Phylogenetic Analysis of Escherichia coli Isolated from Australian Feedlot Cattle in Comparison to Pig Faecal and Poultry/Human Extraintestinal Isolates
Abstract
:1. Introduction
2. Results
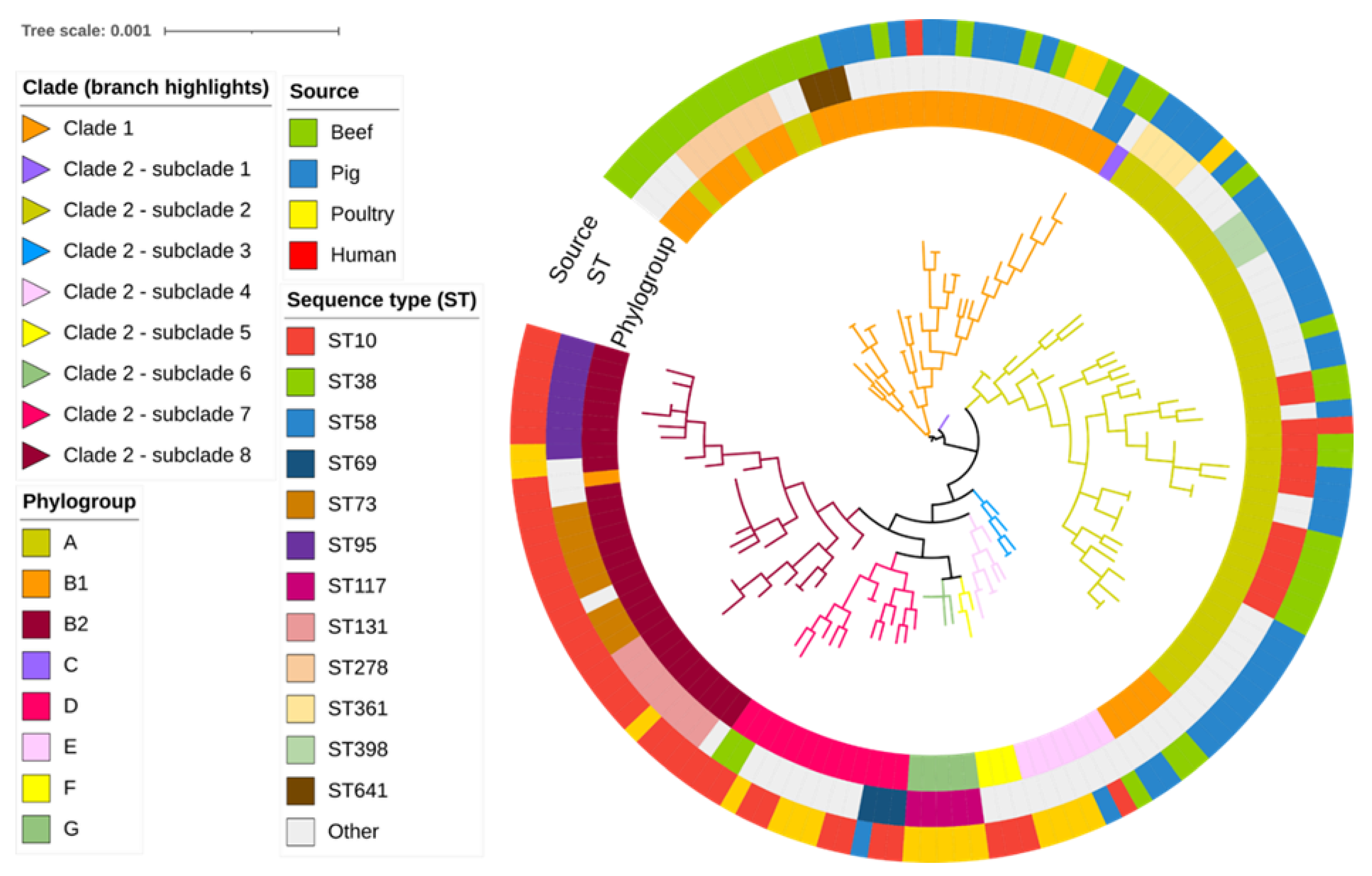
2.1. Phylogroup, Sequence Types and SNP Analyses
2.2. Antimicrobial Resistance Genes
β-Lactam Resistance Genes
2.3. Disinfectant Resistance Genes
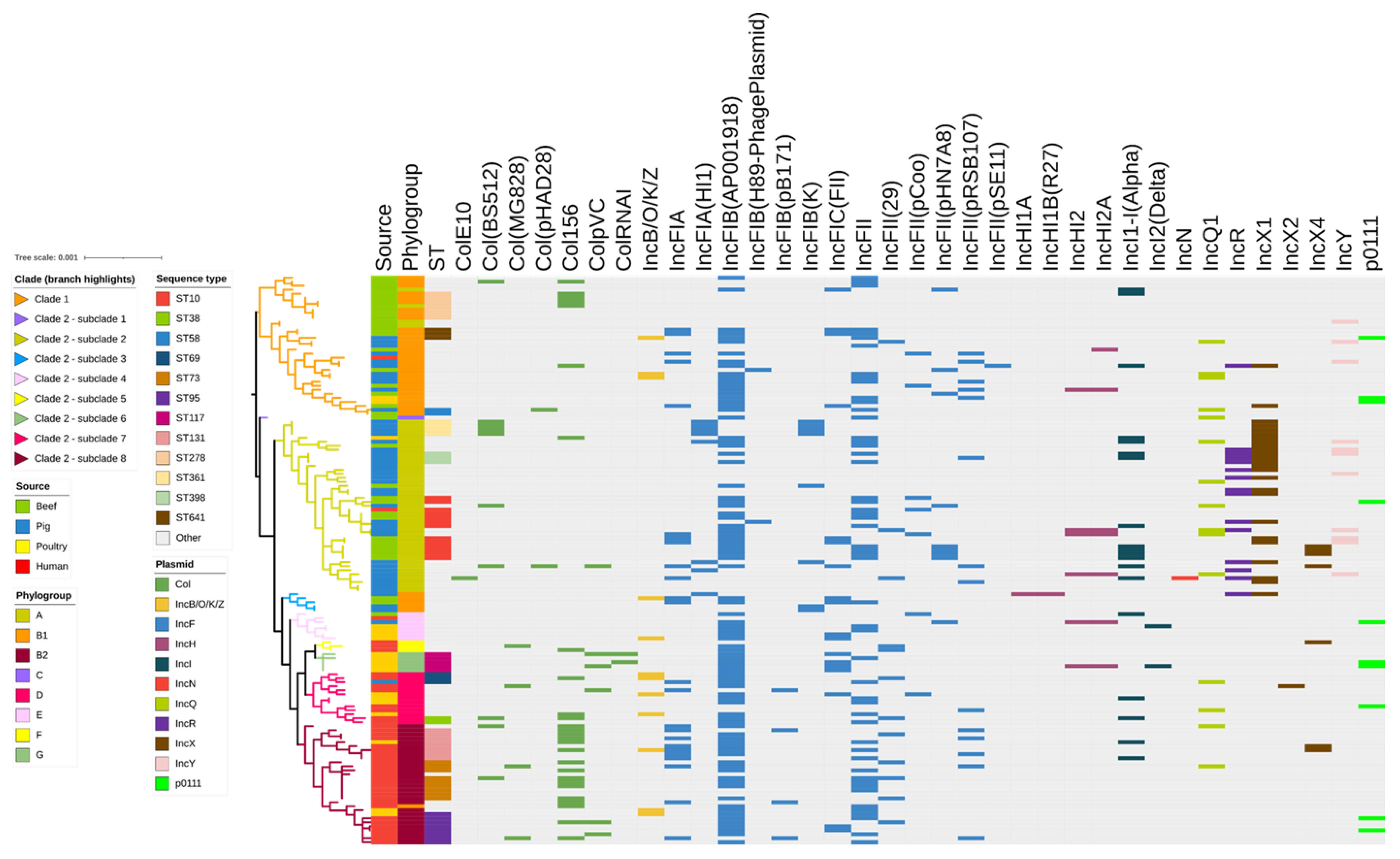
2.4. Identification of Plasmid Replicons
2.5. Agreement between Plasmid Replicons and ARG Arrays
2.6. Virulence Genes
3. Discussion
4. Materials and Methods
4.1. Whole Genome Sequencing and Phylogenetic Analysis
4.2. Determination of Genotypes, Antimicrobial Resistance Genes, and Virulence Genes
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal pathogenic Escherichia coli: Virulence factors and antibiotic resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Banting, G.; Neumann, N.F. A review of the taxonomy, genetics, and biology of the genus Escherichia and the type species Escherichia coli . Can. J. Microbiol. 2021, 67, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Páramo, P.; Le Menac’h, A.; Le Gall, T.; Amorin, C.; Gouriou, S.; Picard, B.; Skurnik, D.; Denamur, E. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. 2006, 8, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Anderson, M.A.; Whitlock, J.E.; Harwood, V.J. Diversity and distribution of Escherichia coli genotypes and antibiotic resistance phenotypes in feces of humans, cattle, and horses. Appl. Environ. Microbiol. 2006, 72, 6914–6922. [Google Scholar] [CrossRef]
- Clermont, O.; Olier, M.; Hoede, C.; Diancourt, L.; Brisse, S.; Keroudean, M.; Glodt, J.; Picard, B.; Oswald, E.; Denamur, E. Animal and human pathogenic Escherichia coli strains share common genetic backgrounds. Infect. Genet. Evol. 2011, 11, 654–662. [Google Scholar] [CrossRef]
- Collignon, P.J.; McEwen, S.A. One health-its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Harada, K.; Asai, T. Role of antimicrobial selective pressure and secondary factors on antimicrobial resistance prevalence in Escherichia coli from food-producing animals in Japan. J. Biomed. Biotechnol. 2010, 2010, 180682. [Google Scholar] [CrossRef]
- Tantoso, E.; Eisenhaber, B.; Kirsch, M.; Shitov, V.; Zhao, Z.; Eisenhaber, F. To kill or to be killed: Pangenome analysis of Escherichia coli strains reveals a tailocin specific for pandemic ST131. BMC Biol. 2022, 20, 146. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli . Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Dobrindt, U.; Chowdary, M.G.; Krumbholz, G.; Hacker, J. Genome dynamics and its impact on evolution of Escherichia coli . Med. Microbiol. Immunol. 2010, 199, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Massella, E.; Reid, C.J.; Cummins, M.L.; Anantanawat, K.; Zingali, T.; Serraino, A.; Piva, S.; Giacometti, F.; Djordjevic, S.P. Snapshot study of whole genome sequences of Escherichia coli from healthy companion animals, livestock, wildlife, humans and food in Italy. Antibiotics 2020, 9, 782. [Google Scholar] [CrossRef]
- Reid, C.J.; DeMaere, M.Z.; Djordjevic, S.P. Australian porcine clonal complex 10 (CC10) Escherichia coli belong to multiple sublineages of a highly diverse global CC10 phylogeny. Microb. Genom. 2019, 5, e000225. [Google Scholar] [CrossRef]
- Matamoros, S.; van Hattem, J.M.; Arcilla, M.S.; Willemse, N.; Melles, D.C.; Penders, J.; Vinh, T.N.; Thi Hoa, N.; Bootsma, M.C.J.; van Genderen, P.J.; et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci. Rep. 2017, 7, 15364. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Shapir, N.; Sadowsky, M.J. Frequency and distribution of tetracycline resistance genes in genetically diverse, nonselected, and nonclinical Escherichia coli strains isolated from diverse human and animal sources. Appl. Environ. Microbiol. 2004, 70, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Junker, E.; Schroeter, A.; Malorny, B.; Lehmann, S.; Helmuth, R. Phenotypic and genotypic characterization of antimicrobial resistance in German Escherichia coli isolates from cattle, swine and poultry. J. Antimicrob. Chemother. 2003, 52, 489–492. [Google Scholar] [CrossRef]
- Adator, E.H.; Narvaez-Bravo, C.; Zaheer, R.; Cook, S.R.; Tymensen, L.; Hannon, S.J.; Booker, C.W.; Church, D.; Read, R.R.; McAllister, T.A. A one Health comparative assessment of antimicrobial resistance in generic and Extended-spectrum cephalosporin-resistant Escherichia coli from beef production, sewage and clinical settings. Microorganisms 2020, 8, 885. [Google Scholar] [CrossRef]
- Nhung, N.T.; Cuong, N.V.; Campbell, J.; Hoa, N.T.; Bryant, J.E.; Truc, V.N.; Kiet, B.T.; Jombart, T.; Trung, N.V.; Hien, V.B.; et al. High levels of antimicrobial resistance among Escherichia coli isolates from livestock farms and synanthropic rats and shrews in the Mekong Delta of Vietnam. Appl. Environ. Microbiol. 2015, 81, 812–820. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial resistance genes in ESBL-producing Escherichia coli isolates from animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Massella, E.; Giacometti, F.; Bonilauri, P.; Reid, C.J.; Djordjevic, S.P.; Merialdi, G.; Bacci, C.; Fiorentini, L.; Massi, P.; Bardasi, L.; et al. Antimicrobial resistance profile and ExPEC virulence potential in commensal Escherichia coli of multiple sources. Antibiotics 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.E.; Bakke, E.; Konopek, N.; Thill, R.; Munson, E.; Hristova, K.R. Antimicrobial resistance traits of Escherichia coli isolated from dairy manure and freshwater ecosystems are similar to one another but differ from associated clinical isolates. Microorganisms 2020, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.; Durso, L.; Ducey, T.F.; Oladeinde, A.; Jackson, C.R.; Frye, J.G.; Dungan, R.; Moorman, T.; Brooks, J.P.; Obayiuwana, A.; et al. Diversity of plasmids and genes encoding resistance to Extended-spectrum β-lactamase in Escherichia coli from different animal sources. Microorganisms 2021, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Turnidge, J.D.; Meleady, K.T. Antimicrobial Use and Resistance in Australia (AURA) surveillance system: Coordinating national data on antimicrobial use and resistance for Australia. Aust. Health Rev. 2018, 42, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Badger, S.; Sullivan, K.; Jordan, D.; Caraguel, C.; Page, S.; Cusack, P.; Frith, D.; Trott, D. Antimicrobial use and stewardship practices on Australian beef feedlots. Aust. Vet. J. 2020, 98, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Silley, P.; Simjee, S.; Schwarz, S. Surveillance and monitoring of antimicrobial resistance and antibiotic consumption in humans and animals. Rev. Sci. Tech. 2012, 31, 105–120. [Google Scholar] [CrossRef]
- Torres, R.T.; Carvalho, J.; Fernandes, J.; Palmeira, J.D.; Cunha, M.V.; Fonseca, C. Mapping the scientific knowledge of antimicrobial resistance in food-producing animals. One Health 2021, 13, 100324. [Google Scholar] [CrossRef]
- Messele, Y.E.; Alkhallawi, M.; Veltman, T.; Trott, D.J.; McMeniman, J.P.; Kidd, S.P.; Low, W.Y.; Petrovski, K.R. Phenotypic and genotypic analysis of antimicrobial resistance in Escherichia coli recovered from feedlot beef cattle in Australia. Animals 2022, 12, 2256. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.K.P.; Hendriksen, R.S.; Okolocha, E.C.; Thakur, S. Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob. Resist. Infect. Control 2021, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, K.; Hölzel, C.; Bauer, J. Resistance gene patterns of tetracycline resistant Escherichia coli of human and porcine origin. Vet. Microbiol. 2010, 142, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.; Bekal, S.; Sanschagrin, F.; Levesque, R.C.; Brousseau, R.; Masson, L.; Larivière, S.; Harel, J. Heterogeneity among virulence and antimicrobial resistance gene profiles of extraintestinal Escherichia coli isolates of animal and human origin. J. Clin. Microbiol. 2004, 42, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S. Escherichia coli ST131-H 22 as a foodborne uropathogen. MBio 2018, 9, e00470-18. [Google Scholar] [CrossRef]
- Jakobsen, L.; Kurbasic, A.; Skjøt-Rasmussen, L.; Ejrnaes, K.; Porsbo, L.J.; Pedersen, K.; Jensen, L.B.; Emborg, H.D.; Agersø, Y.; Olsen, K.E.; et al. Escherichia coli isolates from broiler chicken meat, broiler chickens, pork, and pigs share phylogroups and antimicrobial resistance with community-dwelling humans and patients with urinary tract infection. Foodborne Pathog. Dis. 2010, 7, 537–547. [Google Scholar] [CrossRef]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Ewers, C.; de Jong, A.; Prenger-Berninghoff, E.; El Garch, F.; Leidner, U.; Tiwari, S.K.; Semmler, T. Genomic diversity and virulence potential of ESBL- and AmpC-β-lactamase-producing Escherichia coli strains from healthy food animals across Europe. Front. Microbiol. 2021, 12, 626774. [Google Scholar] [CrossRef]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microbiol. 2015, 81, 5560–5566. [Google Scholar] [CrossRef]
- Manges, A.R.; Harel, J.; Masson, L.; Edens, T.J.; Portt, A.; Reid-Smith, R.J.; Zhanel, G.G.; Kropinski, A.M.; Boerlin, P. Multilocus sequence typing and virulence gene profiles associated with Escherichia coli from human and animal Sources. Foodborne Pathog. Dis. 2015, 12, 302–310. [Google Scholar] [CrossRef]
- Montso, K.P.; Dlamini, S.B.; Kumar, A.; Ateba, C.N. Antimicrobial resistance factors of Extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniae isolated from cattle farms and raw beef in North-West province, South Africa. BioMed Res. Int. 2019, 2019, 4318306. [Google Scholar] [CrossRef]
- Alali, W.Q.; Scott, H.M.; Norby, B.; Gebreyes, W.; Loneragan, G. Quantification of the BlaCMY-2 in feces from beef feedlot cattle administered three different doses of ceftiofur in a longitudinal controlled field trial. Foodborne Pathog. Dis. 2009, 6, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Hörmansdorfer, S.; Messelhäusser, U.; Käsbohrer, A.; Sauter-Louis, C.; Mansfeld, R. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli on Bavarian dairy and beef cattle farms. Appl. Environ. Microbiol. 2013, 79, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, P.M.C.; Graat, E.A.M.; Haenen, A.P.J.; van Santen, M.G.; van Essen-Zandbergen, A.; Mevius, D.J.; van Duijkeren, E.; van Hoek, A.H.A.M. Extended-spectrum and AmpC β-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: Prevalence, risk factors and molecular characteristics. J. Antimicrob. Chemother. 2014, 69, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Mir, R.A.; Park, S.H.; Kim, D.; Kim, H.Y.; Boughton, R.K.; Morris, J.G., Jr.; Jeong, K.C. Prevalence of extended-spectrum beta-lactamases in the local farm environment and livestock: Challenges to mitigate antimicrobial resistance. Crit. Rev. Microbiol. 2020, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Madec, J.Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: A threat for humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef]
- de Been, M.; Lanza, V.F.; de Toro, M.; Scharringa, J.; Dohmen, W.; Du, Y.; Hu, J.; Lei, Y.; Li, N.; Tooming-Klunderud, A.; et al. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLOS Genet. 2014, 10, e1004776. [Google Scholar] [CrossRef]
- Dierikx, C.; van der Goot, J.; Fabri, T.; van Essen-Zandbergen, A.; Smith, H.; Mevius, D. Extended-spectrum-β-lactamase- and AmpC-β-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J. Antimicrob. Chemother. 2012, 68, 60–67. [Google Scholar] [CrossRef]
- Dohmen, W.; Bonten, M.J.M.; Bos, M.E.H.; van Marm, S.; Scharringa, J.; Wagenaar, J.A.; Heederik, D.J.J. Carriage of extended-spectrum β-lactamases in pig farmers is associated with occurrence in pigs. Clin. Microbiol. Infect. 2015, 21, 917–923. [Google Scholar] [CrossRef]
- Huijbers, P.M.C.; van Hoek, A.H.A.M.; Graat, E.A.M.; Haenen, A.P.J.; Florijn, A.; Hengeveld, P.D.; van Duijkeren, E. Methicillin-resistant Staphylococcus aureus and extended-spectrum and AmpC β-lactamase-producing Escherichia coli in broilers and in people living and/or working on organic broiler farms. Vet. Microbiol. 2015, 176, 120–125. [Google Scholar] [CrossRef]
- Dang, S.T.T.; Bortolaia, V.; Tran, N.T.; Le, H.Q.; Dalsgaard, A. Cephalosporin-resistant Escherichia coli isolated from farm workers and pigs in northern Vietnam. Trop. Med. Int. Health 2018, 23, 415–424. [Google Scholar] [CrossRef]
- Dahms, C.; Hübner, N.O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-Producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, W.; Liakopoulos, A.; Bonten, M.J.M.; Mevius, D.J.; Heederik, D.J.J. Longitudinal study of dynamic epidemiology of Extended-spectrum beta-lactamase-producing Escherichia coli in pigs and humans living and/or working on pig farms. Microbiol. Spectr. 2023, 11, e0294722. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.J.; Wyrsch, E.R.; Roy Chowdhury, P.; Zingali, T.; Liu, M.; Darling, A.E.; Chapman, T.A.; Djordjevic, S.P. Porcine commensal Escherichia coli: A reservoir for class 1 integrons associated with IS26. Microb. Genom. 2017, 3, e000143. [Google Scholar] [CrossRef]
- Tadrowski, A.C.; Evans, M.R.; Waclaw, B. Phenotypic switching can speed up microbial evolution. Sci. Rep. 2018, 8, 8941. [Google Scholar] [CrossRef] [PubMed]
- Cvijović, I.; Nguyen Ba, A.N.; Desai, M.M. Experimental studies of evolutionary dynamics in microbes. Trends Genet. 2018, 34, 693–703. [Google Scholar] [CrossRef]
- Boerlin, P.; Travis, R.; Gyles, C.L.; Reid-Smith, R.; Janecko, N.; Lim, H.; Nicholson, V.; McEwen, S.A.; Friendship, R.; Archambault, M. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl. Environ. Microbiol. 2005, 71, 6753–6761. [Google Scholar] [CrossRef]
- Neffe, L.; Abendroth, L.; Bautsch, W.; Häussler, S.; Tomasch, J. High plasmidome diversity of extended-spectrum beta-lactam-resistant Escherichia coli isolates collected during one year in one community hospital. Genomics 2022, 114, 110368. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids in Gram negatives: Molecular typing of resistance plasmids. Int. J. Med. Microbiol. 2011, 301, 654–658. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Popa, L.I.; Bleotu, C.; Chifiriuc, M.C. Targeting plasmids to limit acquisition and transmission of antimicrobial resistance. Front. Microbiol. 2020, 11, 761. [Google Scholar] [CrossRef]
- Balbuena-Alonso, M.G.; Cortés-Cortés, G.; Kim, J.W.; Lozano-Zarain, P.; Camps, M.; del Carmen Rocha-Gracia, R. Genomic analysis of plasmid content in food isolates of E. coli strongly supports its role as a reservoir for the horizontal transfer of virulence and antibiotic resistance genes. Plasmid 2022, 123, 102650. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Nolan, L.K. Pathogenomics of the virulence plasmids of Escherichia coli . Microbiol. Mol. Biol. Rev. 2009, 73, 750–774. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.Q.; Hounmanou, Y.M.G.; Dang, S.T.T.; Olsen, J.E.; Truong, G.T.H.; Tran, N.T.; Scheutz, F.; Dalsgaard, A. Genetic comparison of ESBL-Producing Escherichia coli from workers and pigs at Vietnamese pig farms. Antibiotics 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Mohsin, M.; Johnson, T.J.; Smith, E.A.; Johnson, A.; Umair, M.; Saleemi, M.K.; Sajjad ur, R. Genomic landscape of multi-drug resistant avian pathogenic Escherichia coli recovered from broilers. Vet. Microbiol. 2020, 247, 108766. [Google Scholar] [CrossRef] [PubMed]
- Salinas, L.; Cárdenas, P.; Johnson, T.J.; Vasco, K.; Graham, J.; Trueba, G.; Castanheira, M. Diverse commensal Escherichia coli clones and plasmids disseminate antimicrobial resistance genes in domestic animals and children in a semirural community in Ecuador. mSphere 2019, 4, e00316-19. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Farman, M.; Shah, M.W.; Jiman-Fatani, A.A.; Othman, N.A.; Almasaudi, S.B.; Alawi, M.; Shakil, S.; Al-Abdullah, N.; Ismaeel, N.A. Genomic and antimicrobial resistance genes diversity in multidrug-resistant CTX-M-positive isolates of Escherichia coli at a health care facility in Jeddah. J. Infect. Public Health 2020, 13, 94–100. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Lei, C.-W.; Zeng, J.-X.; Chen, Y.-P.; Kang, Z.-Z.; Wang, Y.-L.; Ye, X.-L.; Zhai, X.-W.; Wang, H.-N. An IncX1 plasmid isolated from Salmonella enterica subsp. enterica serovar Pullorum carrying blaTEM-1B, sul2, arsenic resistant operons. Plasmid 2018, 100, 14–21. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, M.; Shin, M.-K.; Yoo, H.S. Profiling of antimicrobial resistance and plasmid replicon types in β-lactamase producing Escherichia coli isolated from Korean beef cattle. J. Vet. Sci. 2015, 16, 483–489. [Google Scholar] [CrossRef]
- Kocsis, B.; Gulyás, D.; Szabó, D. Emergence and dissemination of extraintestinal pathogenic high-risk international clones of Escherichia coli . Life 2022, 12, 2077. [Google Scholar] [CrossRef]
- Ludden, C.; Raven, K.E.; Jamrozy, D.; Gouliouris, T.; Blane, B.; Coll, F.; Goffau, M.d.; Naydenova, P.; Horner, C.; Hernandez-Garcia, J.; et al. One health genomic surveillance of Escherichia coli demonstrates distinct lineages and mobile genetic elements in isolates from humans versus livestock. mBio 2019, 10, e02693-18. [Google Scholar] [CrossRef]
- Barlow, R.; Mcmillan, K.; Mellor, G.; Duffy, L.; Jordan, D.; Abraham, R.; O’dea, M.; Sahibzada, S.; Abraham, S. Phenotypic and genotypic assessment of antimicrobial resistance in Escherichia coli from Australian cattle populations at slaughter. J. Food Prot. 2022, 85, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Li, Y.; Hao, C. High prevalence of β-lactamase and plasmid-mediated quinolone resistance genes in extended-spectrum cephalosporin-resistant Escherichia coli from dogs in Shaanxi, China. Front. Microbiol. 2016, 7, 1843. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fevre, E.M.; Woolhouse, M.E.; van Bunnik, B.A. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef]
- García, A.; Fox, J.G.; Besser, T.E. Zoonotic enterohemorrhagic Escherichia coli: A one health perspective. Ilar J. 2010, 51, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Sulka, A.C.; Langer, A.J.; Schaben, C.; Crielly, A.S.; Gage, R.; Baysinger, M.; Moll, M.; Withers, G.; Toney, D.M. An outbreak of Escherichia coli O157: H7 infections among visitors to a dairy farm. N. Engl. J. Med. 2002, 347, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Paganini, J.A.; Plantinga, N.L.; Arredondo-Alonso, S.; Willems, R.J.L.; Schürch, A.C. Recovering Escherichia coli plasmids in the absence of long-read sequencing data. Microorganisms 2021, 9, 1613. [Google Scholar] [CrossRef]
- Juraschek, K.; Borowiak, M.; Tausch, S.H.; Malorny, B.; Käsbohrer, A.; Otani, S.; Schwarz, S.; Meemken, D.; Deneke, C.; Hammerl, J.A. Outcome of different sequencing and assembly approaches on the detection of plasmids and localization of antimicrobial resistance genes in commensal Escherichia coli . Microorganisms 2021, 9, 598. [Google Scholar] [CrossRef]
- Zingali, T.; Reid, C.J.; Chapman, T.A.; Gaio, D.; Liu, M.; Darling, A.E.; Djordjevic, S.P. Whole genome sequencing analysis of porcine faecal commensal Escherichia coli carrying class 1 integrons from sows and their offspring. Microorganisms 2020, 8, 843. [Google Scholar] [CrossRef]
- Cummins, M.L.; Reid, C.J.; Roy Chowdhury, P.; Bushell, R.N.; Esbert, N.; Tivendale, K.A.; Noormohammadi, A.H.; Islam, S.; Marenda, M.S.; Browning, G.F.; et al. Whole genome sequence analysis of Australian avian pathogenic Escherichia coli that carry the class 1 integrase gene. Microb. Genom. 2019, 5, 250. [Google Scholar] [CrossRef]
- Hastak, P.; Cummins, M.L.; Gottlieb, T.; Cheong, E.; Merlino, J.; Myers, G.S.A.; Djordjevic, S.P.; Roy Chowdhury, P. Genomic profiling of Escherichia coli isolates from bacteraemia patients: A 3-year cohort study of isolates collected at a Sydney teaching hospital. Microb. Genom. 2020, 6, 371. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Didelot, X.; Wilson, D.J. ClonalFrameML: Efficient inference of recombination in whole bacterial genomes. PLoS Comput. Biol. 2015, 11, e1004041. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genom. 2018, 4, 192. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli . J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Tetzschner, A.M.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F.; Dekker, J.P. In Silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef]
- Néron, B.; Littner, E.; Haudiquet, M.; Perrin, A.; Cury, J.; Rocha, E.P.C. IntegronFinder 2.0: Identification and analysis of integrons across bacteria, with a focus on antibiotic resistance in Klebsiella. Microorganisms 2022, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar] [CrossRef]




| Sample Source | Phylogroup (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B1 | B2 | C | D | E | F | G | |
| Beef (n = 37) | 16 (43.2) | 19 (51.3) | 0 | 1 (2.7) | 0 | 1 (2.7) | 0 | 0 |
| Pig (n = 45) | 29 (64.4) | 14 (31.1) | 0 | 0 | 1 (2.2) | 1 (2.2) | 0 | 0 |
| Poultry (n = 19) | 1 (5.3) | 2 (10.5) | 3 (15.8) | 0 | 4 (21.0) | 4 (21.0) | 0 | 5 (26.3) |
| Human (n = 40) | 1 (2.5) | 2 (5.0) | 26 (65.0) | 0 | 8 (20.0) | 0 | 3 (7.5) | 0 |
| β-Lactam | Sample Source | |||||||
|---|---|---|---|---|---|---|---|---|
| Resistance Genes | Beef Cattle (n = 37) | Pig (n = 45) | Poultry (n = 19) | Human (n = 40) | ||||
| Frequency (%) | Phylogroup (n) | Frequency (%) | Phylogroup (n) | Frequency (%) | Phylogroup (n) | Frequency (%) | Phylogroup (n) | |
| ampC | - | - | - | - | - | - | 1 (2.5) | D (1) |
| blaCARB-2 | - | - | 1 (2.2) | A (1) | 1 (5.3) | G (1) | - | - |
| blaCMY-2 | 2 (5.4) | A (1), E (1) | - | - | - | - | - | - |
| blaCTX-M-15 | 3 (8.1) | A (1), B1 (2) | - | - | - | - | 3 (7.5) | B2 (1), D (2) |
| blaCTX-M-27 | 3 (8.1) | A (2), C (1) | - | - | - | - | 2 (5.0) | B2 (1), D (1) |
| blaOXA-1 | - | - | - | - | - | - | 2 (5.0) | A (1), B2 (1) |
| blaSHV-1 | - | - | - | - | - | - | 1 (2.5) | B2 (1) |
| blaSHV-48 | - | - | - | - | - | - | 1 (2.5) | B2 (1) |
| blaSHV-102 | - | - | - | - | - | - | 1 (2.5) | B2 (1) |
| blaTEM-1A | - | - | - | - | 3 (15.8) | B2 (2), E (1) | - | - |
| blaTEM-1B | 8 (21.6) | A (6), B1 (2) | 42 (93.3) | A (26), B1 (14), D (1), E (1) | 3 (15.8) | A (1), B1 (1), D(1) | 17 (42.5) | B2 (12), D (5) |
| blaTEM-1C | 2 (5.4) | A (1), B1 (1) | - | - | 1 (5.3) | G (1) | 1 (2.5) | B2 (1) |
| blaTEM-33 | - | - | - | - | - | - | 1 (2.5) | B2 (1) |
| blaTEM-34 | - | - | - | - | - | - | 2 (5.0) | B1 (1), B2 (1) |
| blaTEM-57 | - | - | - | - | - | - | 1 (2.5) | B2 (1) |
| blaTEM-141 | - | - | 1 (2.2) | A (1) | - | - | 1 (2.5) | B2 (1) |
| blaTEM-206 | - | - | 2 (4.4) | A (2) | 1 (5.3) | D (1) | 1 (2.5) | B2 (1) |
| blaTEM-209 | - | - | 1 (2.2) | A (1) | - | - | - | - |
| blaTEM-213 | - | - | - | - | - | - | 1 (2.5) | B2 (1) |
| blaTEM-214 | - | - | 2 (4.4) | A (2) | 1 (5.3) | D (1) | - | - |
| blaTEM-216 | - | - | 1 (2.2) | A (1) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messele, Y.E.; Trott, D.J.; Hasoon, M.F.; Veltman, T.; McMeniman, J.P.; Kidd, S.P.; Djordjevic, S.P.; Petrovski, K.R.; Low, W.Y. Phylogenetic Analysis of Escherichia coli Isolated from Australian Feedlot Cattle in Comparison to Pig Faecal and Poultry/Human Extraintestinal Isolates. Antibiotics 2023, 12, 895. https://doi.org/10.3390/antibiotics12050895
Messele YE, Trott DJ, Hasoon MF, Veltman T, McMeniman JP, Kidd SP, Djordjevic SP, Petrovski KR, Low WY. Phylogenetic Analysis of Escherichia coli Isolated from Australian Feedlot Cattle in Comparison to Pig Faecal and Poultry/Human Extraintestinal Isolates. Antibiotics. 2023; 12(5):895. https://doi.org/10.3390/antibiotics12050895
Chicago/Turabian StyleMessele, Yohannes E., Darren J. Trott, Mauida F. Hasoon, Tania Veltman, Joe P. McMeniman, Stephen P. Kidd, Steven P. Djordjevic, Kiro R. Petrovski, and Wai Y. Low. 2023. "Phylogenetic Analysis of Escherichia coli Isolated from Australian Feedlot Cattle in Comparison to Pig Faecal and Poultry/Human Extraintestinal Isolates" Antibiotics 12, no. 5: 895. https://doi.org/10.3390/antibiotics12050895






