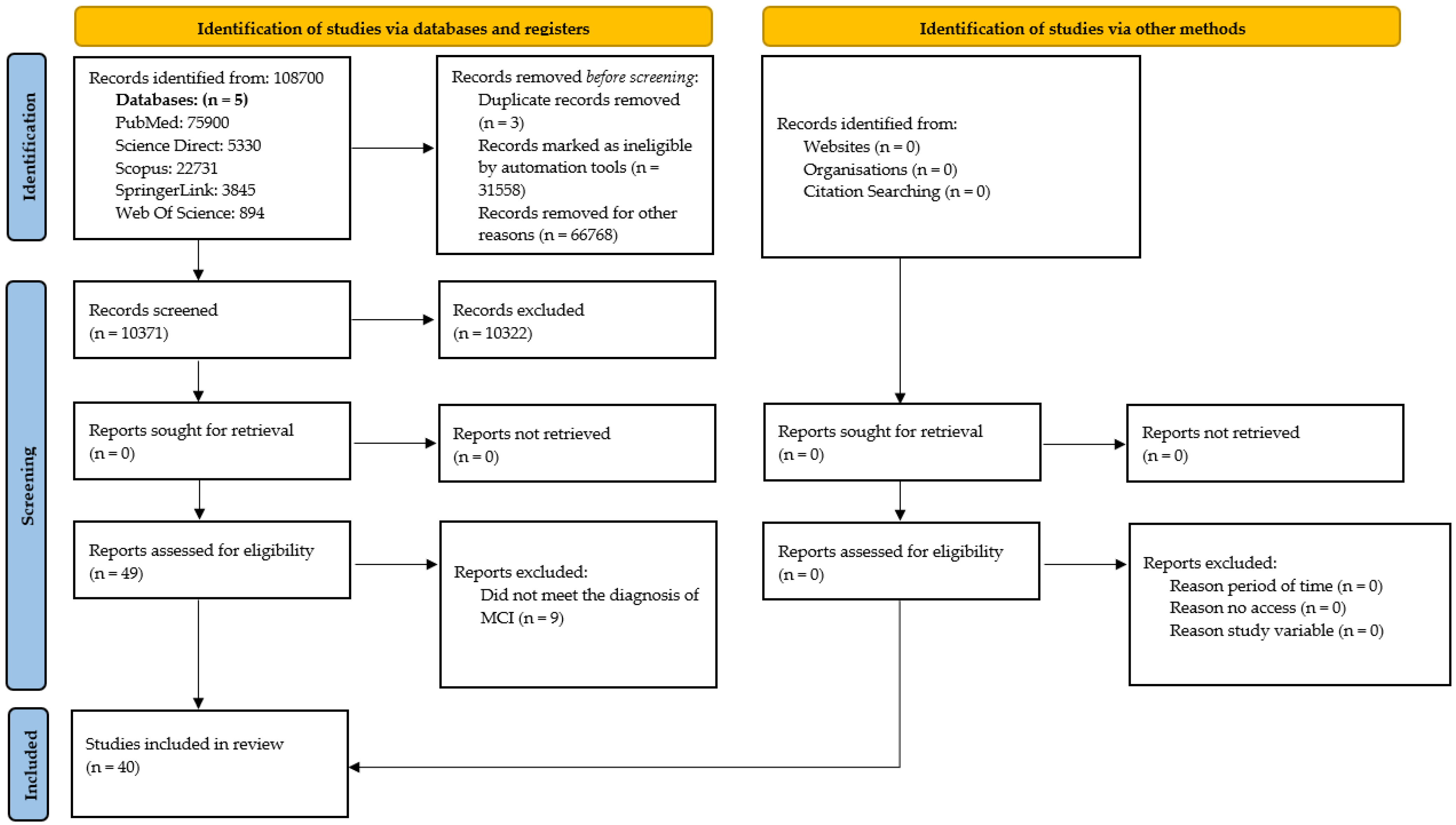
This section presents the selected studies and the findings related to interventions for MCI, following a rigorous process of search, selection, and analysis of the studies included in the systematic review. Based on a carefully planned methodological strategy, relevant evidence was identified that addresses the objectives of the research, ensuring transparency and traceability throughout the entire process.
3.2. Risk of Bias Analysis
The risk of bias assessment was conducted using Cochrane’s RoB 2 tool for a total of 37 randomised controlled trials (RCTs). As for the remaining three studies, which did not meet the methodological criteria of a controlled clinical trial, the ROBINS-I tool was employed in order to rigorously evaluate their methodological quality and responsibly integrate their evidence into this review.
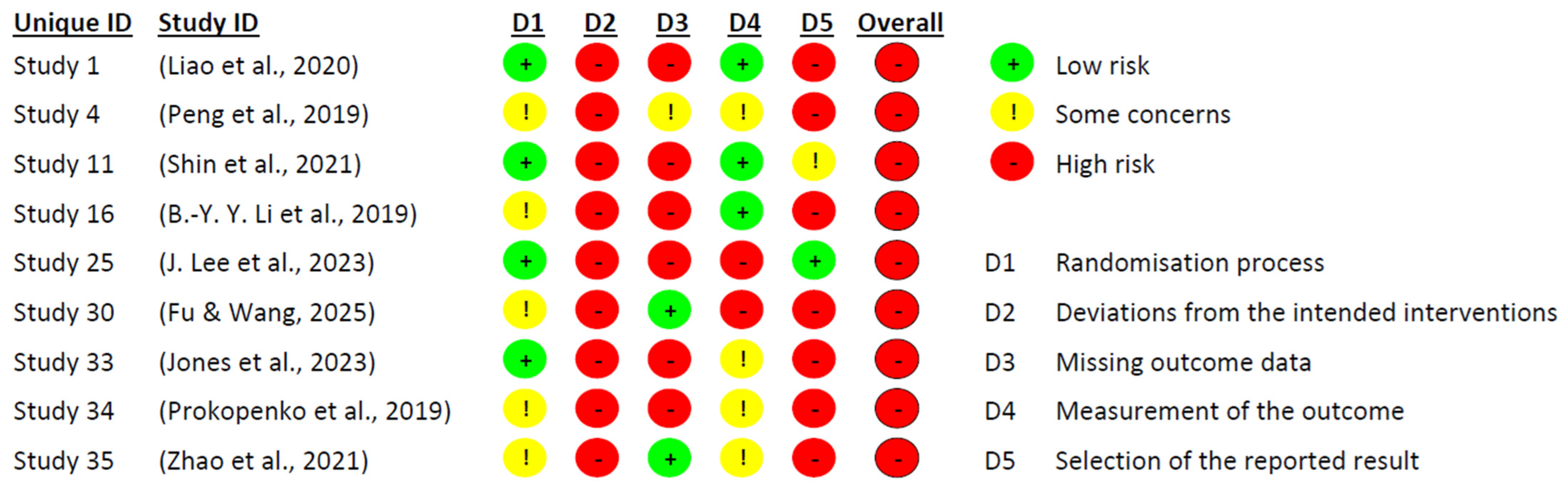
Figure 2 and
Figure 3 present the risk of bias evaluation for the non-randomised studies. Although the primary focus of this systematic review was on randomised controlled trials, the inclusion of these three studies was considered appropriate, as they examined potentially relevant interventions in the context of pharmacological and combined treatment approaches. Their inclusion broadened the spectrum of available evidence, particularly regarding the use of medication, since most experimental studies tend to focus on dementia interventions, and few address MCI.
As expected, the three non-randomised studies displayed methodological limitations inherent to their design. The most affected domains were: confounding (D1), selection of participants (D2), and handling of missing data (D5). Issues identified included the lack of comparison groups, unclear participant selection procedures, and inadequate (or unreported) management of attrition and missing data.
Nevertheless, the domain related to selective reporting (D7) showed no signs of bias in any of the three studies, suggesting that outcomes were reported completely and without manipulation. Additionally, the domains concerning intervention during the study (D3) and deviations from intended intervention (D4) did not reveal significant bias, indicating that the intervention was administered appropriately and without major deviations.
Following the comprehensive evaluation, it was concluded that the three studies presented an overall moderate risk of bias. Accordingly, their findings should be interpreted with caution, close attention to detail, and avoidance of unwarranted generalisations. However, we argue that these findings should not be disregarded, as they offer valuable insights into interventions that are seldom explored in research on MCI, particularly within the pharmacological domain.
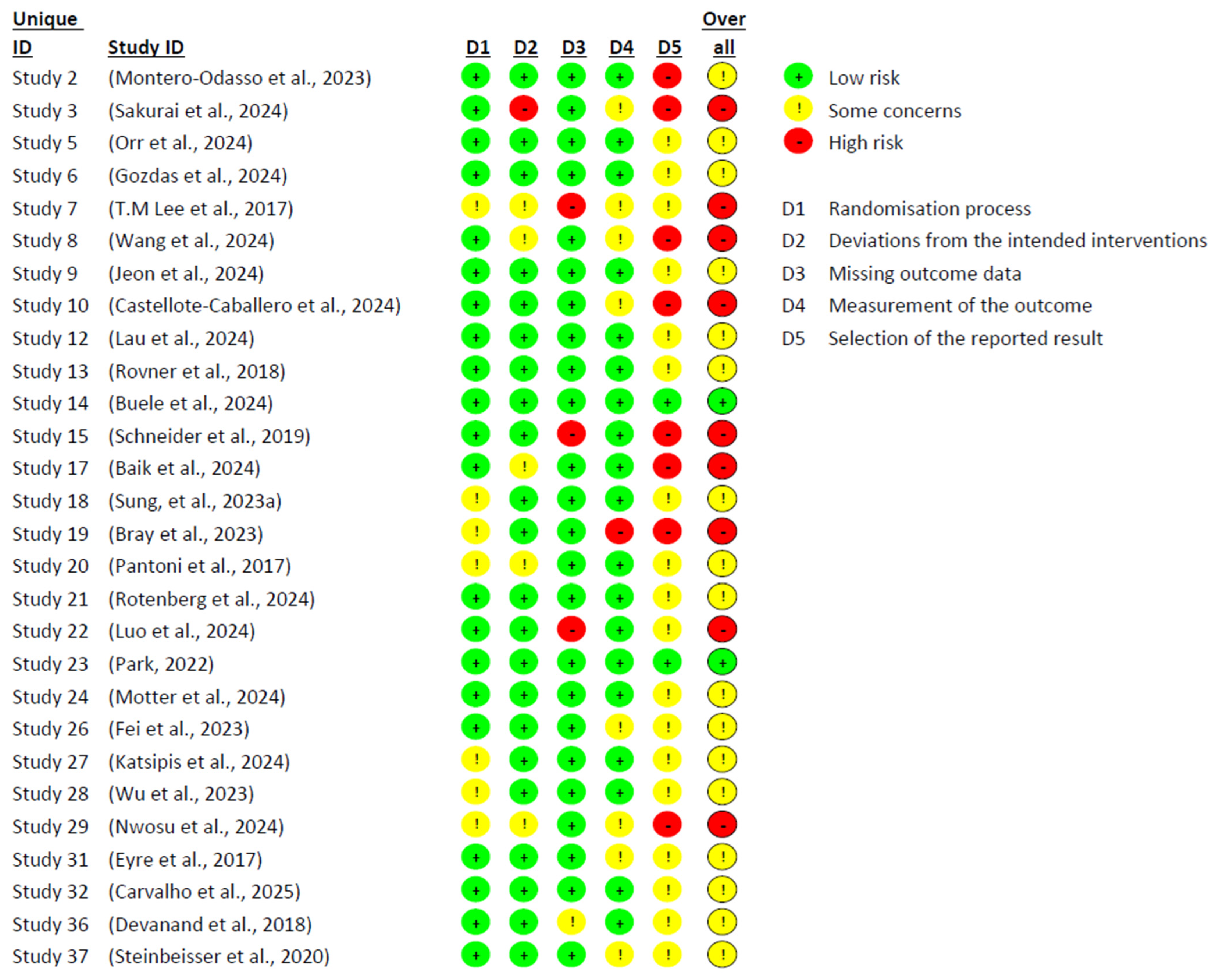
Figure 4 and
Figure 5 present the risk of bias analysis, conducted using Cochrane’s RoB 2 tool, which enabled an assessment of the methodological quality of the 37 included studies. Significant limitations were identified in the design and implementation of the trials, which affected the reliability of the findings and reduced their internal validity. Only 2 studies were classified as having a low risk of bias across all domains, highlighting the persistent methodological challenges in research on MCI. One of the most critical sources of bias was the type of statistical analysis employed. While 28 studies used an intention-to-treat approach, 9 conducted per-protocol analyses. The latter were generally associated with a higher risk of bias, likely due to the exclusion of participants who did not complete the intervention, potentially introducing systematic distortions in the outcomes.
Domain 5—related to outcome reporting—was the most frequently compromised. In many studies, pre-specified protocols were either not declared or not accessible, making it difficult to compare planned and reported outcomes. Furthermore, signs of post hoc decisions and unreported exploratory analyses were identified, which may have artificially inflated the observed effects. In contrast, Domains 1 (random sequence generation), 2 (deviations from intended interventions), and 3 (missing outcome data) showed comparatively lower risk, though not without issues. Instances such as lack of allocation concealment, absence of blinding among intervention staff, participant attrition, and poor adherence to protocols were still observed. Many of these limitations appeared to be closely linked to the nature of non-pharmacological interventions—such as cognitive training, physical exercise, or psychosocial programmes—in which blinding is often unfeasible and adherence tends to fluctuate considerably.
3.3. Characteristics of the Included Studies
Following a rigorous and systematic literature review, 40 studies were identified that met the established eligibility criteria. The majority of these (n = 37) were randomised controlled trials (RCTs), while two were single-arm studies and one employed a quasi-experimental design.
Table 5 provides a summary of the main methodological characteristics and the most relevant findings reported in each of the included studies.
The studies included in this review display a diverse geographical distribution, with a predominance in countries across Asia, North America, and Europe. Latin America, by contrast, was underrepresented, highlighting a significant research gap in the region. China, South Korea, the United States, and Canada (
Jeon et al., 2024;
Montero-Odasso et al., 2023;
Peng et al., 2019;
Rovner et al., 2018) accounted for the highest concentration of studies, which limits the generalisability of findings and underscores the need to strengthen scientific output on MCI in Latin American contexts.
Regarding methodological approaches, most studies employed clinical trials as their primary design, in accordance with the inclusion criteria established. However, only a small number of studies adopted alternative designs, such as single-arm or quasi-experimental approaches (
Hassan et al., 2021;
Nakagawa et al., 2024). This indicates a predominance of controlled investigations, while also suggesting the need to broaden methodological perspectives in order to better capture the complexity of the phenomenon.
The duration of interventions varied considerably, ranging from 4 to 156 weeks. Nevertheless, the majority of trials were concentrated within periods of 8 to 26 weeks, suggesting that short- to medium-term interventions may be sufficient to produce cognitive improvements (
Buele et al., 2024;
Jones et al., 2023;
Lau et al., 2024). This observation reinforces the value of implementing accessible and sustainable programmes, particularly in settings with limited resources.
In terms of therapeutic strategies, a wide variety of non-pharmacological treatments were identified. Cognitive training was the most frequently used intervention and also reported the most favourable outcomes in terms of cognitive function enhancement (
Gozdas et al., 2024;
Sung et al., 2023b), a finding that aligns with the specialised literature. Additional interventions such as transcranial magnetic stimulation, yoga, the use of probiotics, and the administration of vortioxetine were also evaluated. These showed promising effects by targeting biological mechanisms related to oxidative stress, brain inflammation, and neural connectivity (
Eyre et al., 2017;
Fei et al., 2023;
Lau et al., 2024;
Tan & Tan, 2021).
Sample sizes varied considerably across studies: while some trials involved small groups (20 to 50 participants), others engaged much larger populations (
Sakurai et al., 2024;
Steinbeisser et al., 2020). Despite this variability, most investigations reported statistically significant improvements following the intervention, particularly in domains such as memory, attention, processing speed, and verbal fluency. However, not all studies yielded positive results; notably, some trials involving pharmacological interventions—such as ladostigil, donepezil, and nicotinamide riboside—did not demonstrate significant effects compared with control or placebo groups (
Devanand et al., 2018;
Orr et al., 2024;
Schneider et al., 2019).
Multimodal interventions based on lifestyle changes also emerged as a particularly relevant strategy. The combination of physical activity, healthy nutrition, and sleep hygiene not only showed cognitive benefits but also supported a holistic approach to wellbeing, with potential for both preventive and therapeutic applications (
Wang et al., 2024).
Finally, the criteria used to diagnose MCI varied across studies, reflecting the absence of a universally accepted diagnostic standard. Among the most commonly used instruments were the Mini-Mental State Examination (MMSE), the MoCA, clinical dementia rating scales, as well as the criteria proposed by Petersen, Marilyn Albert, and Winblad. While this methodological diversity enriches the field, it also poses challenges for comparing studies and standardising diagnostic protocols.
Table 6 presents the studies that implemented various therapeutic strategies based on neurocognitive intervention modalities.
A notable diversity of approaches is evident, with conventional cognitive training being the most frequently employed modality. This intervention was delivered through both computerised formats and virtual reality, and in several cases was combined with techniques such as transcranial magnetic stimulation. These findings reflect an ongoing trend towards the use of non-invasive treatments, which are widely accepted in both clinical and experimental settings.
Most studies focused on intervening in specific cognitive functions, particularly memory (in its various forms), attention, and language. This focus suggests that these functions are the most commonly affected during the early stages of cognitive decline. Moreover, there was a consistent interest in assessing executive functions globally, highlighting the importance of considering functional performance in daily life as a key clinical criterion in the diagnosis of MCI.
Among the most relevant findings, several studies (
Castellote-Caballero et al., 2024;
Gozdas et al., 2024;
T. M. Lee et al., 2017;
Liao et al., 2020;
Montero-Odasso et al., 2023;
Peng et al., 2019;
Wang et al., 2024) reported significant improvements in global cognitive functioning, with notable gains in attention, memory, and verbal fluency. In contrast, some trials (
Carvalho et al., 2025;
Jones et al., 2023) demonstrated only moderate clinical effects, while other investigations (
Bray et al., 2023;
Pantoni et al., 2017;
Rotenberg et al., 2024;
Sakurai et al., 2024) reported no significant benefits. These included interventions such as metacognitive therapy (
Rotenberg et al., 2024), standalone cognitive training (
Pantoni et al., 2017), a hybrid combination of physical exercise, cognitive training, and vitamin D3 supplementation (
Bray et al., 2023), as well as nutritional counselling focused on cardiovascular risk management (
Sakurai et al., 2024).
Table 7 presents the two studies that evaluated non-pharmacological interventions with psychotherapeutic components aimed at individuals with MCI. Both studies integrated behavioural and emotional strategies to promote cognitive and functional well-being (
J. Lee et al., 2023;
Rovner et al., 2018).
The first study, conducted by (
Rovner et al., 2018), implemented a behavioural activation intervention in a sample of older African American adults. The intervention aimed to prevent the progression of MCI through the structured increase of meaningful activities—such as reading, walking with neighbours, and weekly phone calls—supported by personalised action plans and visual reminders. After a two-year follow-up, only 1.2% of participants in the intervention group showed memory decline, compared to 9.3% in the control group. Moreover, participants who received the behavioural activation intervention maintained their ability to carry out everyday tasks such as managing finances and using a mobile phone, whereas those in the control group experienced a decline in these functions. The study also found a significant improvement in problem-solving speed, with treated participants showing an increase of 13 s per year compared to controls.
The second study, conducted by (
J. Lee et al., 2023), implemented a comprehensive programme aimed at enhancing self-efficacy by combining emotional strategies with physical and cognitive interventions. The programme included social dialogue sessions, practical workbook activities, verbal persuasion about MCI, and emotional regulation through music and physical exercise. Results indicated significant improvements in the experimental group in terms of dementia-related knowledge (F = 4.582,
p = 0.005), levels of self-efficacy (F = 5.547,
p = 0.002), and frequency of preventive behaviours (F = 6.376,
p = 0.001). Additionally, the study reported an improvement in global cognitive function (F = 13.880,
p < 0.001). However, as the intervention was multifaceted, the clinical effects could not be attributed specifically to individual components.
The eight studies presented in
Table 8 employed physical interventions as a non-pharmacological therapeutic strategy. Generally, these interventions were structured as multimodal programmes incorporating aerobic, resistance, and balance exercises (
Bray et al., 2023;
Montero-Odasso et al., 2023). The most common frequency was two to three sessions per week, although the study by (
Hassan et al., 2021) implemented a more intensive schedule of five sessions per week. Session durations ranged from 40 to 120 min. Notably, the protocol by (
Buele et al., 2024) was the shortest, while that of (
Steinbeisser et al., 2020) was the longest. Other studies, such as those by (
Montero-Odasso et al., 2023), (
Bray et al., 2023), and (
J. Lee et al., 2023), maintained an average session length of 60 min.
In the case of (
Montero-Odasso et al., 2023), although meaningful improvements were observed when the physical intervention was combined with other components of the experimental protocol, the effects did not reach statistical significance compared to the control group when analysed in isolation (
p = 0.19). Similarly, the studies by (
Castellote-Caballero et al., 2024), (
J. Lee et al., 2023), and (
Steinbeisser et al., 2020) also reported post-intervention improvements within their respective groups; however, their clinical effectiveness appears to rely more on a comprehensive approach than on physical exercise alone.
In contrast, the studies by (
Buele et al., 2024) and (
Hassan et al., 2021) demonstrated significant effects on cognitive variables following exclusively physical interventions. (
Buele et al., 2024) reported improvements in cognitive function as measured by the MoCA, with an increase in mean scores from 20.83 (±1.8) to 23.67 (±2.24) (
p < 0.001), alongside a reduction in depressive symptoms from 5.58 (±2.07) to 2.75 (±1.42) (
p < 0.001). Similarly, (
Hassan et al., 2021) found that resistance training led to significant cognitive gains across various neuropsychological tests, including: MoCA (from 17.60 ± 1.35 to 21.93 ± 1.57;
p < 0.01), Mini-Mental State Examination (from 20.60 ± 1.35 to 23.20 ± 1.69;
p < 0.01), and the Trail Making Tests A (from 1.47 ± 0.03 to 1.23 ± 0.04;
p < 0.01) and B (from 2.51 ± 0.04 to 2.08 ± 0.04;
p < 0.01).
Conversely, the study by (
Bray et al., 2023) did not report cognitive improvements, even when physical intervention was combined with other treatments. In a different vein, (
Katsipis et al., 2024) did not directly assess cognitive performance but did report improvements in biomarkers associated with the progression of MCI and inflammatory processes. Although these results do not provide direct evidence of cognitive impact, they suggest a potential neuroprotective effect of physical activity in the early stages of cognitive decline.
Table 9 presents information from recent studies that have explored the effectiveness of non-pharmacological interventions in the treatment and management of MCI. These strategies, ranging from nutritional supplementation to mind-body therapies and creative expression programmes, have been developed as alternatives or complements to conventional treatments.
In the field of nutrition, the use of vitamin D as a therapeutic supplement stands out. (
Montero-Odasso et al., 2023) and (
Bray et al., 2023) administered doses of 10,000 international units three times per week. However, in neither study was the effect of vitamin D evaluated in isolation as monotherapy, which limits the ability to draw definitive conclusions about its specific efficacy. In fact, Bray and colleagues did not report significant improvements in cognitive function, suggesting that, while vitamin D may play a complementary role in combined interventions, its isolated effect appears to be limited in this clinical context.
Traditional medicine has also been explored as a promising therapeutic avenue. The study by (
Shin et al., 2021) examined the effects of Kami-guibi-tang, a Korean herbal formula composed of 15 medicinal plants, administered three times daily over a 24-week period. The results showed significant improvements in cognitive performance, measured by the Seoul Neuropsychological Dementia Battery, with the average score increasing from 176.00 (±24.76) at baseline to 198.47 (±31.29) at the end of the intervention (
p < 0.001). Moreover, the treatment was well tolerated, with no reports of serious adverse effects, supporting its viability as a safe complementary therapy.
One of the most consistent findings has emerged from research on the gut-brain axis, particularly in interventions targeting the gut microbiota. In this regard, (
Fei et al., 2023) assessed the daily administration of 2 g of probiotics composed of 18 different bacterial strains. The results demonstrated statistically significant improvements in cognitive function: The MMSEscore increased from 21.75 ± 2.57 to 24.75 ± 2.47 (
p < 0.001), and the MoCA score improved from 19.80 ± 1.85 to 22.05 ± 2.14 (
p < 0.001). The most notable improvements were observed in recall memory and visuospatial function, supporting the relevance of the gut-brain axis as a therapeutic pathway in neurodegenerative processes.
Among mind-body therapies, two approaches have shown particularly positive outcomes. The study by (
Eyre et al., 2017) implemented a daily yoga routine involving mindful breathing, gentle finger movements, and moments of relaxation. This practice, carried out for just 12 min a day, resulted in significant improvements in verbal memory (d = 0.95) and in executive functions, as measured by the Trail Making Test (d = −0.75) and the Stroop Test (d = 0.71). In a separate study, (
Fu & Wang, 2025) combined intensive acupuncture sessions at three cranial points—the vertex, crown, and forehead—with virtual reality training, five times per week. Although improvements were noted in MMSE and MoCA scores, the combined nature of the intervention prevents a clear attribution of effects solely to acupuncture, highlighting the need for further studies that assess its isolated impact.
Creative interventions have also emerged as effective alternatives for stimulating cognitive, emotional, and social functions. The programme designed by (
Luo et al., 2024) incorporated activities such as painting, clay modelling, collage-making, and the creation of artistic narratives, delivered in one-hour sessions twice a week. This intervention led to statistically significant improvements in verbal fluency (
p = 0.021). Complementarily, (
Zhao et al., 2021) implemented a programme focused on storytelling and drawing, in which participants constructed narratives based on their illustrations and shared reflections in group settings. This had a positive impact on cognitive processing speed, a critical function in individuals with MCI.
Comparatively, interventions that address multiple dimensions of human functioning—such as probiotics, yoga, or expressive arts—tend to yield more consistent and sustained outcomes. In contrast, unidimensional approaches, such as isolated vitamin D supplementation, while safe and well-tolerated, show limitations in terms of effectiveness. Likewise, interventions that are applied with greater frequency and regularity—such as daily yoga, intensive acupuncture, or continuous probiotic intake—appear to be associated with more pronounced cognitive benefits.
As shown in
Table 10, six studies were identified that evaluated the use of pharmacological interventions in individuals with MCI. Most of these studies investigated compounds with neuroprotective or antidepressant properties, aiming to exert a favourable impact on cognitive function. The pharmacological agents examined included nicotinamide riboside (
Orr et al., 2024), vortioxetine (
Tan & Tan, 2021), choline alfoscerate (
Jeon et al., 2024), ladostigil (
Schneider et al., 2019), cholinesterase inhibitors, and, in combination, donepezil with antidepressants such as citalopram and venlafaxine (
Devanand et al., 2018).
Of these studies, only two reported clinically meaningful effects in terms of cognitive improvement or deceleration of decline (
Jeon et al., 2024;
Tan & Tan, 2021). In the first, vortioxetine was considered a promising therapeutic option; however, the authors emphasised the need to replicate the findings through randomised clinical trials, given that the absence of a comparative group limited the strength of the conclusions. The second study, focused on choline alfoscerate, demonstrated significant improvements in specific domains such as language and memory, supporting its potential use in cases of amnestic MCI.
In contrast, the clinical trial involving ladostigil (
Schneider et al., 2019) showed a statistically significant reduction in global brain volume loss (
p = 0.025) and hippocampal atrophy (
p = 0.043); however, it failed to demonstrate clinically relevant benefits in terms of progression to dementia, based on post-treatment neuropsychological assessments. Similarly, the study involving nicotinamide riboside (
Orr et al., 2024) did not report improvements in global cognitive function when compared to the placebo group.
The study that examined the combination of donepezil with citalopram and venlafaxine (
Devanand et al., 2018) in participants with MCI and depressive symptoms not only showed limited clinical efficacy but also reported the highest number of adverse effects among the studies reviewed. The most frequently reported symptoms included fatigue, insomnia, headache, and dizziness, raising concerns about the safety of this combined approach in this vulnerable population.
On the other hand, the intervention that integrated cholinesterase inhibitors with a structured resistance exercise programme (
Hassan et al., 2021) yielded positive results compared to the control group. However, the observed benefits were attributed primarily to the physical component of the intervention, as details regarding the specific pharmacological agent used were not reported.
Regarding the dosages administered, two studies showed variability. The nicotinamide riboside protocol (
Orr et al., 2024) employed a progressive titration strategy, starting with 250 mg/day and reaching 1 g/day from the fourth week onwards. Likewise, the dosage of donepezil (
Devanand et al., 2018) was adjusted according to each participant’s individual tolerance, highlighting the importance of personalised dosing strategies in the pharmacological management of MCI. In terms of safety, most studies did not report serious adverse events. The exception was the trial by Devanand et al. (
Devanand et al., 2018), in which a significant burden of side effects was documented.