Evaluating the Effects of Sensorimotor Training on the Physical Capacities of Older People
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Ethics
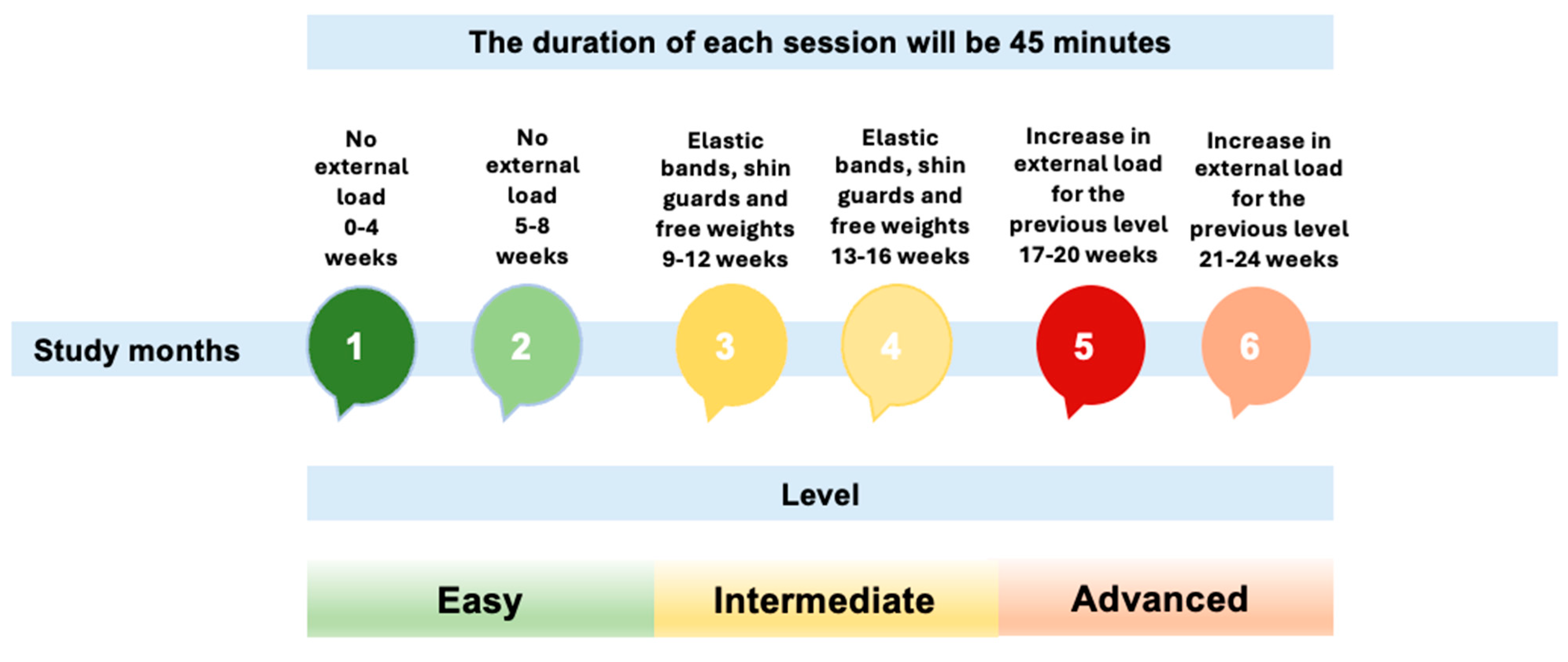
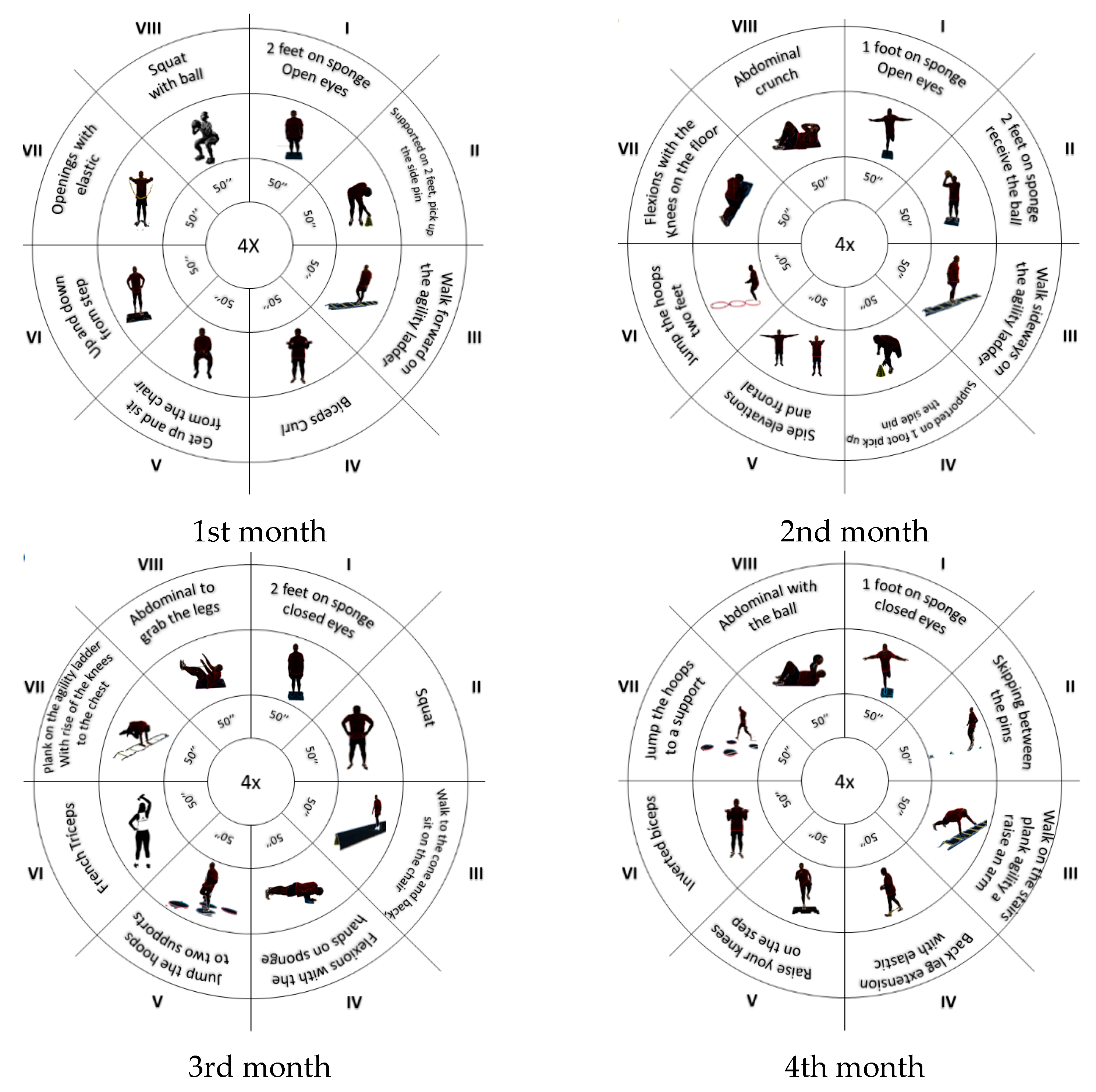
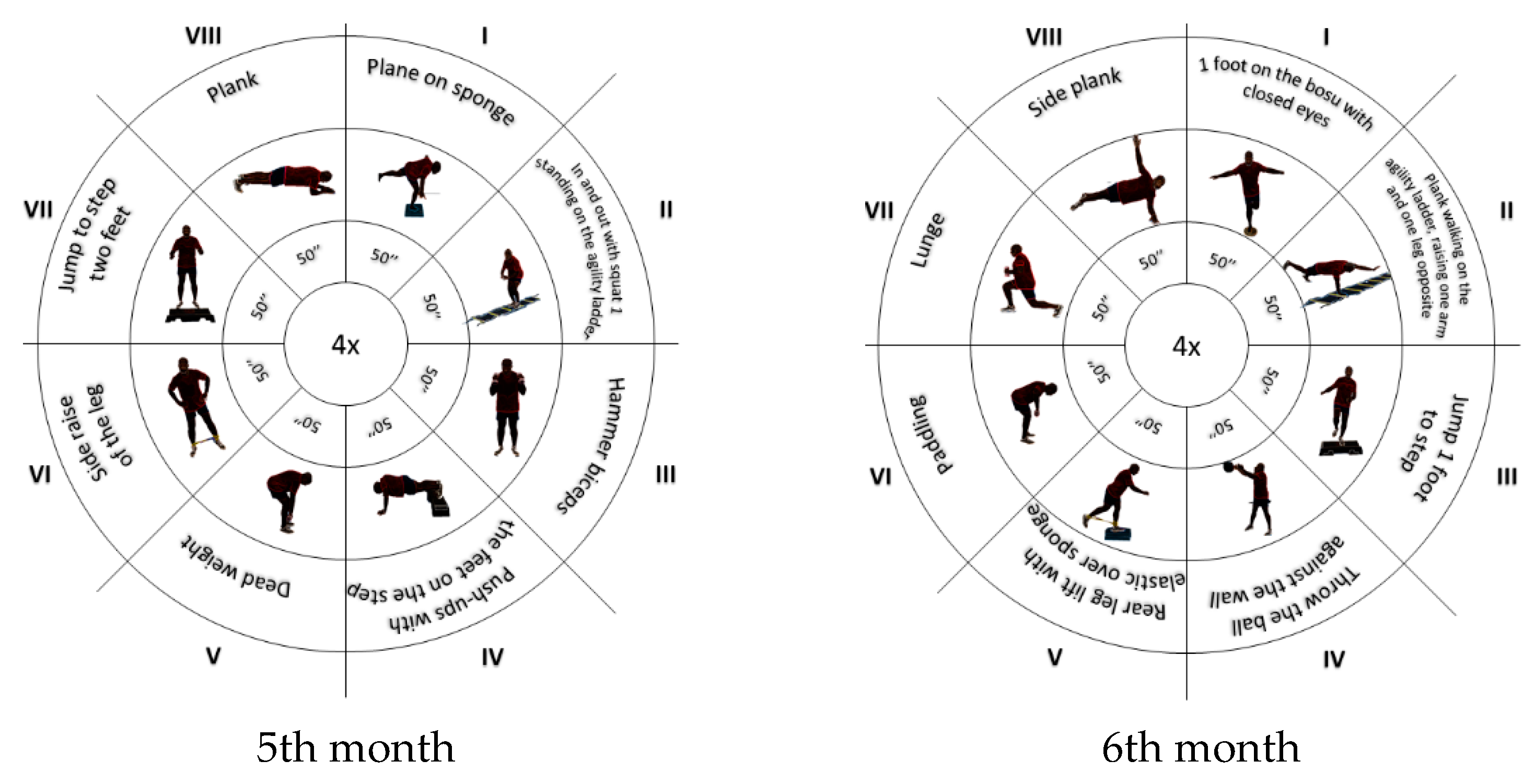
2.4. Intervention
2.5. Measuring Instruments
Evaluations
- Bodyweight and height. The participants were instructed to take off their heavy outerwear (coats, sweaters, etc.), shoes, and socks before the measurements. In addition, they were instructed to take off belts and other accessories (such as necklaces and bands) and to empty their pockets. A stadiometer (Seca 22, Hamburg, Germany) was used to measure their height. The measuring scale on this device was positioned perpendicular to the ground on a vertical surface. The participants were asked to stand with their arms relaxed along their bodies and their shoulders balanced. The height was measured in centimeters and rounded to the closest millimeter. A scale was used to measure their body weight. Weight (Kg)/height2 was the formula used to compute the BMI when the body weight was recorded in kilograms.
- Agility and execution speed were assessed through the TUG test, which involved getting out of a chair, walking three meters in a straight line, going back, and then sitting down again, which was used to measure speed (Prasad et al., 2021).
- Muscular endurance was evaluated using functional tasks such as rising from a chair or performing repeated bending and straightening movements for 30 s. These exercises targeted the lower limb strength and endurance, focusing on key muscle groups like the vastus medialis obliquus (VMO) and vastus lateralis (VL). The performance was quantified by reference to the number of repetitions completed within the 30 s, providing a measure of endurance and strength in the lower extremities, as outlined in Dunsky (2019).
- The upper limb strength was assessed by counting the number of repetitions that a participant could perform in 30 s, using a specified weight during arm flexion–extension exercises. This measure provided a functional evaluation of the participants’ upper limb strength and endurance, emphasizing the capacity to sustain repetitive motion and muscular power. Additional details of the specific weight used and the positioning during the exercise would further clarify this methodology (Dunsky, 2019).
- The lower limb flexibility was assessed using the “sit and reach” test, in which the participants gently bent over while sitting with one leg out in front of them, and then moved their hands down their leg till they touched (or passed) their toes (Mueller et al., 2021).
- The upper limb flexibility was assessed using the “behind the back reach” test, which consisted of measuring with a ruler the distance between (or the overlap of) the middle fingers behind the back (Dunsky, 2019).
2.6. Statistical Analysis
3. Results
3.1. Descriptive and Inferential Analysis Considering the EG
3.2. Descriptive and Inferential Analysis Considering the CG
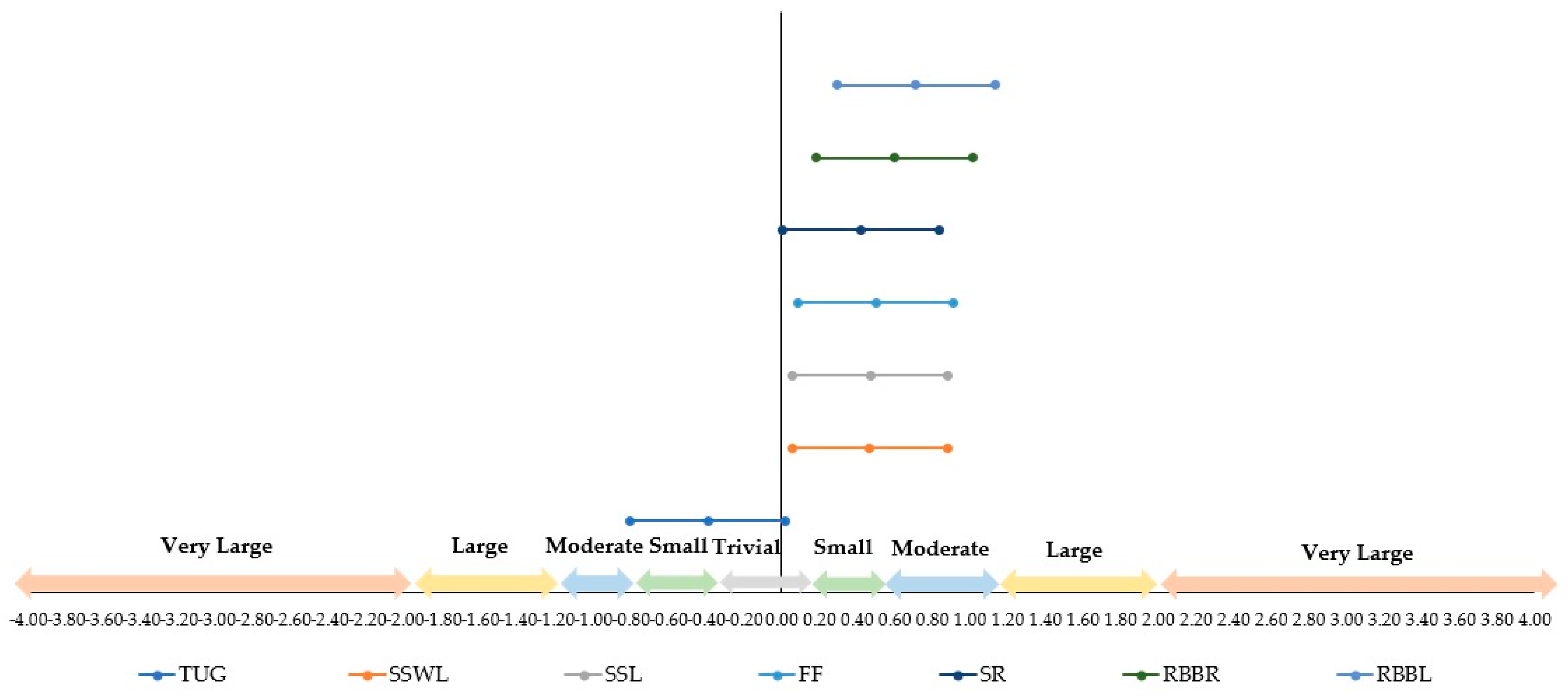
3.3. Analysis of the Effect Size
3.4. Analysis of the MANOVA
- TUG test: F = 79.0907, indicating a substantial variance between group means and a strong effect.
- Stand and sit without leaning and forearm flexion: F = 8.1732 and F = 9.4793, respectively, highlighting a more pronounced variance in this measure.
- Reach behind your back (left): F = 12.7230, showing a considerable difference between the groups.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aman, J. E., Elangovan, N., Yeh, I.-L., & Konczak, J. (2015). The effectiveness of proprioceptive training for improving motor function: A systematic review. Frontiers in Human Neuroscience, 8, 1075. [Google Scholar] [CrossRef]
- Andrade, L. C. A., Costa, G. L. dos A., Diogenes, L. G. B., & Pimentel, P. H. R. (2021). Timed up and go teste na avaliação do risco de quedas em idosos: Uma revisão de literatura. Research, Society and Development, 10(13), e321101321615. [Google Scholar] [CrossRef]
- Asan, A. S., McIntosh, J. R., & Carmel, J. B. (2022). Targeting sensory and motor integration for recovery of movement after CNS injury. Frontiers in Neuroscience, 15, 791824. [Google Scholar] [CrossRef]
- Baptista, F., & Sardinha, L. B. (2005). Avaliação da aptidão física e do equilíbrio de pessoas idosas—Baterias de fullerton. Faculdade de Motricidade Humana. [Google Scholar]
- Barry, E., Galvin, R., Keogh, C., Horgan, F., & Fahey, T. (2014). Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatrics, 14(1), 14. [Google Scholar] [CrossRef]
- Bretan, O., Elias Silva, J., Ribeiro, O. R., & Corrente, J. E. (2013). Risk of falling among elderly persons living in the community: Assessment by the Timed up and go test. Brazilian Journal of Otorhinolaryngology, 79(1), 18–21. [Google Scholar] [CrossRef]
- Cabo, C. A., Fernandes, O., Mendoza-Muñoz, M., Barrios-Fernandez, S., Muñoz-Bermejo, L., Gómez-Galán, R., & Parraca, J. A. (2022). An active retirement programme, a randomized controlled trial of a sensorimotor training programme for older adults: A study protocol. Healthcare, 11(1), 86. [Google Scholar] [CrossRef]
- Cabo, C. A., Hernández-Beltrán, V., Gamonales, J. M., Fernandes, O., Espada, M. C., & Parraca, J. A. (2024). Evolution of documents related to the influence of physical activity and functional capacity throughout the aging process: A bibliometric review. Frontiers in Physiology, 15, 1427038. [Google Scholar] [CrossRef]
- Carvalho, W. V. D., Katakura, E. A. L. B., Carvalho, T. L. R. B. D., Koga, P. M., Kawamoto, A. B. S. S., Cardoso, R. B. C. M., Tashima, C. M., & Alarcon, M. F. S. (2023). Benefit of pleasurable physical activity for the elderly: An integrative review. Em A Look at development (1.a ed.). Seven Editora. [Google Scholar] [CrossRef]
- Cho, K. H., Bok, S. K., Kim, Y.-J., & Hwang, S. L. (2012). Effect of lower limb strength on falls and balance of the elderly. Annals of Rehabilitation Medicine, 36(3), 386. [Google Scholar] [CrossRef]
- Choi, J. I., Cho, Y. H., Kim, Y. J., Lee, S. Y., Lee, J. G., Yi, Y. H., Tak, Y. J., Hwang, H. R., Lee, S. H., Park, E. J., Lee, Y. I., Ra, Y. J., & Lee, S. J. (2021). The relationship of sitting time and physical activity on the quality of life in elderly people. International Journal of Environmental Research and Public Health, 18(4), 1459. [Google Scholar] [CrossRef]
- Chua, K. Y., Lim, W. S., Lin, X., Yuan, J.-M., & Koh, W.-P. (2020). Handgrip strength and timed up-and-go (tug) test are predictors of short-term mortality among elderly in a population-based cohort in singapore. The Journal of Nutrition, Health and Aging, 24(4), 371–378. [Google Scholar] [CrossRef]
- Ciumărnean, L., Milaciu, M. V., Negrean, V., Orășan, O. H., Vesa, S. C., Sălăgean, O., Iluţ, S., & Vlaicu, S. I. (2021). Cardiovascular risk factors and physical activity for the prevention of cardiovascular diseases in the elderly. International Journal of Environmental Research and Public Health, 19(1), 207. [Google Scholar] [CrossRef]
- Correa, L. da P., Bento, T. P. F., Guariglia, D. A., Rodrigues, G. F., & Conti, M. H. S. D. (2022). Effects of functional training on pain and functional capacity in elderly women. Fisioterapia Em Movimento, 35, e35149. [Google Scholar] [CrossRef]
- de Oliveira, F. N., Damião, E. P., dos Santos, L., Galvão, L. L., Machado, H. R., Silva, R. R., Tribess, S., Virtuoso Júnior, J. S., & de Assis Teles Santos, D. (2024). Prevalence and factors associated with low functional mobility in older adults. Aging Medicine, 7(3), 292–300. [Google Scholar] [CrossRef]
- Donisi, L., Coccia, A., Amitrano, F., Mercogliano, L., Cesarelli, G., & D’Addio, G. (2020, June 1–July 1). Backpack influence on kinematic parameters related to timed up and go (tug) test in school children. 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA) (pp. 1–5), Bari, Italy. [Google Scholar] [CrossRef]
- Dunsky, A. (2019). The effect of balance and coordination exercises on quality of life in older adults: A mini-review. Frontiers in Aging Neuroscience, 11, 318. [Google Scholar] [CrossRef]
- Fabris, E., & Sinagra, G. (2022). Physical activity in older people: Better late than never, but better early than late. Heart, 108(5), 328–329. [Google Scholar] [CrossRef]
- Field, A. (2013). Discovering statistics using IBM SPSS statistics. Sage Publications. [Google Scholar]
- Fiorilli, G., Buonsenso, A., Centorbi, M., Calcagno, G., Iuliano, E., Angiolillo, A., Ciccotelli, S., Di Cagno, A., & Di Costanzo, A. (2022). Long term physical activity improves quality of life perception, healthy nutrition, and daily life management in elderly: A randomized controlled trial. Nutrients, 14(12), 2527. [Google Scholar] [CrossRef]
- Fletcher, G. F., Landolfo, C., Niebauer, J., Ozemek, C., Arena, R., & Lavie, C. J. (2018). Reprint of: Promoting physical activity and exercise. Journal of the American College of Cardiology, 72(23), 3053–3070. [Google Scholar] [CrossRef]
- Freire, I., & Seixas, A. (2024). Effectiveness of a sensorimotor exercise program on proprioception, balance, muscle strength, functional mobility and risk of falls in older people. Frontiers in Physiology, 15, 1309161. [Google Scholar] [CrossRef]
- Ghram, A., Briki, W., Mansoor, H., Al-Mohannadi, A. S., Lavie, C. J., & Chamari, K. (2021). Home-based exercise can be beneficial for counteracting sedentary behavior and physical inactivity during the COVID-19 pandemic in older adults. Postgraduate Medicine, 133(5), 469–480. [Google Scholar] [CrossRef]
- Gomiero, A. B., Kayo, A., Abraão, M., Peccin, M. S., Grande, A. J., & Trevisani, V. F. (2018). Sensory-motor training versus resistance training among patients with knee osteoarthritis: Randomized single-blind controlled trial. Sao Paulo Medical Journal, 136(1), 44–50. [Google Scholar] [CrossRef]
- Goodarzi, S., Teymouri Athar, M. M., Beiky, M., Fathi, H., Nakhaee, Z., Omran, S. P., & Shafiee, A. (2024). Effect of physical activity for reducing anxiety symptoms in older adults: A meta-analysis of randomized controlled trials. BMC Sports Science, Medicine and Rehabilitation, 16(1), 153. [Google Scholar] [CrossRef] [PubMed]
- Granacher, U., Muehlbauer, T., Gollhofer, A., Kressig, R. W., & Zahner, L. (2011). An intergenerational approach in the promotion of balance and strength for fall prevention—A mini-review. Gerontology, 57(4), 304–315. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W. G., Marshall, S. W., Batterham, A. M., & Hanin, J. (2009). Progressive statistics for studies in sports medicine and exercise science. Medicine & Science in Sports & Exercise, 41(1), 3–12. [Google Scholar] [CrossRef]
- Janssen, J. C., & Le-Ngoc, L. (2009). Intratester reliability and validity of concentric measurements using a new hand-held dynamometer. Archives of Physical Medicine and Rehabilitation, 90(9), 1541–1547. [Google Scholar] [CrossRef]
- Jones, J., & Rikli, R. E. (2022). Measuring functional. The Journal on Active Aging, 25–30. [Google Scholar]
- Koo, T. K., & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. [Google Scholar] [CrossRef]
- Kuş, G., Tarakçı, E., Razak Ozdincler, A., & Erçin, E. (2023). Sensory-motor training versus resistance training in the treatment of knee osteoarthritis: A randomized controlled trial. Clinical Rehabilitation, 37(5), 636–650. [Google Scholar] [CrossRef]
- La Greca, S., Rapali, M., Ciaprini, G., Russo, L., Vinciguerra, M. G., & Di Giminiani, R. (2022). Acute and chronic effects of supervised flexibility training in older adults: A comparison of two different conditioning programs. International Journal of Environmental Research and Public Health, 19(24), 16974. [Google Scholar] [CrossRef]
- Lesinski, M., Hortobágyi, T., & Granacher, U. (2015). Effects of balance training on balance performance in healthy older adults: A systematic review and meta-analysis. Sports Medicine, 45(12), 1721–1738. [Google Scholar] [CrossRef]
- Li, R. C., Jasiewicz, J. M., Middleton, J., Condie, P., Barriskill, A., Hebnes, H., & Purcell, B. (2006). The development, validity, and reliability of a manual muscle testing device with integrated limb position sensors. Archives of Physical Medicine and Rehabilitation, 87(3), 411–417. [Google Scholar] [CrossRef]
- Lindsay Smith, G., Banting, L., Eime, R., O’Sullivan, G., & Van Uffelen, J. G. Z. (2017). The association between social support and physical activity in older adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 56. [Google Scholar] [CrossRef] [PubMed]
- Lord, S. R., Sherrington, C., Menz, H. B., & Close, C. T. (2002). Falls in older people: Risk factors and strategies for prevention. Cambridge University Press. [Google Scholar]
- Martinez, B. P., Santos, M. R. dos, Simões, L. P., Ramos, I. R., Oliveira, C. S. de, Forgiarini Júnior, L. A., Camelier, F. W. R., & Camelier, A. A. (2016). Segurança e reprodutibilidade do teste timed up and go em idosos hospitalizados. Revista Brasileira de Medicina Do Esporte, 22(5), 408–411. [Google Scholar] [CrossRef]
- Martinez, M., Brezun, J., Zennou-Azogui, Y., Baril, N., & Xerri, C. (2009). Sensorimotor training promotes functional recovery and somatosensory cortical map reactivation following cervical spinal cord injury. European Journal of Neuroscience, 30(12), 2356–2367. [Google Scholar] [CrossRef]
- McGarrigle, L., Boulton, E., & Todd, C. (2020). Map the apps: A rapid review of digital approaches to support the engagement of older adults in strength and balance exercises. BMC Geriatrics, 20(1), 483. [Google Scholar] [CrossRef]
- Melchiorri, G., Triossi, T., Viero, V., Marroni, S., D’Arcangelo, G., & Tancredi, V. (2022). A study about a new standardized method of home-based exercise in elderly people aged 65 and older to improve motor abilities and well-being: Feasibility, functional abilities and strength improvements. Geriatrics, 7(6), 134. [Google Scholar] [CrossRef]
- Montero, I., & León, O. G. (2007). A guide for naming research studies in Psychology. International Journal of Clinical and Health Psychology, 7(3), 847–862. [Google Scholar]
- Muehlbauer, T., Gollhofer, A., & Granacher, U. (2015). Associations between measures of balance and lower-extremity muscle strength/power in healthy individuals across the lifespan: A systematic review and meta-analysis. Sports Medicine, 45(12), 1671–1692. [Google Scholar] [CrossRef]
- Mueller, D., Redkva, P. E., Fernando De Borba, E., Barbosa, S. C., Krause, M. P., & Gregorio Da Silva, S. (2021). Effect of mat vs. Apparatus pilates training on the functional capacity of elderly women. Journal of Bodywork and Movement Therapies, 25, 80–86. [Google Scholar] [CrossRef]
- Musdalifah Ahmad, S. (2023). A systematic review of the impact of physical activity on elderly mental health. Journal of Psychiatric Nursing, 14, 248–255. [Google Scholar] [CrossRef]
- Nikitas, C., Kikidis, D., Bibas, A., Pavlou, M., Zachou, Z., & Bamiou, D.-E. (2022). Recommendations for physical activity in the elderly population: A scoping review of guidelines. Journal of Frailty, Sarcopenia and Falls, 7(1), 18–28. [Google Scholar] [CrossRef]
- O’Donoghue, P. (2012). Statistics for sport and exercise studies. Routledge. [Google Scholar]
- Parraca, J. A., Adsuar, J. C., Domínguez-Muñoz, F. J., Barrios-Fernandez, S., & Tomas-Carus, P. (2022). Test-retest reliability of isokinetic strength measurements in lower limbs in elderly. Biology, 11(6), 802. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D. H., Robertson, R. J., & Ray, R. L. (1987). Bilateral isokinetic peak torque, torque acceleration energy, power, and work relationships in athletes and nonathletes. Journal of Orthopaedic & Sports Physical Therapy, 9(5), 184–189. [Google Scholar] [CrossRef]
- Pfeifer, C. E., Ross, L. M., Weber, S. R., Sui, X., & Blair, S. N. (2022). Are flexibility and muscle-strengthening activities associated with functional limitation? Sports Medicine and Health Science, 4(2), 95–100. [Google Scholar] [CrossRef]
- Pizzigalli, L., Filippini, A., Ahmaidi, S., Jullien, H., & Rainoldi, A. (2011). Prevention of Falling Risk in Elderly People: The Relevance of Muscular Strength and Symmetry of Lower Limbs in Postural Stability. Journal of Strength and Conditioning Research, 25(2), 567–574. [Google Scholar] [CrossRef]
- Podsiadlo, D., & Richardson, S. (1991). The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39(2), 142–148. [Google Scholar] [CrossRef]
- Prasad, L., Fredrick, J., & Aruna, R. (2021). The relationship between physical performance and quality of life and the level of physical activity among the elderly. Journal of Education and Health Promotion, 10(1), 68. [Google Scholar] [CrossRef]
- Pšeničnik Sluga, S., & Kozinc, Z. (2024). Sensorimotor and proprioceptive exercise programs to improve balance in older adults: A systematic review with meta-analysis. European Journal of Translational Myology, 34, 12010. [Google Scholar] [CrossRef]
- Rikli, R. E., & Jones, C. J. (1999). Development and validation of a functional fitness test for community-residing older adults. Journal of Aging and Physical Activity, 7(2), 129–161. [Google Scholar] [CrossRef]
- Rizwan Sajid, M., Muhammad, N., Shahbaz, A., & Zakaria, R. (2021). A statistical study on the prevalence of physical inactivity among cardiovascular diseases patients: The predictive role of demographic and socioeconomic factors. Research Journal of Pharmacy and Technology, 14, 3679–3684. [Google Scholar] [CrossRef]
- Ruivo, R. (2015). Manual de avaliação e prescrição de exercício. Self. [Google Scholar]
- Sherrington, C., Fairhall, N. J., Wallbank, G. K., Tiedemann, A., Michaleff, Z. A., Howard, K., & Clemson, L. (2017). Exercise for preventing falls in older people living in the community: An abridged Cochrane systematic review. British Journal of Sports Medicine, 51(24), 1750–1758. [Google Scholar] [PubMed]
- Shirazi, F., Jaberi, A., & Zahedian Nasab, N. (2023). Effect of a technology-based exercise program on physical fitness and activities of daily living in the elderly with balance impairment: A clinical trial. Salmand, 18(2), 178–191. [Google Scholar] [CrossRef]
- Takai, Y., Ohta, M., Akagi, R., Kanehisa, H., Kawakami, Y., & Fukunaga, T. (2009). Sit-to-stand test to evaluate knee extensor muscle size and strength in the elderly: A novel approach. Journal of Physiological Anthropology, 28(3), 123–128. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, K., Tsuji, T., Jindo, T., Tokunaga, S., & Okura, T. (2022). Changes in the physical fitness of elderly people in the COVID-19 pandemic: An examination using performance tests. Nippon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics, 59(4), 491. [Google Scholar] [CrossRef]
- Vagetti, G. C., Barbosa Filho, V. C., Moreira, N. B., Oliveira, V. D., Mazzardo, O., & Campos, W. D. (2014). Association between physical activity and quality of life in the elderly: A systematic review, 2000-2012. Revista Brasileira de Psiquiatria, 36(1), 76–88. [Google Scholar] [CrossRef]
- Warburton, D. E., & Bredin, S. S. (2017). Health benefits of physical activity: A systematic review of current systematic reviews. Current Opinion in Cardiology, 32(5), 541–556. [Google Scholar] [CrossRef]
- Woessner, M. N., Tacey, A., Levinger-Limor, A., Parker, A. G., Levinger, P., & Levinger, I. (2021). The evolution of technology and physical inactivity: The good, the bad, and the way forward. Frontiers in Public Health, 9, 655491. [Google Scholar] [CrossRef]
- Wróblewska, Z., Chmielewski, J. P., Florek-Łuszczki, M., Nowak-Starz, G., Wojciechowska, M., & Wróblewska, I. M. (2023). Assessment of functional capacity of the elderly. Annals of Agricultural and Environmental Medicine, 30(1), 156–163. [Google Scholar]
| N | Age (year) | Weight (kg) | Height (m) | BMI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Control Group | 44 | 73.70 | 6.08 | 70.10 | 12.70 | 1.58 | 0.08 | 28.10 | 4.69 |
| Experimental Group | 46 | 72.40 | 6.88 | 68.40 | 14.20 | 1.58 | 0.09 | 27.40 | 5.03 |
| Variables | Mean | SD | Student’s t Test | Mean Differences | p-Values |
|---|---|---|---|---|---|
| Timed up and go (pre) (s) | 7.26 | ±1.23 | 3.90 | 0.416 | <0.001 |
| Timed up and go (post) (s) | 6.85 | ±0.81 | |||
| Stand and sit with leaning (pre) (rep) | 13.00 | ±2.30 | −3.64 | −1.043 | <0.0001 |
| Stand and sit with leaning (post) (rep) | 14.00 | ±1.97 | |||
| Stand and sit without leaning (pre) (rep) | 15.30 | ±2.95 | −5.04 | −1.370 | <0.001 |
| Stand and sit without leaning (post) (rep) | 16.60 | ±2.57 | |||
| Forearm flexion (pre) (rep) | 17.30 | ±5.83 | −3.33 | −2.522 | 0.002 |
| Forearm flexion (post) (rep) | 19.80 | ±4.04 | |||
| Sitting and reaching (pre) (rep) | −2.54 | ±8.70 | −4.43 | −3.565 | <0.001 |
| Sitting and reaching (post) (rep) | 1.02 | ±8.24 | |||
| Reach behind your back (right) (pre) (m) | −13.80 | ±11.70 | −7.29 | −6.261 | <0.001 |
| Reach behind your back (right) (post) (m) | −7.50 | ±9.12 | |||
| Reach behind your back (left) (pre) (m) | −18.50 | ±11.00 | −8.97 | −7.391 | <0.001 |
| Reach behind your back (left) (post) (m) | −11.20 | ±9.43 |
| Variables | Mean | SD | Student’s t Test | Mean Differences | p-Values |
|---|---|---|---|---|---|
| Timed up and go (pre) (s) | 8.15 | ±2.89 | 0.763 | 0.076 | 0.450 |
| Timed up and go (post) (s) | 8.08 | ±2.94 | |||
| Stand and sit with leaning (pre) (rep) | 13.50 | ±3.42 | −0.947 | −0.318 | 0.349 |
| Stand and sit with leaning (post) (rep) | 13.80 | ±2.96 | |||
| Stand and sit without leaning (pre) (rep) | 15.50 | ±4.31 | −0.120 | −0.045 | 0.905 |
| Stand and sit without leaning (post) (rep) | 15.60 | ±3.63 | |||
| Forearm flexion (pre) (rep) | 18.30 | ±4.69 | 1.007 | 0.386 | 0.319 |
| Forearm flexion (post) (rep) | 17.90 | ±4.11 | |||
| Sitting and reaching (pre) (rep) | −0.61 | ±9.97 | 1.446 | 1.204 | 0.155 |
| Sitting and reaching (post) (rep) | −1.82 | ±9.37 | |||
| Reach behind your back (right) (pre) (m) | −9.32 | ±12.50 | 1.100 | 2.636 | 0.277 |
| Reach behind your back (right) (post) (m) | −12.00 | ±20.40 | |||
| Reach behind your back (left) (pre) (m) | −15.80 | ±12.90 | −1.310 | −1.056 | 0.197 |
| Reach behind your back (left) (post) (m) | −14.80 | ±11.90 |
| Variables | Pre-Intervention | Post-Intervention | ES | σ | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | M | SD | n | LL | UL | |||
| Times up and go | 7.26 | 1.23 | 46 | 6.85 | 0.81 | 46 | −0.39 | 0.210524737 | −0.81 | 0.02 |
| Stand and sit with leaning | 13.00 | 2.3 | 46 | 14.00 | 1.97 | 46 | 0.47 | 0.211337371 | 0.05 | 0.88 |
| Stand and sit without leaning | 15.30 | 2.95 | 46 | 16.60 | 2.57 | 46 | 0.47 | 0.211372433 | 0.06 | 0.88 |
| Forearm flexion | 17.30 | 5.83 | 46 | 19.80 | 4.04 | 46 | 0.50 | 0.211727587 | 0.08 | 0.91 |
| Sitting and reaching | −2.54 | 8.7 | 46 | 1.02 | 8.24 | 46 | 0.42 | 0.210802398 | 0.01 | 0.83 |
| Reach behind your back (right) | −13.80 | 11.7 | 46 | −7.50 | 9.12 | 46 | 0.60 | 0.213163448 | 0.18 | 1.02 |
| Reach behind your back (left) | −18.50 | 11 | 46 | −11.20 | 9.43 | 46 | 0.71 | 0.215029136 | 0.29 | 1.13 |
| Pre-Intervention | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD | Pre-Intervention p-Value | Post-Intervention p-Value |
| Timed up and go (pre) (s) | 7.26 | ±1.23 | 8.15 | ±2.89 | 0.059 | 0.008 |
| Timed up and go (post) (s) | 6.85 | ±0.81 | 8.08 | ±2.94 | ||
| Stand and sit with leaning (pre) (rep) | 13.00 | ±2.30 | 13.50 | ±3.42 | 0.395 | 0.702 |
| Stand and sit with leaning (post) (rep) | 14.00 | ±1.97 | 13.80 | ±2.96 | ||
| Stand and sit without leaning (pre) (rep) | 15.30 | ±2.95 | 15.50 | ±4.31 | 0.737 | 0.111 |
| Stand and sit without leaning (post) (rep) | 16.60 | ±2.57 | 15.60 | ±3.63 | ||
| Forearm flexion (pre) (rep) | 17.30 | ±5.83 | 18.30 | ±4.69 | 0.389 | 0.026 |
| Forearm flexion (post) (rep) | 19.80 | ±4.04 | 17.90 | ±4.11 | ||
| Sitting and reaching (pre) (rep) | −2.54 | ±8.70 | −0.61 | ±9.97 | 0.330 | 0.130 |
| Sitting and reaching (post) (rep) | 1.02 | ±8.24 | −1.82 | ±9.37 | ||
| Reach behind your back (right) (pre) (m) | −13.80 | ±11.70 | −9.32 | ±12.50 | 0.086 | 0.182 |
| Reach behind your back (right) (post) (m) | −7.50 | ±9.12 | −12.00 | ±20.40 | ||
| Reach behind your back (left) (pre) (m) | −18.50 | ±11.00 | −15.80 | ±12.90 | 0.285 | 0.113 |
| Reach behind your back (left) (post) (m) | −11.20 | ±9.43 | −14.80 | ±11.90 | ||
| Variables | Sum of Squares | Mean Square | F-Values | p-Values |
|---|---|---|---|---|
| Timed up and go (post) (s) | 6.85 | ±0.81 | 79.0907 | <0.001 |
| Stand and sit with leaning (post) (rep) | 14.00 | ±1.97 | 0.3351 | 0.564 |
| Stand and sit without leaning (post) (rep) | 16.60 | ±2.57 | 8.1732 | 0.005 |
| Forearm flexion (post) (rep) | 19.80 | ±4.04 | 9.4793 | 0.003 |
| Sitting and reaching (post) (rep) | 1.02 | ±8.24 | 7.1769 | 0.009 |
| Reach behind your back (right) (post) (m) | −7.50 | ±9.12 | 3.7447 | 0.056 |
| Reach behind your back (left) (post) (m) | −11.20 | ±9.43 | 12.7130 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the University Association of Education and Psychology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabo, C.A.; Hernández-Beltrán, V.; Fernandes, O.; Mendes, C.; Gamonales, J.M.; Espada, M.C.; Parraca, J.A. Evaluating the Effects of Sensorimotor Training on the Physical Capacities of Older People. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 50. https://doi.org/10.3390/ejihpe15040050
Cabo CA, Hernández-Beltrán V, Fernandes O, Mendes C, Gamonales JM, Espada MC, Parraca JA. Evaluating the Effects of Sensorimotor Training on the Physical Capacities of Older People. European Journal of Investigation in Health, Psychology and Education. 2025; 15(4):50. https://doi.org/10.3390/ejihpe15040050
Chicago/Turabian StyleCabo, Carolina A., Víctor Hernández-Beltrán, Orlando Fernandes, Cláudia Mendes, José M. Gamonales, Mário C. Espada, and José A. Parraca. 2025. "Evaluating the Effects of Sensorimotor Training on the Physical Capacities of Older People" European Journal of Investigation in Health, Psychology and Education 15, no. 4: 50. https://doi.org/10.3390/ejihpe15040050
APA StyleCabo, C. A., Hernández-Beltrán, V., Fernandes, O., Mendes, C., Gamonales, J. M., Espada, M. C., & Parraca, J. A. (2025). Evaluating the Effects of Sensorimotor Training on the Physical Capacities of Older People. European Journal of Investigation in Health, Psychology and Education, 15(4), 50. https://doi.org/10.3390/ejihpe15040050










