Cutaneous Epithelioid Angiomatous Nodule: Report of a New Case and Literature Review
Abstract
1. Introduction
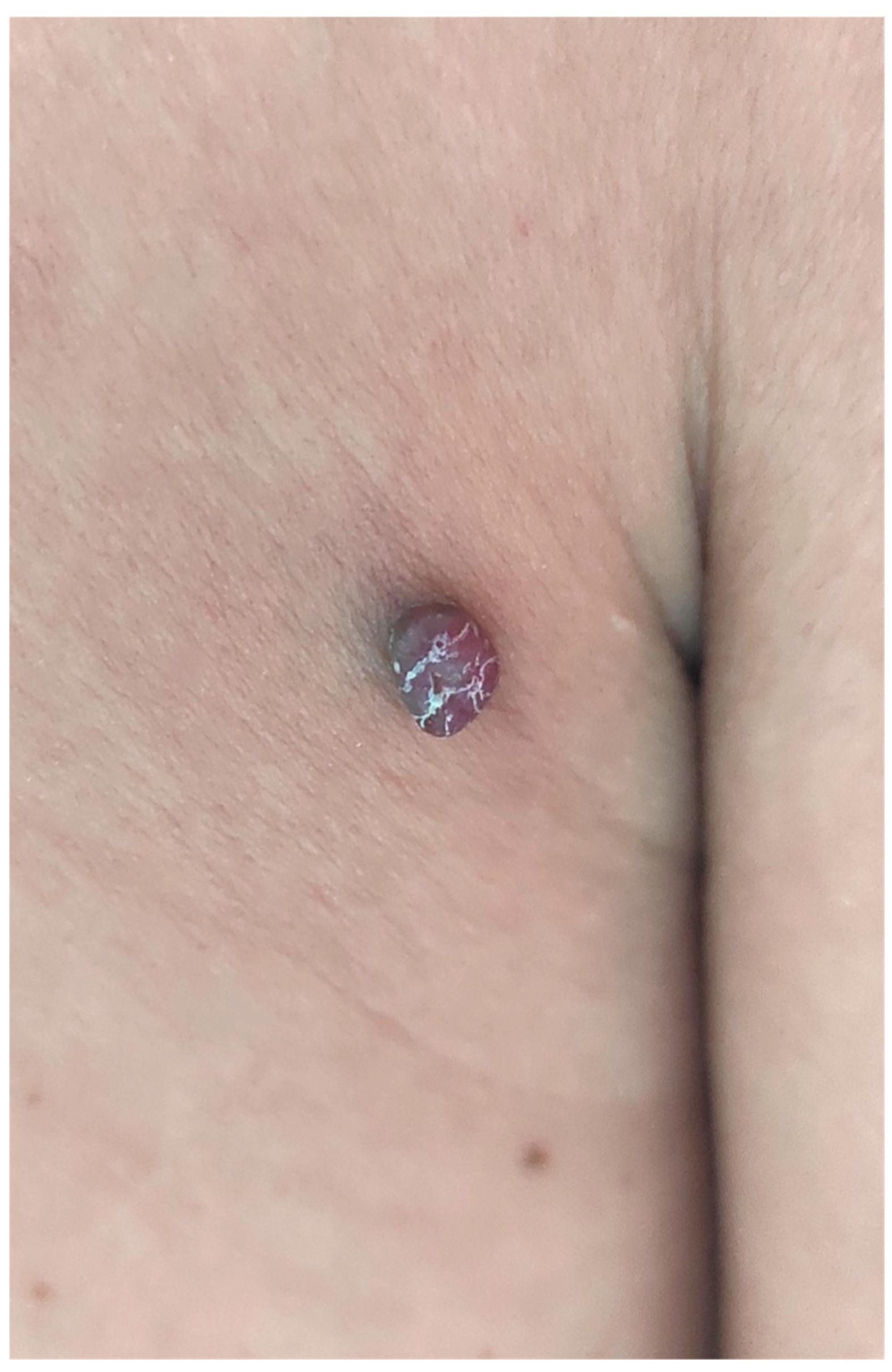
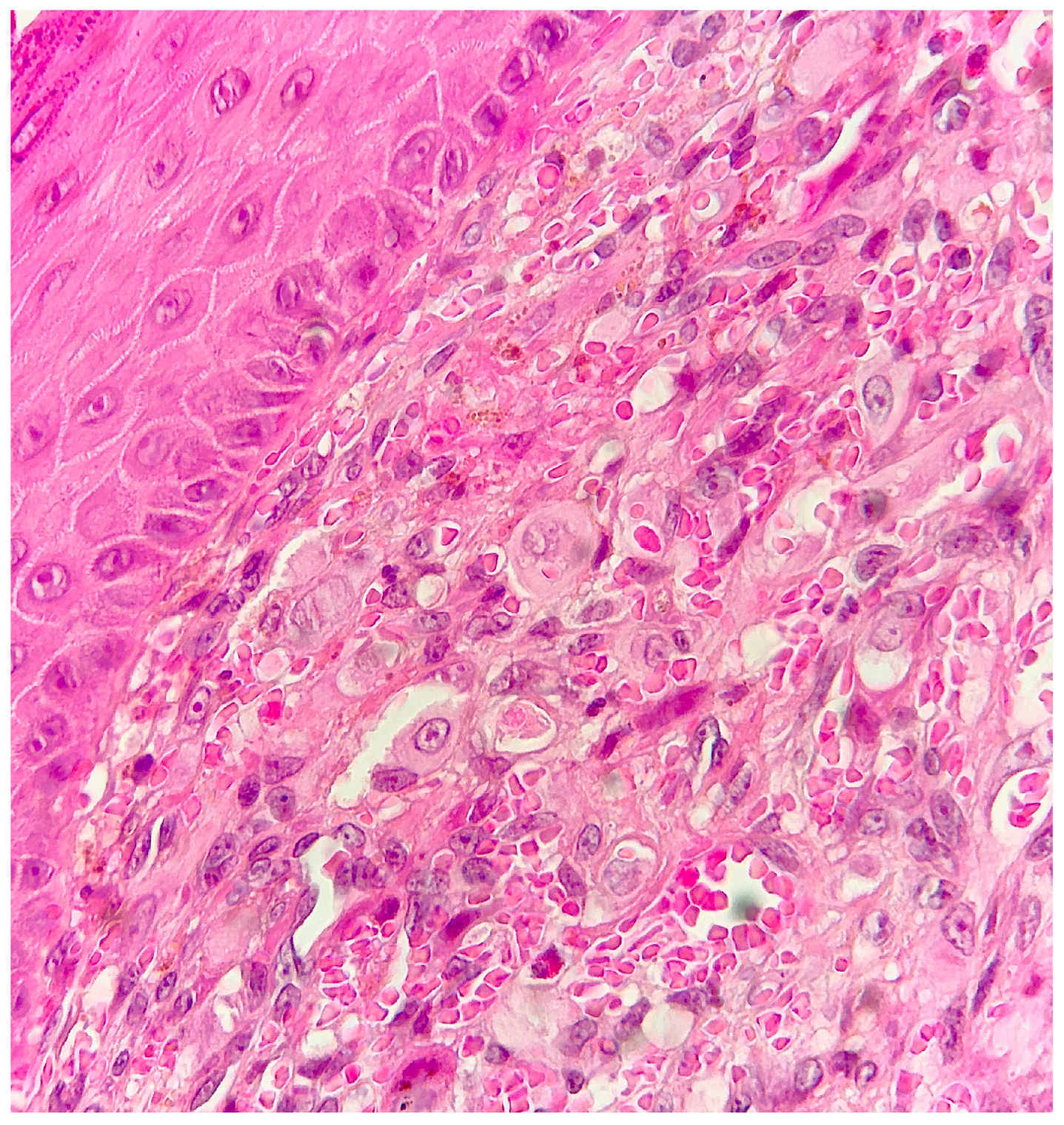
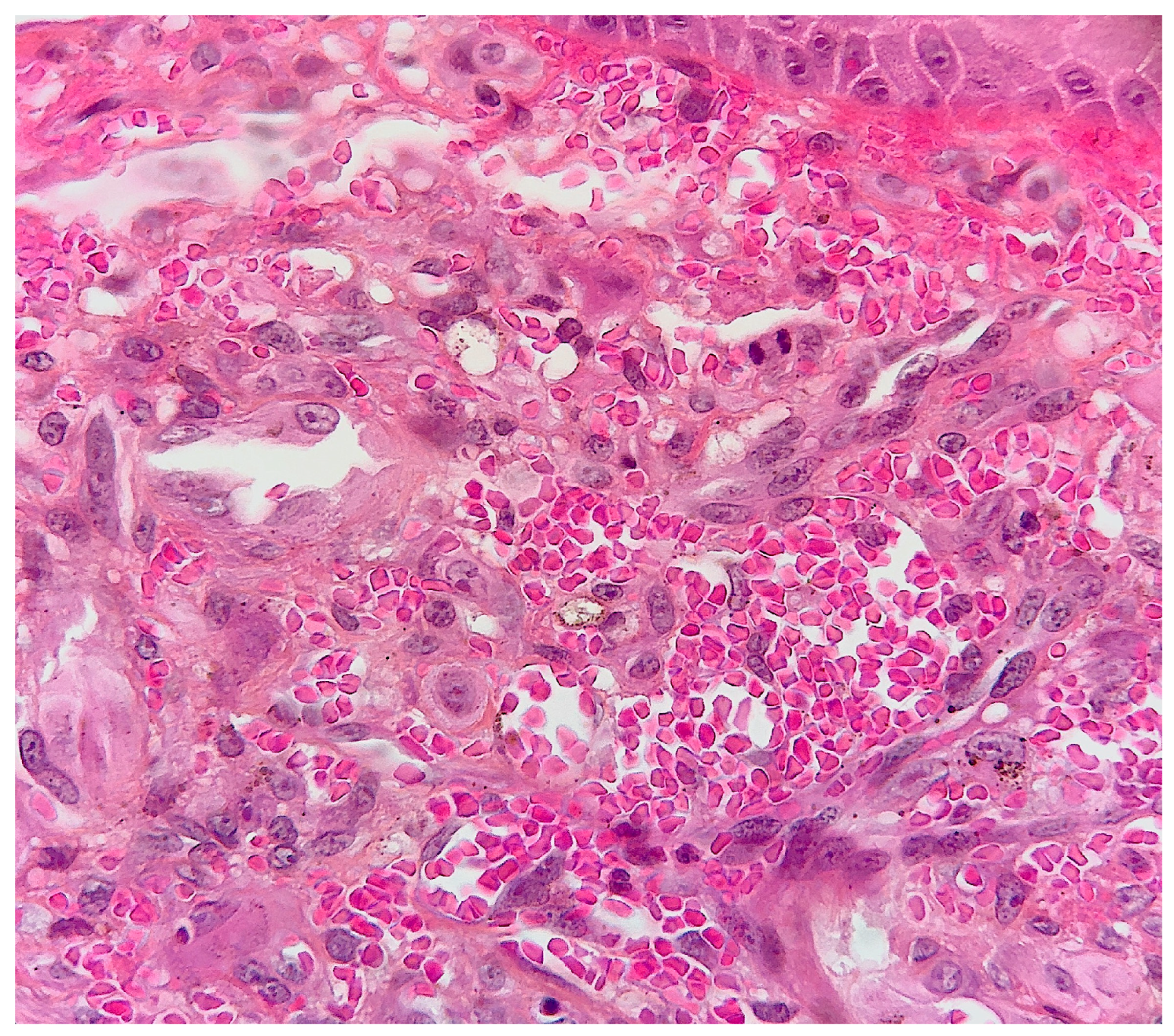
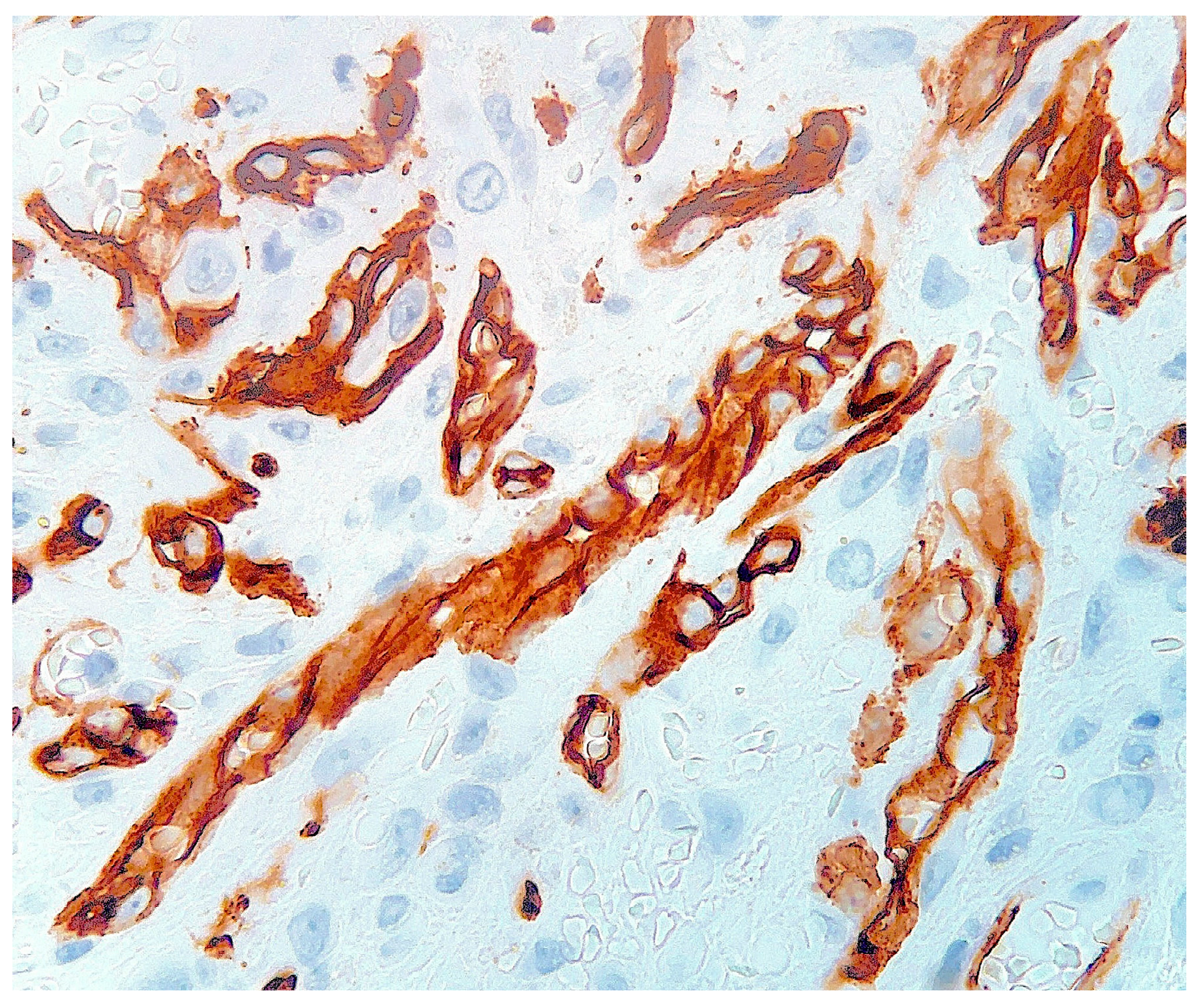
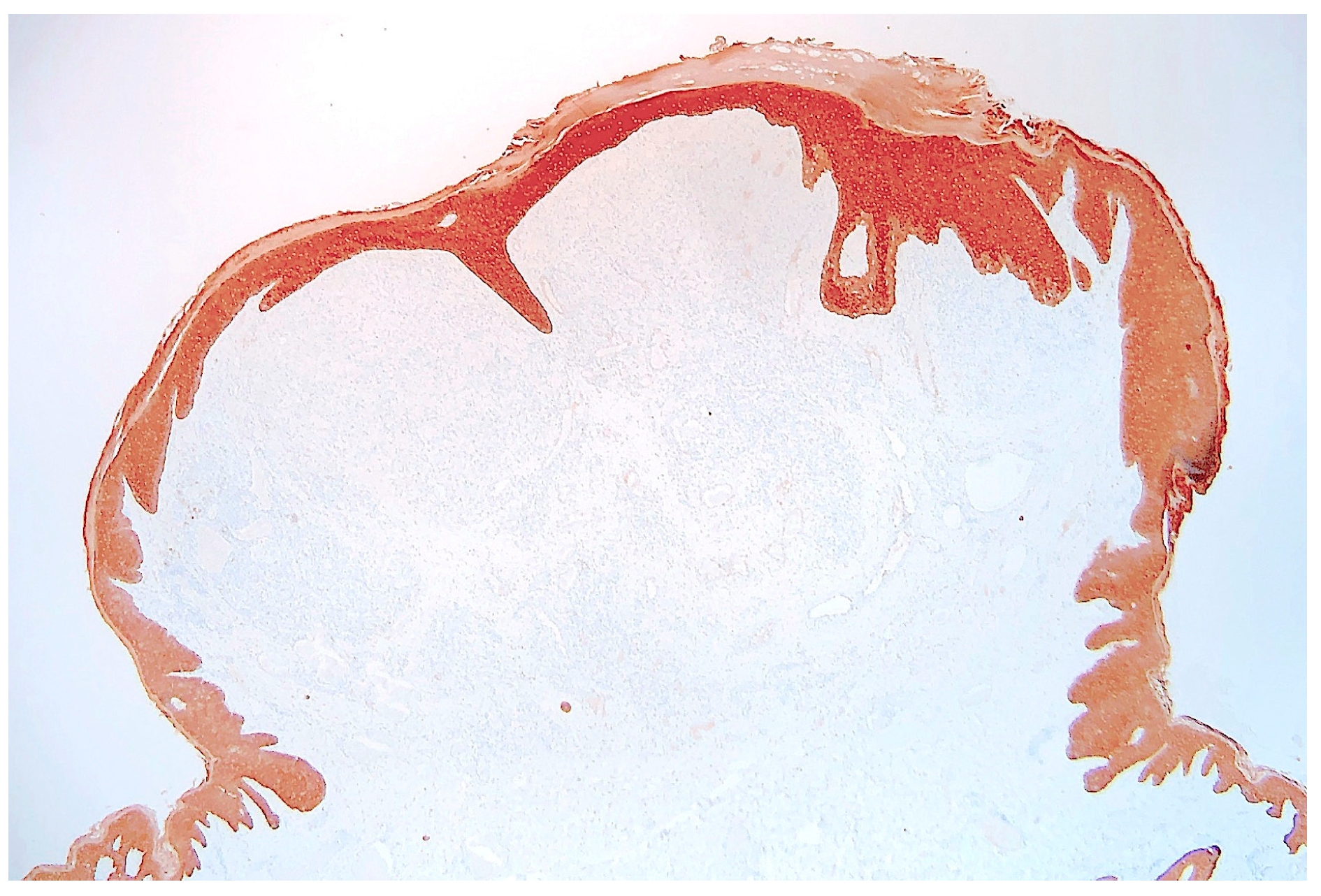
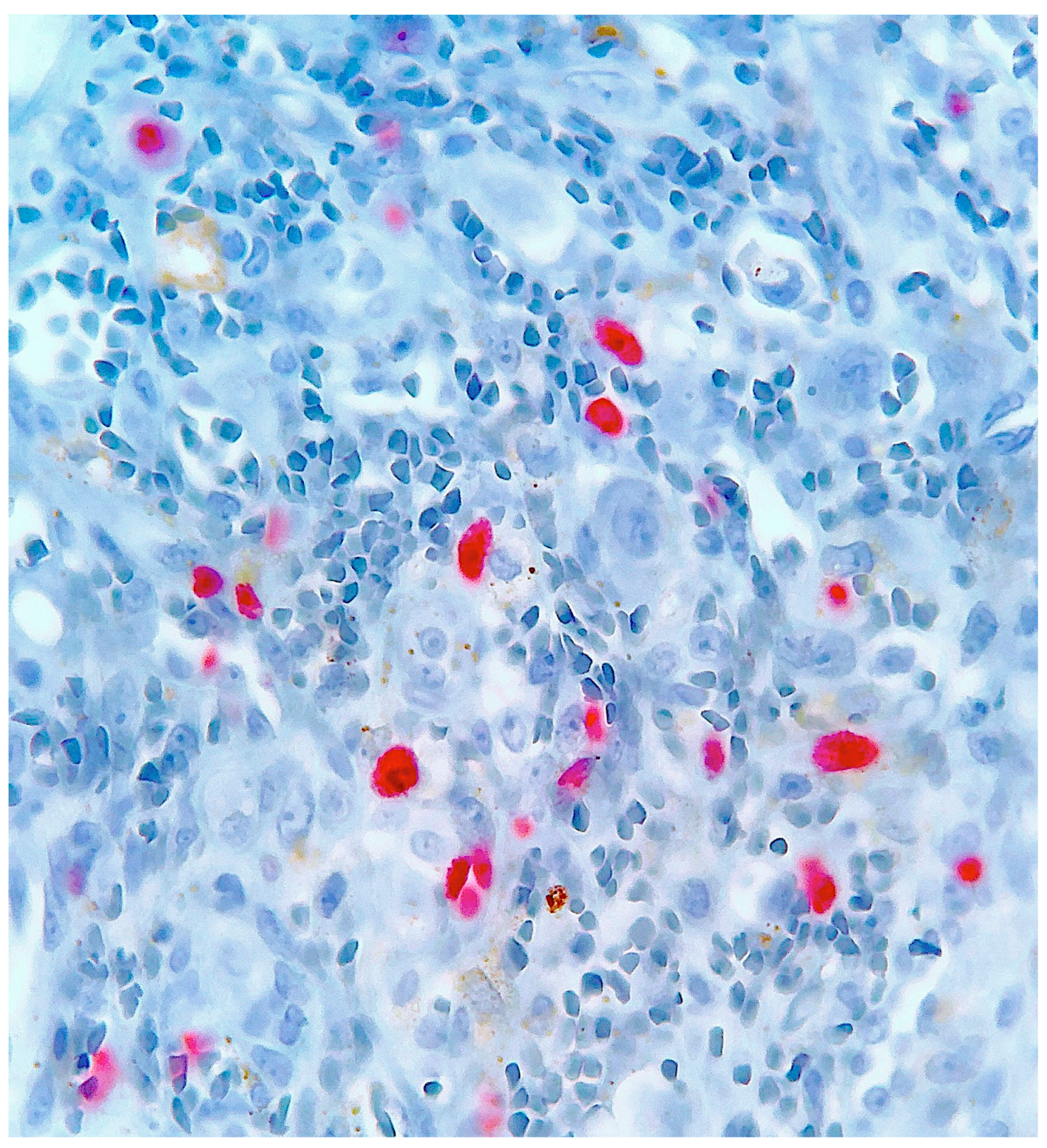
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenn, T.; Fletcher, C.D.M. Cutaneous Epithelioid Angiomatous Nodule: A Distinct Lesion in the Morphologic Spectrum of Epithelioid Vascular Tumors. Am. J. Dermatopathol. 2004, 26, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Argüelles-Cabrera, H.; Guimerá-Martín-Neda, F.; Carrasco, J.; Garc’A-Castro, M.; Hernández-León, C.; D’Az-Flores, L. Cutaneous epithelioid angiomatous nodule. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1383–1385. [Google Scholar] [CrossRef]
- Blackwood, L.; Chapman, I.; Lyon, M.; Hernandez, C. Multifocal eruptive cutaneous epithelioid angiomatous nodules. JAAD Case Rep. 2016, 2, 454–456. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tsai, Y.-L.; Shih, P.-Y.; Chiu, C.-S. Large cutaneous epithelioid angiomatous nodule on the glabella repaired using a medially based upper eyelid myocutaneous flap. Dermatol. Surg. 2009, 35, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.-J.; Zheng, X.-Y.; Tang, S.-F. Large cutaneous epithelioid angiomatous nodules in a patient with nephrotic syndrome: A case report. World J. Clin. Cases 2020, 8, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R.; Kamil, Z.S.; Wang, A.; Al Habeeb, A.; Ghazarian, D. Cutaneous epithelioid angiomatous nodule: A report of a series including a case with moderate cytologic atypia and immunosuppression. Diagn. Pathol. 2018, 13, 50. [Google Scholar] [CrossRef]
- Chokoeva, A.; Maximov, G.; Wollina, U.; Patterson, J.; Tchernev, G. Solitary Cutaneous Epithelioid Angiomatous Nodule Associated with Unilateral Capillary Malformation. Acta Derm. Venereol. 2017, 97, 135–136. [Google Scholar] [CrossRef]
- Dastgheib, L.; Aslani, F.S.; Sepaskhah, M.; Saki, N.; Motevalli, D. A young woman with multiple cutaneous epithelioid angiomatous nodules on her forearm: A case report and follow-up of therapeutic intervention. Dermatol. Online J. 2013, 19, 1. [Google Scholar] [CrossRef]
- Fernandez-Flores, A. D2-40 and Cutaneous Epithelioid Angiomatous Nodule. Am. J. Dermatopathol. 2008, 30, 302–304. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Cassarino, D.S. Myxoid Cutaneous Epithelioid Angiomatous Nodule. Am. J. Dermatopathol. 2019, 41, 82–84. [Google Scholar] [CrossRef]
- Goto, K.; Ogawa, K.; Fukai, T.; Miura, K.; Yanagihara, S.; Honma, K.; Motoi, T. Categorization of cutaneous epithelioid angiomatous nodule as epithelioid hemangioma or angiolymphoid hyperplasia with eosinophilia: Clinicopathologic, immunohistochemical, and molecular analyses of seven lesions. J. Cutan. Pathol. 2022, 49, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kumari, R.; Jacob, S.; Rajesh, N.; Thappa, D. Cutaneous epithelioid angiomatous nodule versus epithelioid hemangioma: A dilemma. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 99–101. [Google Scholar] [CrossRef]
- Ide, F.; Tanaka, A.; Kusama, K. Two unusual vascular lesions: Epithelioid angiomatous nodule and spindle cell hemangiomatosis. J. Oral Pathol. Med. 2008, 37, 252–254. [Google Scholar] [CrossRef]
- Kantrow, S.; Martin, J.D.; Vnencak-Jones, C.L.; Boyd, A.S. Cutaneous epithelioid angiomatous nodule: Report of a case and absence of microsatellite instability. J. Cutan. Pathol. 2007, 34, 515–516. [Google Scholar] [CrossRef]
- Kaushal, S.; Sharma, M.C.; Ramam, M.; Kumar, U. Multifocal cutaneous epithelioid angiomatous nodules of the penis. J. Cutan. Pathol. 2011, 38, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Labbène, I.; Rammeh, S.; Znaidi, N.; Fazaa, B.; Zermani, R. A case of multiple epithelioid angiomatous nodules. Dermatol. Online J. 2012, 18, 8. [Google Scholar] [CrossRef]
- Leroy, X.; Mortuaire, G.; Chevalier, D.; Aubert, S. Epithelioid angiomatous nodule of the nasal cavity. Pathol. Res. Pract. 2008, 204, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-S.; Lee, M.-C. Case of a cutaneous epithelioid angiomatous nodule on the foot. J. Dermatol. 2013, 40, 480–481. [Google Scholar] [CrossRef]
- McLemore, M.S.; Huo, L.; Deavers, M.T.; Curry, J.L.; Torres-Cabala, C.A.; Wang, W.-L.; Prieto, V.G. Cutaneous epithelioid angiomatous nodule of the chest wall with expression of estrogen receptor: A mimic of carcinoma and a potential diagnostic pitfall. J. Cutan. Pathol. 2011, 38, 818–822. [Google Scholar] [CrossRef]
- Misago, N.; Inoue, T.; Narisawa, Y. Subcutaneous epithelioid angiomatous nodule: A variant of epithelioid hemangioma. J. Dermatol. 2006, 33, 73–74. [Google Scholar] [CrossRef]
- Okano, S.; Takahara, M.; Tanaka, M.; Nakahara, T.; Suzuki, H.; Fujii, H.; Shirabe, K.; Oda, Y.; Maehara, Y.; Furue, M. Cutaneous epithelioid angiomatous nodule in a patient with a history of multiple pyogenic granulomas. Eur. J. Dermatol. 2015, 25, 268–269. [Google Scholar] [CrossRef]
- Pavlidakey, P.G.; Burroughs, C.; Karrs, T.; Somach, S.C. Cutaneous Epithelioid Angiomatous Nodule: A Case with Metachronous Lesions. Am. J. Dermatopathol. 2011, 33, 831–834. [Google Scholar] [CrossRef]
- Samal, S.; Monohar, D.B.; Adhya, A.K.; Sinha, M.; Mitra, S. Cutaneous Epithelioid Angiomatous Nodule of Breast. Indian Dermatol. Online J. 2019, 10, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Sangüeza, O.P.; Walsh, S.N.; Sheehan, D.J.; Orland, A.F.; Llombart, B.; Requena, L. Cutaneous Epithelioid Angiomatous Nodule: A Case Series and Proposed Classification. Am. J. Dermatopathol. 2008, 30, 16–20. [Google Scholar] [CrossRef]
- Shiomi, T.; Kaddu, S.; Yoshida, Y.; Yamamoto, O.; Yamane, T.; Shomori, K.; Ito, H. Cutaneous epithelioid angiomatous nodule arising in capillary malformation. J. Cutan. Pathol. 2011, 38, 372–375. [Google Scholar] [CrossRef]
- Sorrells, T.C.; Winn, A.; Sulit, D.J. Granular cell cutaneous epithelioid angiomatous nodule. J. Cutan. Pathol. 2019, 46, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-J.; Cai, Y.; Zhao, Y.-F.; Hu, X.; Zhang, W.; Chen, X.-M.; Lai, F.M.-M. Epithelioid angiomatous nodule of head and neck. Pathol. Res. Pract. 2009, 205, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Lim, D.H.; Ong, C.W. Epithelioid angiomatous nodule of the nasal cavity: Report of 2 cases. Auris Nasus Larynx 2015, 42, 341–344. [Google Scholar] [CrossRef]
- Zamecnik, M. Relationship between cutaneous epithelioid angiomatous nodule and epithelioid hemangioma. Am. J. Dermatopathol. 2004, 26, 351–352. [Google Scholar] [CrossRef]
- Bren, T.; Kutzner, H.; Sangueza, O. Cutaneous epithelioid angiomatous nodule. In WHO Classification of Skin Tumors; Elder, D., Massi, D., Scolyer, R., Willemze, R., Eds.; IARC Press: Lyon, France, 2018; p. 345. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubus, M.; Kanitakis, J. Cutaneous Epithelioid Angiomatous Nodule: Report of a New Case and Literature Review. Dermatopathology 2023, 10, 112-119. https://doi.org/10.3390/dermatopathology10010017
Dubus M, Kanitakis J. Cutaneous Epithelioid Angiomatous Nodule: Report of a New Case and Literature Review. Dermatopathology. 2023; 10(1):112-119. https://doi.org/10.3390/dermatopathology10010017
Chicago/Turabian StyleDubus, Margaux, and Jean Kanitakis. 2023. "Cutaneous Epithelioid Angiomatous Nodule: Report of a New Case and Literature Review" Dermatopathology 10, no. 1: 112-119. https://doi.org/10.3390/dermatopathology10010017
APA StyleDubus, M., & Kanitakis, J. (2023). Cutaneous Epithelioid Angiomatous Nodule: Report of a New Case and Literature Review. Dermatopathology, 10(1), 112-119. https://doi.org/10.3390/dermatopathology10010017






