Technology-Supported Behavior Change—Applying Design Thinking to mHealth Application Development
Abstract
1. Introduction
1.1. Technology-Supported Health-Related Interventions
1.2. The Challenge of Dropouts
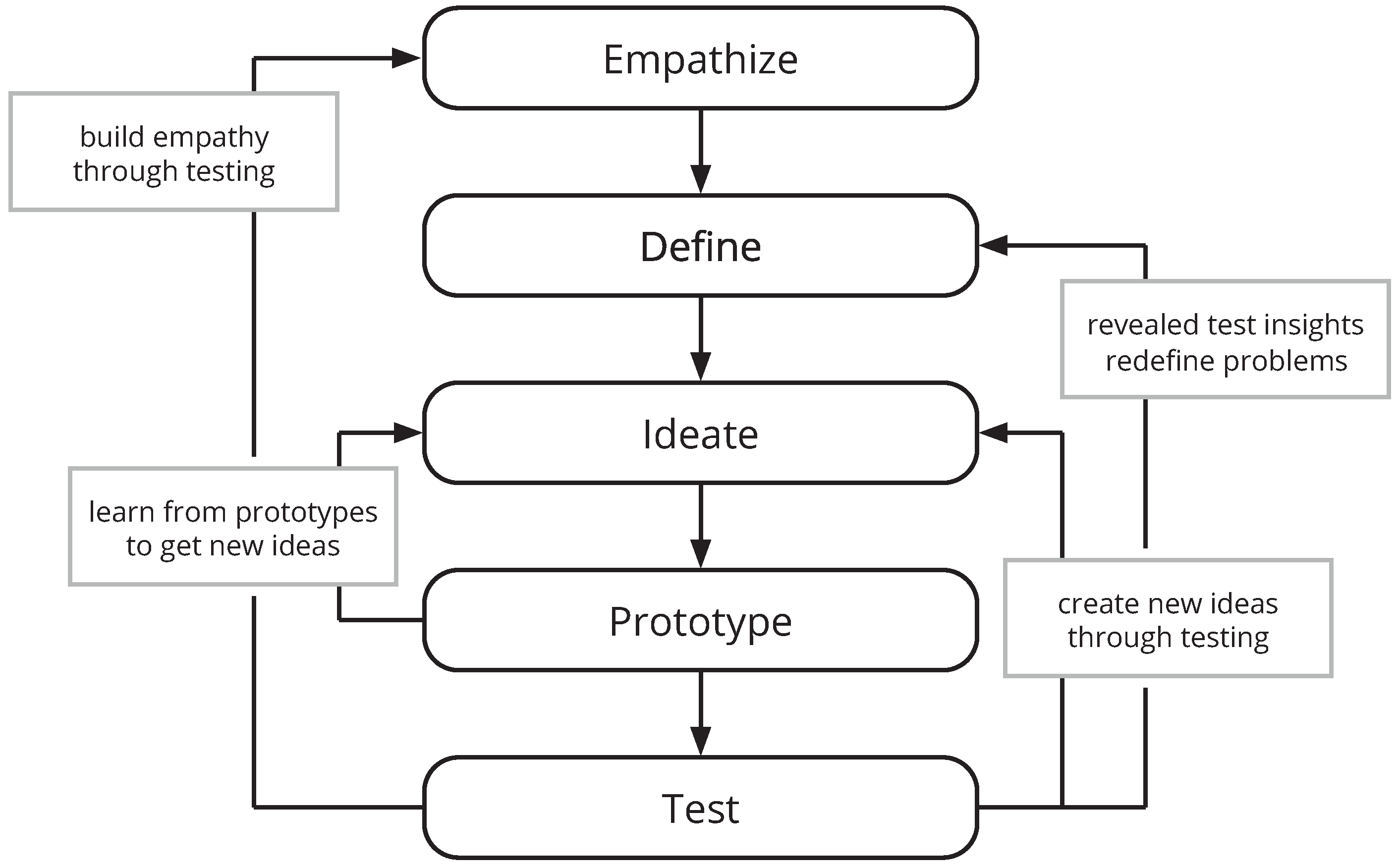
1.3. Building User-Centered mHealth Applications
1.4. Research Goal and Questions
- What are the distinct needs and challenges of key users of this mHealth application (DT modes: Empathize and Define)?
- How can these needs be transferred to and implemented by an mHealth application (DT modes: Ideate and Prototype)?
- Which usability problems can be identified by a first low-fidelity prototype (DT mode: Test)?
- What can be learned form this first iteration that should be considered in follow-up design cycles (evaluation of the overall process)?
2. Methodology
2.1. Analysis Phase
2.2. Conception Phase
2.3. Development Phase
2.4. Evaluation Phase
2.4.1. Participants
2.4.2. Procedures
2.4.3. Think-Aloud
2.4.4. Task Completion
2.4.5. Post-Test Interview
3. Results
3.1. Conception: Embedding a Theory of Change into mHealth
3.1.1. The Health Belief Model
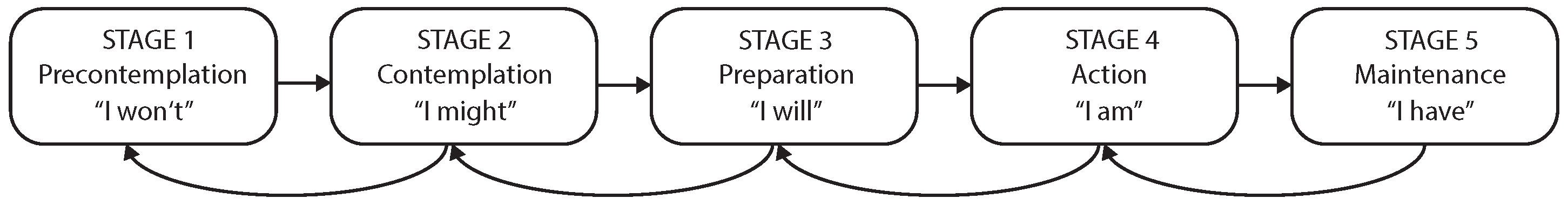
3.1.2. The Transtheoretical Model of Change
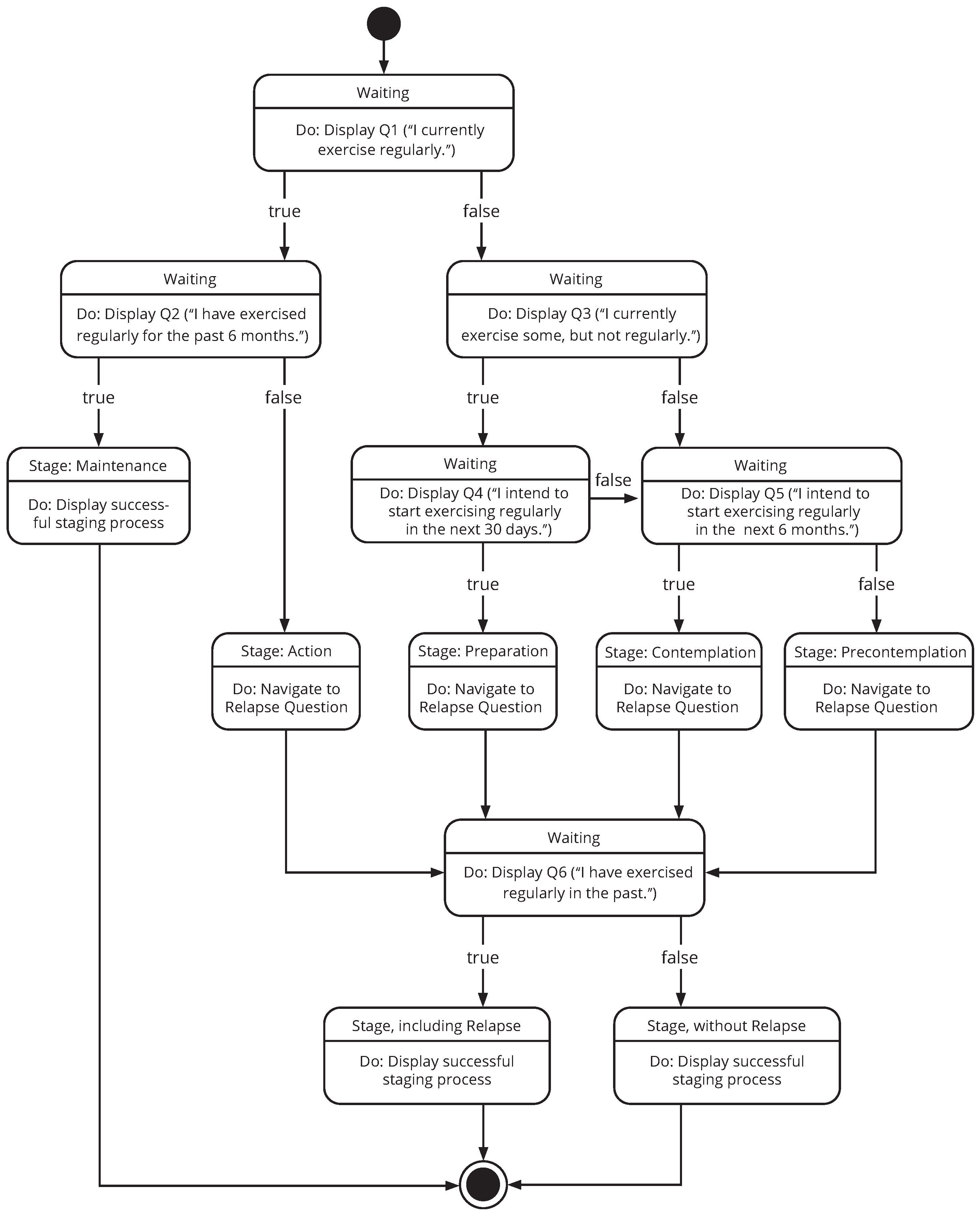
3.1.3. A TTM-Based Staging Algorithm for mHealth
3.2. Development: Implementing an Initial Prototype
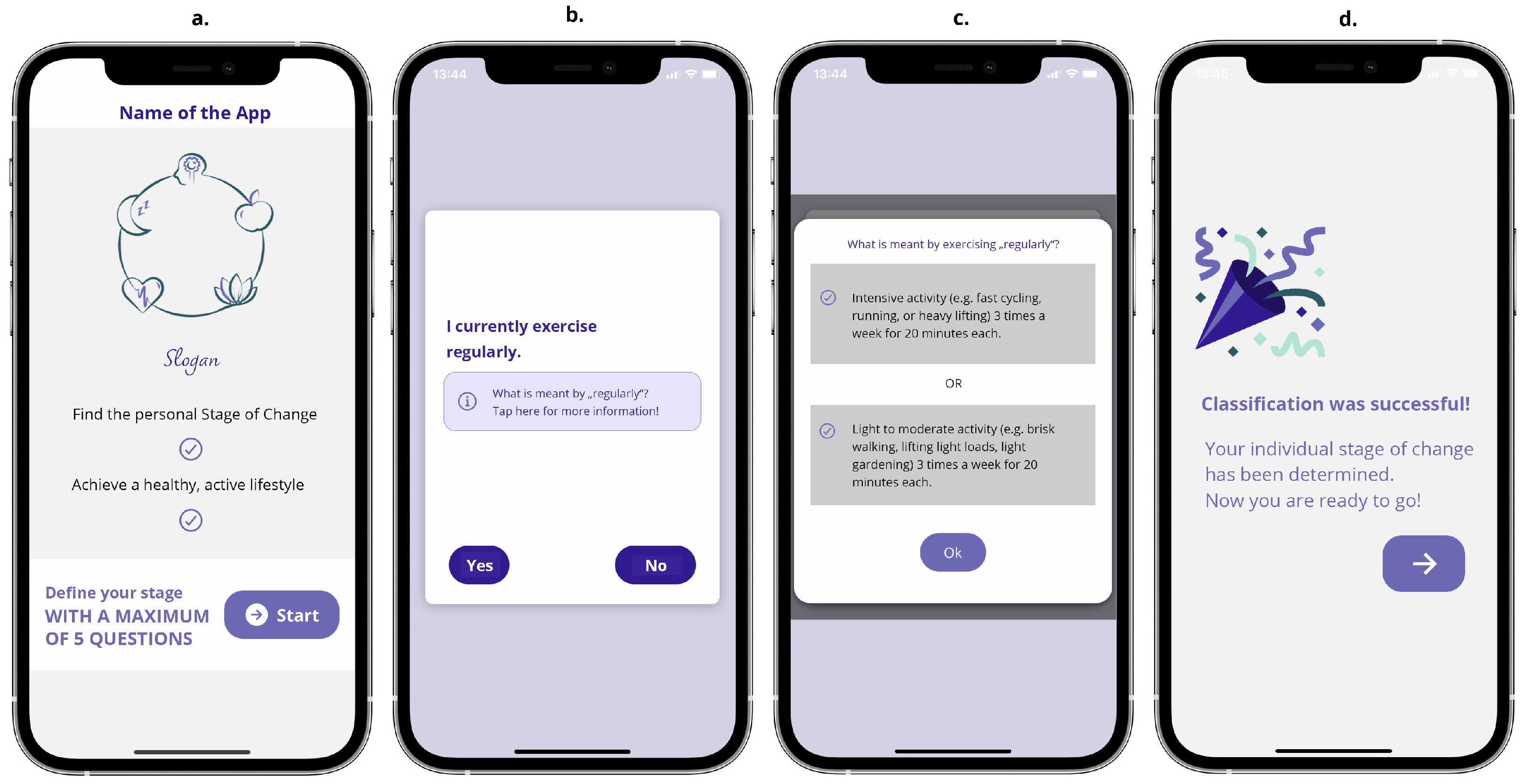
3.2.1. The Staging Process Screen Unit
3.2.2. The Stages Screen Unit
3.2.3. The Content Screen Unit
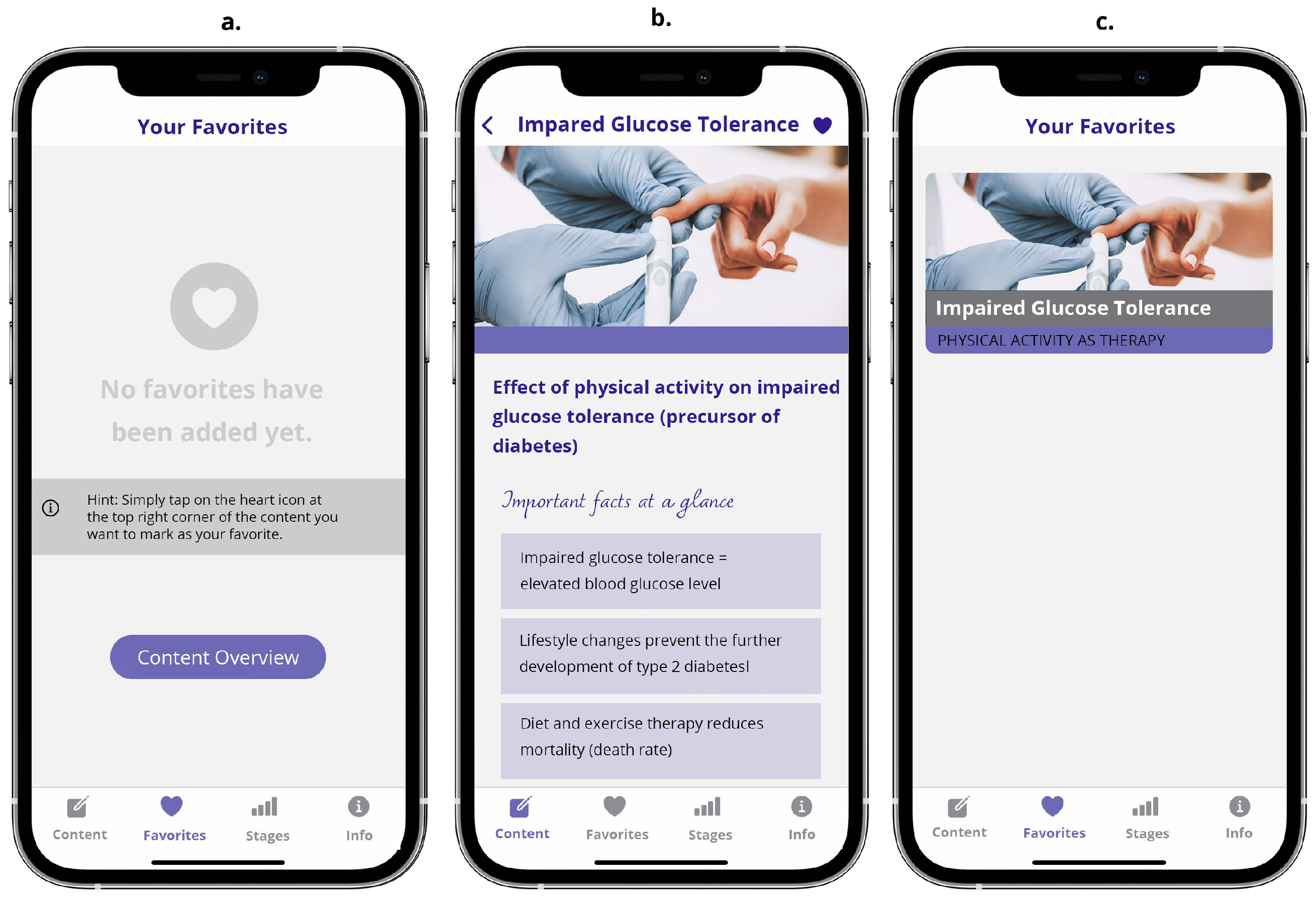
3.2.4. The Favorites Screen Unit
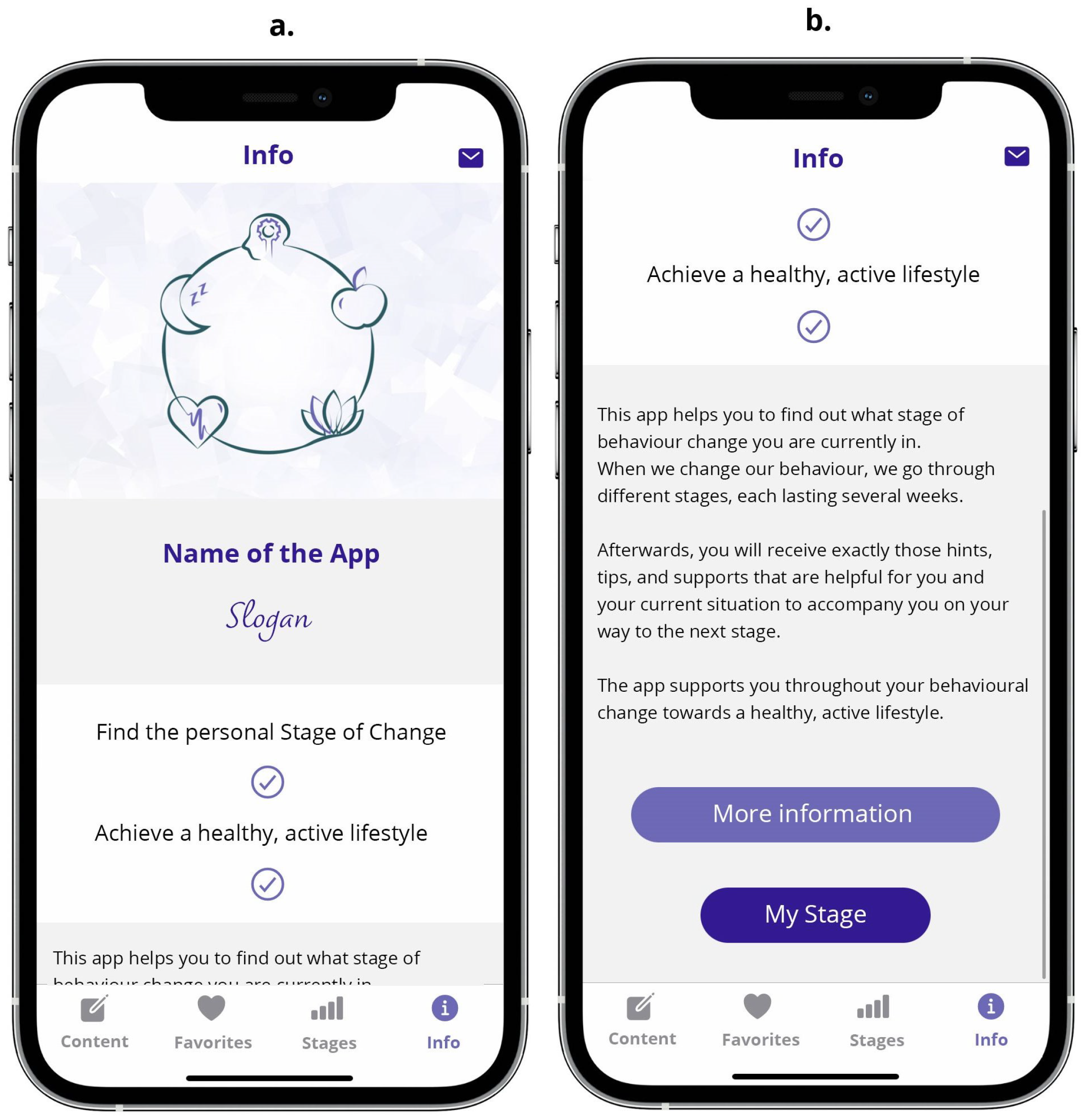
3.2.5. The Info Screen Unit
3.3. Evaluation: Collecting Feedback from Six Potential mHealth Users
3.3.1. Satisfaction
The staging process was great. It was easy - not time-consuming. I was surprised how fast I accessed the app content. That was pleasant, because I don’t like to answer too many questions.[P4]
[After the staging process] Wow, I am already done with the questions. Now I am ready to go. (…) It is easy to understand.[P1]
The image is very nice [Icon after staging process]. I think that’s good. People like that too, the feeling that you’ve achieved something.[P5]
I like that I have an immediate overview and that I can still filter and see exactly what I am interested in.[P2]
I believe that it helps people when they are given something to take with them [application] that helps them along the way.[P6]
There is one comfort thing - Dark Mode. (…) But I wouldn’t force it, but leave it open to chose. Some people prefer the light version.[P3]
I’m just missing a user profile yet. I always want to see everything at a glance.[P4]
3.3.2. Learnability
I think the menu at the bottom is comfortable. I find it convenient. You can see it right away.[P4]
It’s actually really easy to use. It is very self-explanatory.[P2]
Really simple, even for me - because I’m actually not an app person at all.[P1]
I would have to take a little more time - I have already noticed that. (…) Yes, a tutorial certainly adds to the simplicity.[P6]
3.3.3. Error Potential
Even if you tap somewhere else, it does not matter - you just go back.[P2]
If someone does not want to do a new staging process, but has already tapped the button, there is a hint [Alert] - I think that’s good.[P5]
3.3.4. Cognitive Load
I find it very advantageous that there is not too much input at once. Everything is clearly arranged on one page and it’s easy to find everything.[P1]
I like that there are only four items here [main menu / bottom tab navigator]. Then it is not so cluttered. Also the icons are well done - you recognize right away what it is about.[P5]
You just have to try it out a bit. (…) So I think it’s applicable - even for people who don’t have that much experience with mobile phones.[P6]
With the favorites, for example, it’s also good that it’s a heart - then it’s like people have it in their minds.[P5]
3.3.5. Effectiveness
4. Discussion
4.1. The Importance of Theory
4.2. The Importance of Processes
4.3. The Importance of Users
4.4. The Importance of Interdisciplinary Teams
5. Conclusions, Limitations, and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DT | Design Thinking |
| HBM | Health Belief Model |
| NCD | Non-communicable diseases |
| TTM | Transtheoretical Model of Change |
| UCD | user-centered design |
| WHO | World Health Organization |
References
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 2016, 354, i3857. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1· 9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; Van Mechelen, W.; Pratt, M.; for the Lancet Physical Activity Series 2 Executive Committee. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Fogg, B.J. Persuasive technology: Using computers to change what we think and do. Ubiquity 2002, 2002, 89–120. [Google Scholar] [CrossRef]
- Schoeppe, S.; Alley, S.; Van Lippevelde, W.; Bray, N.A.; Williams, S.L.; Duncan, M.J.; Vandelanotte, C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Dennison, L.; Morrison, L.; Conway, G.; Yardley, L. Opportunities and challenges for smartphone applications in supporting health behavior change: Qualitative study. J. Med. Internet Res. 2013, 15, e2583. [Google Scholar] [CrossRef] [PubMed]
- Oinas-Kukkonen, H. A foundation for the study of behavior change support systems. Pers. Ubiquitous Comput. 2013, 17, 1223–1235. [Google Scholar] [CrossRef]
- Guertler, D.; Vandelanotte, C.; Kirwan, M.; Duncan, M.J. Engagement and nonusage attrition with a free physical activity promotion program: The case of 10,000 steps Australia. J. Med. Internet Res. 2015, 17, e4339. [Google Scholar] [CrossRef]
- World Health Organization. Global Observatory for eHealth Series, Vol. 3: MHealth: New Horizons for Health through Mobile Technologies; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Milne-Ives, M.; Lam, C.; De Cock, C.; Van Velthoven, M.H.; Meinert, E. Mobile apps for health behavior change in physical activity, diet, drug and alcohol use, and mental health: Systematic review. JMIR mHealth uHealth 2020, 8, e17046. [Google Scholar] [CrossRef]
- Miller, G. The smartphone psychology manifesto. Perspect. Psychol. Sci. 2012, 7, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Riley, W.T.; Rivera, D.E.; Atienza, A.A.; Nilsen, W.; Allison, S.M.; Mermelstein, R. Health behavior models in the age of mobile interventions: Are our theories up to the task? Transl. Behav. Med. 2011, 1, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Direito, A.; Carraça, E.; Rawstorn, J.; Whittaker, R.; Maddison, R. mHealth technologies to influence physical activity and sedentary behaviors: Behavior change techniques, systematic review and meta-analysis of randomized controlled trials. Ann. Behav. Med. 2017, 51, 226–239. [Google Scholar] [PubMed]
- Glanz, K.; Bishop, D.B. The role of behavioral science theory in development and implementation of public health interventions. Annu. Rev. Public Health 2010, 31, 399–418. [Google Scholar] [CrossRef]
- Laranjo, L.; Ding, D.; Heleno, B.; Kocaballi, B.; Quiroz, J.C.; Tong, H.L.; Chahwan, B.; Neves, A.L.; Gabarron, E.; Dao, K.P.; et al. Do smartphone applications and activity trackers increase physical activity in adults? Systematic review, meta-analysis and metaregression. Br. J. Sport. Med. 2021, 55, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Spring, B.J.; Riper, H.; Morrison, L.G.; Crane, D.H.; Curtis, K.; Merchant, G.C.; Naughton, F.; Blandford, A. Understanding and promoting effective engagement with digital behavior change interventions. Am. J. Prev. Med. 2016, 51, 833–842. [Google Scholar] [CrossRef]
- Odendaal, W.A.; Watkins, J.A.; Leon, N.; Goudge, J.; Griffiths, F.; Tomlinson, M.; Daniels, K. Health workers’ perceptions and experiences of using mHealth technologies to deliver primary healthcare services: A qualitative evidence synthesis. Cochrane Database Syst. Rev. 2020, 3, CD011942. [Google Scholar] [CrossRef]
- Maramba, I.; Chatterjee, A.; Newman, C. Methods of usability testing in the development of eHealth applications: A scoping review. Int. J. Med. Inform. 2019, 126, 95–104. [Google Scholar] [CrossRef]
- Pohl, M. mHealth APP Economics 2017/2018: Current Status and Future Trends in Mobile Health; Research2Guidance: Berlin, Germany, 2017. [Google Scholar]
- Gilliland, J.; Sadler, R.; Clark, A.; O’Connor, C.; Milczarek, M.; Doherty, S. Using a smartphone application to promote healthy dietary behaviours and local food consumption. BioMed Res. Int. 2015, 2015, 841368. [Google Scholar] [CrossRef]
- Yerrakalva, D.; Yerrakalva, D.; Hajna, S.; Griffin, S. Effects of mobile health app interventions on sedentary time, physical activity, and fitness in older adults: Systematic review and meta-analysis. J. Med. Internet Res. 2019, 21, e14343. [Google Scholar] [CrossRef]
- Greaves, C.; Sheppard, K.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P.; The IMAGE Study Group. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011, 11, 119–130. [Google Scholar] [CrossRef]
- Lyon, A.R.; Koerner, K. User-centered design for psychosocial intervention development and implementation. Clin. Psychol. Sci. Pract. 2016, 23, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Or, C.K.; Karsh, B.T. A systematic review of patient acceptance of consumer health information technology. J. Am. Med. Inform. Assoc. 2009, 16, 550–560. [Google Scholar] [CrossRef]
- Courage, C.; Baxter, K. Understanding Your Users: A Practical Guide to User Requirements Methods, Tools, and Techniques; Gulf Professional Publishing: Houston, TX, USA, 2005. [Google Scholar]
- Norman, D.A.; Draper, S.W. User Centered System Design: New Perspectives on Human-Computer Interaction; New York L. Erlbaum Associates Inc.: Mahwah, NJ, USA, 1986. [Google Scholar]
- ISO 9141-210; Ergonomics of Human-System Interaction—Part 210: Human-Centred Design for Interactive Systems. ISO: Geneva, Switzerland, 2010.
- Searl, M.M.; Borgi, L.; Chemali, Z. It is time to talk about people: A human-centered healthcare system. Health Res. Policy Syst. 2010, 8, 35. [Google Scholar] [CrossRef]
- Andersen, T.O.; Bansler, J.P.; Kensing, F.; Moll, J.; Mønsted, T.; Nielsen, K.D.; Nielsen, O.W.; Petersen, H.H.; Svendsen, J.H. Aligning concerns in telecare: Three concepts to guide the design of patient-centred E-health. Comput. Support. Coop. Work (CSCW) 2019, 28, 1039–1072. [Google Scholar] [CrossRef]
- Altman, M.; Huang, T.T.; Breland, J.Y. Peer reviewed: Design thinking in health care. Prev. Chronic Dis. 2018, 15, E117. [Google Scholar] [CrossRef]
- Doorley, S.; Holcomb, S.; Kliebahn, P.; Segovia, K.; Utley, J. Design Thinking Bootleg. 2018. Available online: https://dschool.stanford.edu/resources/design-thinking-bootleg (accessed on 23 February 2024).
- Roberts, J.P.; Fisher, T.R.; Trowbridge, M.J.; Bent, C. A design thinking framework for healthcare management and innovation. Healthcare 2016, 4, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Gordon, V.S.; Bieman, J.M. Rapid prototyping: Lessons learned. IEEE Softw. 1995, 12, 85–95. [Google Scholar] [CrossRef]
- Higgins, J.P. Smartphone applications for patients’ health and fitness. Am. J. Med. 2016, 129, 11–19. [Google Scholar] [CrossRef]
- Sefelin, R.; Tscheligi, M.; Giller, V. Paper prototyping-what is it good for? A comparison of paper-and computer-based low-fidelity prototyping. In Proceedings of the CHI’03 Extended Abstracts on Human Factors in Computing Systems, Fort Lauderdale, FL, USA, 5–10 April 2003; pp. 778–779. [Google Scholar]
- Hanington, B.; Martin, B. Universal Methods of Design Expanded and Revised: 125 Ways to Research Complex Problems, Develop Innovative Ideas, and Design Effective Solutions; Rockport Publishers: Gloucester, MA, USA, 2019. [Google Scholar]
- Design Council. What Is the Framework for Innovation? Design Council’s Evolved Double Diamond. [Internet]; Design Council: London, UK, 2015. [Google Scholar]
- Norman, D. The Design of Everyday Things: Revised and Expanded Edition; Basic Books: New York, NY, USA, 2013. [Google Scholar]
- Brown, T.; Wyatt, J. Design thinking for social innovation. Stanf. Soc. Innov. Rev. 2010, 12, 29–43. [Google Scholar] [CrossRef]
- Giacomin, J. What is human centred design? Des. J. 2014, 17, 606–623. [Google Scholar] [CrossRef]
- Dam, R.F.; Siang, T. Stages in the Design Thinking Process; Interaction Design Foundation: Aarhus, Denmark, 2016; Available online: https://www.interaction-design.org/literature/topics/design-thinking (accessed on 23 February 2024).
- Thoring, K.; Müller, R.M. Understanding the creative mechanisms of design thinking: An evolutionary approach. In Proceedings of the Second Conference on Creativity and Innovation in Design, Eindhoven, The Netherlands, 19–21 October 2011; pp. 137–147. [Google Scholar]
- Pagliari, C. Design and evaluation in eHealth: Challenges and implications for an interdisciplinary field. J. Med. Internet Res. 2007, 9, e614. [Google Scholar] [CrossRef]
- Andrianda, J.M.; Dhewanto, W. Research, Development and Design for Product Development SAC: Interaction Design Mobile Web Application Using User Centered Design Methodology. Int. J. Curr. Sci. Res. Rev. 2023, 6. [Google Scholar] [CrossRef]
- Witteman, H.O.; Dansokho, S.C.; Colquhoun, H.; Coulter, A.; Dugas, M.; Fagerlin, A.; Giguere, A.; Glouberman, S.; Haslett, L.; Hoffman, A.; et al. User-centered design and the development of patient decision aids: Protocol for a systematic review. Syst. Rev. 2015, 4, 11. [Google Scholar] [CrossRef]
- Kushniruk, A. Evaluation in the design of health information systems: Application of approaches emerging from usability engineering. Comput. Biol. Med. 2002, 32, 141–149. [Google Scholar] [CrossRef]
- Degerli, M. Design Considerations of Mobile Applications for Healthy Living. In mHealth and Human-Centered Design towards Enhanced Health, Care, and Well-Being; Scataglini, S., Imbesi, S., Marques, G., Eds.; Springer Nature: Singapore, 2023; pp. 101–117. [Google Scholar] [CrossRef]
- Barnum, C. The ‘magic number 5’: Is it enough for web-testing? Inf. Des. J. 2002, 11, 160–170. [Google Scholar] [CrossRef]
- Nielsen, J. How many test users in a usability study. Nielsen Norman Group 2012, 4. Available online: https://www.nngroup.com/articles/how-many-test-users/ (accessed on 23 February 2024).
- Alwashmi, M.F.; Hawboldt, J.; Davis, E.; Fetters, M.D. The iterative convergent design for mobile health usability testing: Mixed methods approach. JMIR mHealth uHealth 2019, 7, e11656. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Magnusson, S.L.; Fortney, J.C.; Sayre, G.G.; Whooley, M.A. VA FitHeart, a mobile app for cardiac rehabilitation: Usability study. JMIR Hum. Factors 2018, 5, e8017. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.; Lewis, J.; Nicholls, C.M.; Ormston, R. (Eds.) Qualitative Research Practice: A Guide for Social Science Students and Researchers; Sage: New York, NY, USA, 2013. [Google Scholar]
- Glanz, K.; Rimer, B.K.; Viswanath, K. Health Behavior and Health Education: Theory, Research, and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Painter, J.E.; Borba, C.P.; Hynes, M.; Mays, D.; Glanz, K. The use of theory in health behavior research from 2000 to 2005: A systematic review. Ann. Behav. Med. 2008, 35, 358–362. [Google Scholar] [CrossRef]
- Champion, V.L.; Skinner, C.S. The health belief model. Health Behav. Health Educ. Theory, Res. Pract. 2008, 4, 45–65. [Google Scholar]
- Prochoska, J.; Velicer, W. The transtheoritical model of health behaviour change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Rosenstock, I.M. Historical origins of the health belief model. Health Educ. Monogr. 1974, 2, 328–335. [Google Scholar] [CrossRef]
- Marshall, S.J.; Biddle, S.J. The transtheoretical model of behavior change: A meta-analysis of applications to physical activity and exercise. Ann. Behav. Med. 2001, 23, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.; DiClemente, C.; Norcross, J. In search of how people change: Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef]
- Prochaska, J.O.; DiClemente, C.C. Stages and processes of self-change of smoking: Toward an integrative model of change. J. Consult. Clin. Psychol. 1983, 51, 390. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M.E.P. Learned Helplessness. Annu. Rev. Med. 1972, 23, 407–412. [Google Scholar] [CrossRef]
- Simons-Morton, B.; McLeroy, K.; Wendel, M. Behavior Theory in Health Promotion Practice and Research; Jones & Bartlett Learning: Burlington, MA, USA, 2012. [Google Scholar]
- Nakabayashi, J.; Melo, G.R.I.; Toral, N. Transtheoretical model-based nutritional interventions in adolescents: A systematic review. BMC Public Health 2020, 20, 1543. [Google Scholar] [CrossRef] [PubMed]
- Balmford, J.; Borland, R.; Burney, S. Is contemplation a separate stage of change to precontemplation? Int. J. Behav. Med. 2008, 15, 141–148. [Google Scholar] [CrossRef]
- Petrocelli, J.V. Processes and stages of change: Counseling with the transtheoretical model of change. J. Couns. Dev. 2002, 80, 22–30. [Google Scholar] [CrossRef]
- Bouton, M. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2000, 19, 57–63. [Google Scholar] [CrossRef]
- Reed, G.R.; Velicer, W.F.; Prochaska, J.O.; Rossi, J.S.; Marcus, B.H. What makes a good staging algorithm: Examples from regular exercise. Am. J. Health Promot. 1997, 12, 57–66. [Google Scholar] [CrossRef]
- Spencer, L.; Adams, T.B.; Malone, S.; Roy, L.; Yost, E. Applying the transtheoretical model to exercise: A systematic and comprehensive review of the literature. Health Promot. Pract. 2006, 7, 428–443. [Google Scholar] [CrossRef]
- Curry, L.; Nunez-Smith, M. Mixed Methods in Health Sciences Research: A Practical Primer; Sage Publications: Washington, DC, USA, 2014; Volume 1. [Google Scholar]
- Olson, E.L.; Geir, B. Bringing the Voice of the Customer into the New Product Development Process of a High Technology Firm with the Lead User Method: A Longitudinal Study of Intentions versus Actions. J. Prod. Innov. Manag. 2001, 18, 388–395. [Google Scholar] [CrossRef]
- Van Genugten, L.; Dusseldorp, E.; Webb, T.L.; Van Empelen, P. Which combinations of techniques and modes of delivery in internet-based interventions effectively change health behavior? A meta-analysis. J. Med. Internet Res. 2016, 18, e4218. [Google Scholar] [CrossRef] [PubMed]
- Cowan, L.T.; Van Wagenen, S.A.; Brown, B.A.; Hedin, R.J.; Seino-Stephan, Y.; Hall, P.C.; West, J.H. Apps of steel: Are exercise apps providing consumers with realistic expectations? A content analysis of exercise apps for presence of behavior change theory. Health Educ. Behav. 2013, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- McEwan, D.; Beauchamp, M.R.; Kouvousis, C.; Ray, C.M.; Wyrough, A.; Rhodes, R.E. Examining the active ingredients of physical activity interventions underpinned by theory versus no stated theory: A meta-analysis. Health Psychol. Rev. 2019, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- West, J.H.; Hall, P.C.; Hanson, C.L.; Barnes, M.D.; Giraud-Carrier, C.; Barrett, J. There’s an app for that: Content analysis of paid health and fitness apps. J. Med. Internet Res. 2012, 14, e1977. [Google Scholar] [CrossRef] [PubMed]
- Middelweerd, A.; Mollee, J.S.; van der Wal, C.N.; Brug, J.; Te Velde, S.J. Apps to promote physical activity among adults: A review and content analysis. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 97. [Google Scholar] [CrossRef]
- Abroms, L.C.; Padmanabhan, N.; Thaweethai, L.; Phillips, T. iPhone apps for smoking cessation: A content analysis. Am. J. Prev. Med. 2011, 40, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, W.; Kumar, S.; Shar, A.; Varoquiers, C.; Wiley, T.; Riley, W.T.; Pavel, M.; Atienza, A.A. Advancing the science of mHealth. J. Health Commun. 2012, 17, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Massa, P. Transtheoretical model for designing technologies supporting an active lifestyle. In Proceedings of the Biannual Conference of the Italian Chapter of SIGCHI, Trento, Italy, 16 September 2013; pp. 1–8. [Google Scholar]
- He, H.A.; Greenberg, S.; Huang, E.M. One size does not fit all: Applying the transtheoretical model to energy feedback technology design. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Atlanta, GA, USA, 10–15 April 2010; pp. 927–936. [Google Scholar]
- Sheeran, P. Intention–behavior relations: A conceptual and empirical review. Eur. Rev. Soc. Psychol. 2002, 12, 1–36. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.A.; Min, Y.H. Transtheoretical model-based nursing intervention on lifestyle change: A review focused on intervention delivery methods. Asian Nurs. Res. 2015, 9, 158–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change: Applications to addictive behaviors. Addict. Nurs. Netw. 1993, 5, 2–16. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Tan, H.; Huang, S. Transtheoretical model-based mobile health application for PCOS. Reprod. Health 2022, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.; Shim, J.E.; Sung, E.; Kang, J.H.; Hwang, J.Y. Development of tailored nutrition information messages based on the transtheoretical model for smartphone application of an obesity prevention and management program for elementary-school students. Nutr. Res. Pract. 2017, 11, 247–256. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, J.B.; Lee, S.; Lee, S.J.; Shin, S.E.; Park, S.H.; Park, E.J.; Kim, W.; Na, J.O.; Choi, C.U.; et al. A smartphone app (AnSim) with various types and forms of messages using the transtheoretical model for cardiac rehabilitation in patients with coronary artery disease: Development and usability study. JMIR Med. Inform. 2021, 9, e23285. [Google Scholar] [CrossRef]
- Nielsen, J. Enhancing the explanatory power of usability heuristics. In Proceedings of the SIGCHI conference on Human Factors in Computing Systems, Boston, MA, USA, 24–28 April 1994; pp. 152–158. [Google Scholar]
- Kujala, S.; Kauppinen, M. Identifying and selecting users for user-centered design. In Proceedings of the Third Nordic Conference on Human-Computer Interaction, Tampere, Finland, 23–27 October 2004; pp. 297–303. [Google Scholar]
- Cooper, A. The Inmates Are Running the Asylum; Sams Publishing: Indianapolis, IN, USA, 2004. [Google Scholar]
- Breton, E.R.; Fuemmeler, B.F.; Abroms, L.C. Weight loss—There is an app for that! But does it adhere to evidence-informed practices? Transl. Behav. Med. 2011, 1, 523–529. [Google Scholar] [CrossRef]
- Davies, C.A.; Spence, J.C.; Vandelanotte, C.; Caperchione, C.M.; Mummery, W.K. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 52. [Google Scholar] [CrossRef]
- Gordon, C.R.; Rezzadeh, K.S.; Li, A.; Vardanian, A.; Zelken, J.; Shores, J.T.; Sacks, J.M.; Segovia, A.L.; Jarrahy, R. Digital mobile technology facilitates HIPAA-sensitive perioperative messaging, improves physician-patient communication, and streamlines patient care. Patient Saf. Surg. 2015, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, D.; Marsden, N.; Kübler, P.; Leonhardt, C.; Thomanek, S.; Jung, H.; Becker, A. Specifying computer-based counseling systems in health care: A new approach to user-interface and interaction design. J. Biomed. Inform. 2009, 42, 347–355. [Google Scholar] [CrossRef] [PubMed]


| Design Principle | Questions |
|---|---|
| Satisfaction | “How did you find the integration of various functions in this app?” “How can we make it better?” “Would you need the support of a technical person to be able to use this system?” “How would you contact them: phone, email, or messaging?” |
| Learnability | “How did you learn to use the app?” “How can we reduce the time it takes to learn the app?” |
| Error Potential | “Did you have any troubles when using the app?” “Where?” “How can we fix it?” “What can we do to help users avoid the same error?” |
| Cognitive Load | “How do you feel about the complexity of the app?” “How can we simplify it?” “Do you have any recommendations to make the wording and interface easier to use?” “How did you feel about the consistency of the app?” “How can we simplify it?”’ |
| Effectiveness | Depending on the observed effectiveness of a user: “I noticed that you did not complete the task (…)” “Other users were struggling with completing the task (…)” “What can we do to enhance the completion rate?” |
| No | Age Group | Weekly App Use | Types of App Use | Health App Use | Future Health App Use |
|---|---|---|---|---|---|
| P1 | 35–44 | 4–9 h | Communication Social Media News/Information Education | No | Yes |
| P2 | 55–64 | 4–9 h | Communication Social Media | No | Yes |
| P3 | 18–24 | >40 h | Communication Social Media Games/Entertainment News/Information Education Lifestyle | Yes (Fitness apps) | Yes |
| P4 | 25–34 | 10–19 h | Communication Social Media News/Information Education Lifestyle Utility/Productivity | Yes (Gymodo) | Yes |
| P5 | 45–55 | 20–40 h | Communication Social Media Games/Entertainment News/Information Education Lifestyle Utility/Productivity | Yes (Yazio, Fitnesspoint) | Yes |
| P6 | >65 | 10–19 h | Communication Social Media News/Information Education Utility/Productivity | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweitzer, R.; Schlögl, S.; Schweitzer, M. Technology-Supported Behavior Change—Applying Design Thinking to mHealth Application Development. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 584-608. https://doi.org/10.3390/ejihpe14030039
Schweitzer R, Schlögl S, Schweitzer M. Technology-Supported Behavior Change—Applying Design Thinking to mHealth Application Development. European Journal of Investigation in Health, Psychology and Education. 2024; 14(3):584-608. https://doi.org/10.3390/ejihpe14030039
Chicago/Turabian StyleSchweitzer, Ramona, Stephan Schlögl, and Marco Schweitzer. 2024. "Technology-Supported Behavior Change—Applying Design Thinking to mHealth Application Development" European Journal of Investigation in Health, Psychology and Education 14, no. 3: 584-608. https://doi.org/10.3390/ejihpe14030039
APA StyleSchweitzer, R., Schlögl, S., & Schweitzer, M. (2024). Technology-Supported Behavior Change—Applying Design Thinking to mHealth Application Development. European Journal of Investigation in Health, Psychology and Education, 14(3), 584-608. https://doi.org/10.3390/ejihpe14030039








