]
- Campbell, E.H.; Becknell, C. Spiny keratoderma exposes underlying renal cell carcinoma. JAAD Case Rep. 2018, 4, 382–383. [Google Scholar] [CrossRef]
- Hillion, B.; Le Bozec, P.; Moulonguet-Michau, I.; Blanchet-Bardon, C.; Petit, A.; Stephan, J.; Civatte, J. Filiform palmoplantar hyperkeratosis and cancer of the breast. Ann. Dermatol. Venereol. 1990, 117, 834–836. [Google Scholar]
- Beylot, C.; Taïeb, A.; Bioulac, P.; Doutre, M.S.; Foix, P. Filiform palmoplantar hyperkeratosis and visceral neoplasia. Ann. Dermatol. Venereol. 1982, 109, 747–748. [Google Scholar]
- Fegueux, S.; Bilet, S.; Crickx, B.; Perron, J.; Grossin, M.; Belaïch, S. Filiform palmar hyperkeratosis and rectosigmoid cancer. Ann. Dermatol. Venereol. 1988, 115, 1145–1146. [Google Scholar]
- Nazzaro, P.; Argentieri, R.; Balus, L.; Bassetti, F.; Fazio, M.; Giacalone, B.; Ponno, R. Paraneoplastic syndrome with papulo-keratosic lesions of the extremities and diffuse spinulose pilar ker-atosis. Ann. Dermatol. Syphiligr. 1974, 101, 411–413. [Google Scholar]
- Tomasini, C.; Michelerio, A.; Brazzelli, V. Eruptive ulcerative follicular spicules heralding progression of smoldering multiple myeloma. J. Cutan. Pathol. 2019, 46, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Böckers, M.; Pfeifle, J. Pathogenesis of Paraneoplastic Follicular Hyperkeratotic Spicules in Multiple Myeloma. Arch. Dermatol. 1990, 126, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Orlandi, A.; Iraci, S.; Spagnoli, L.; Nini, G. Punctate porokeratotic keratoderma-its occurrence with internal neoplasia. Clin. Exp. Dermatol. 1994, 19, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Shimoura, T.; Hozumi, Y.; Aso, K. Punctate porokeratotic keratoderma: Some pathogenetic analyses of hy-perproliferation and parakeratosis. Acta Derm. Venereol. 1990, 70, 478–482. [Google Scholar]
- Hashimoto, K.; Toi, Y.; Horton, S.; Sun, T.-T. Spiny keratoderma--a demonstration of hair keratin and hair type keratinization. J. Cutan. Pathol. 1999, 26, 25–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukhalskaya, T.; Mully, T.W.; Wei, M.L. Spiny Keratoderma in Association with Melanoma. Dermatopathology 2023, 10, 142-146. https://doi.org/10.3390/dermatopathology10020021
Pukhalskaya T, Mully TW, Wei ML. Spiny Keratoderma in Association with Melanoma. Dermatopathology. 2023; 10(2):142-146. https://doi.org/10.3390/dermatopathology10020021
Chicago/Turabian StylePukhalskaya, Tatsiana, Thaddeus W. Mully, and Maria L. Wei. 2023. "Spiny Keratoderma in Association with Melanoma" Dermatopathology 10, no. 2: 142-146. https://doi.org/10.3390/dermatopathology10020021
APA StylePukhalskaya, T., Mully, T. W., & Wei, M. L. (2023). Spiny Keratoderma in Association with Melanoma. Dermatopathology, 10(2), 142-146. https://doi.org/10.3390/dermatopathology10020021






