Streptococcus salivarius as an Important Factor in Dental Biofilm Homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in Mixed Biofilm
Abstract
1. Introduction
2. Results
2.1. Mixed Biofilm
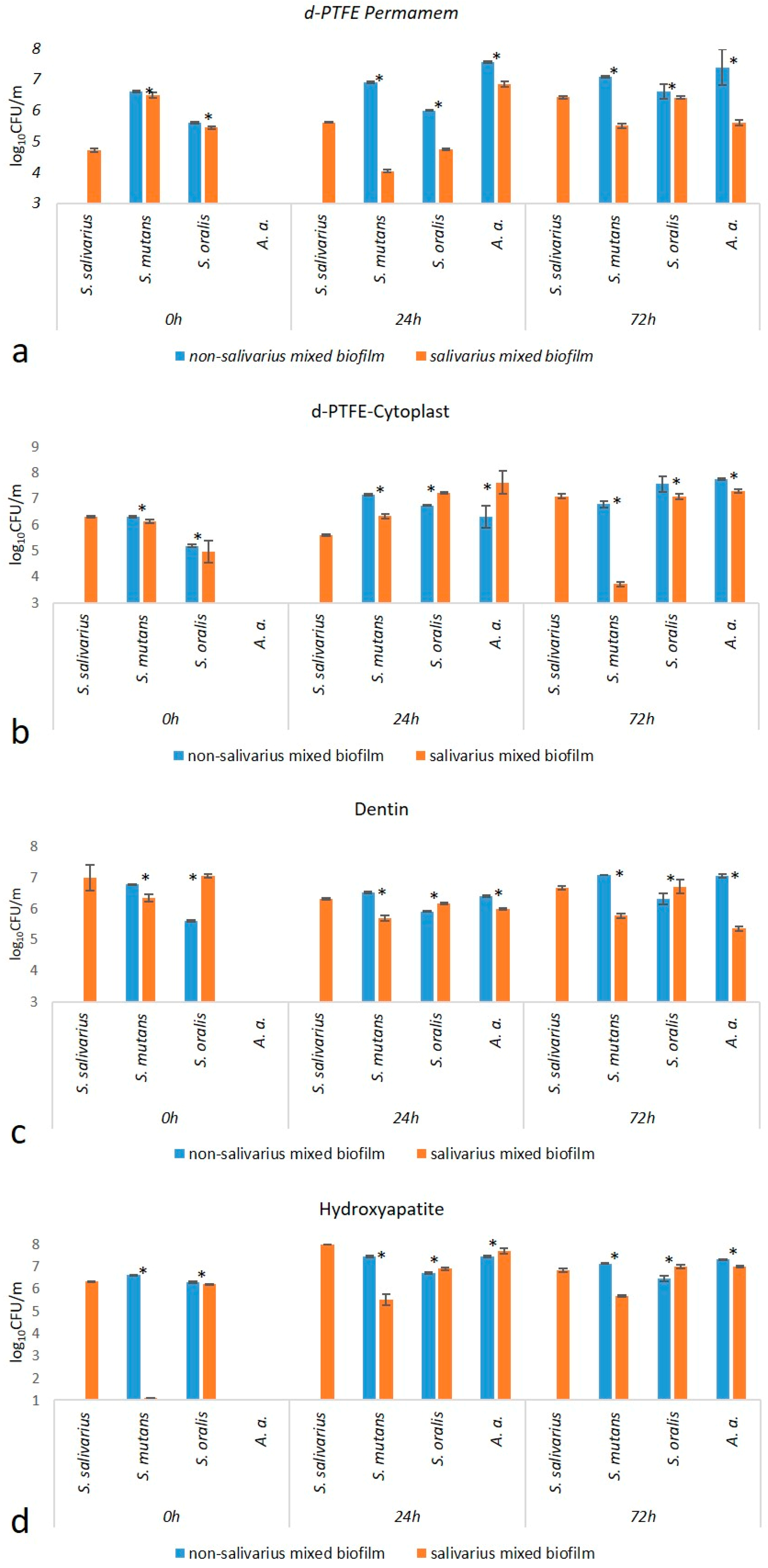
2.1.1. Total Number of Bacteria
2.1.2. Number of Individual Bacterial Species
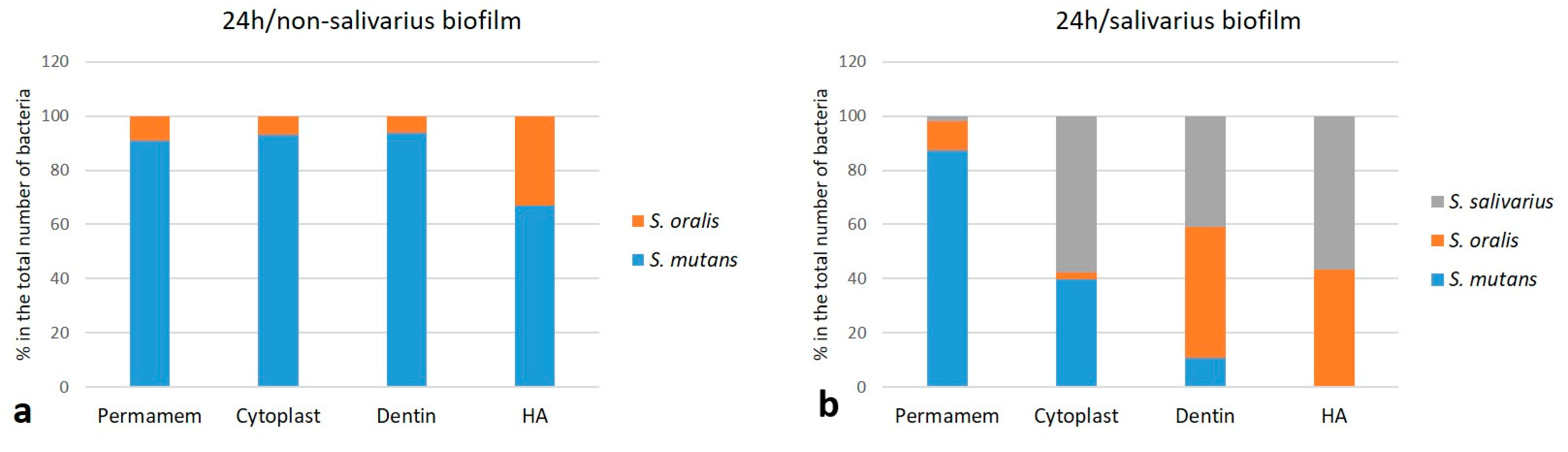
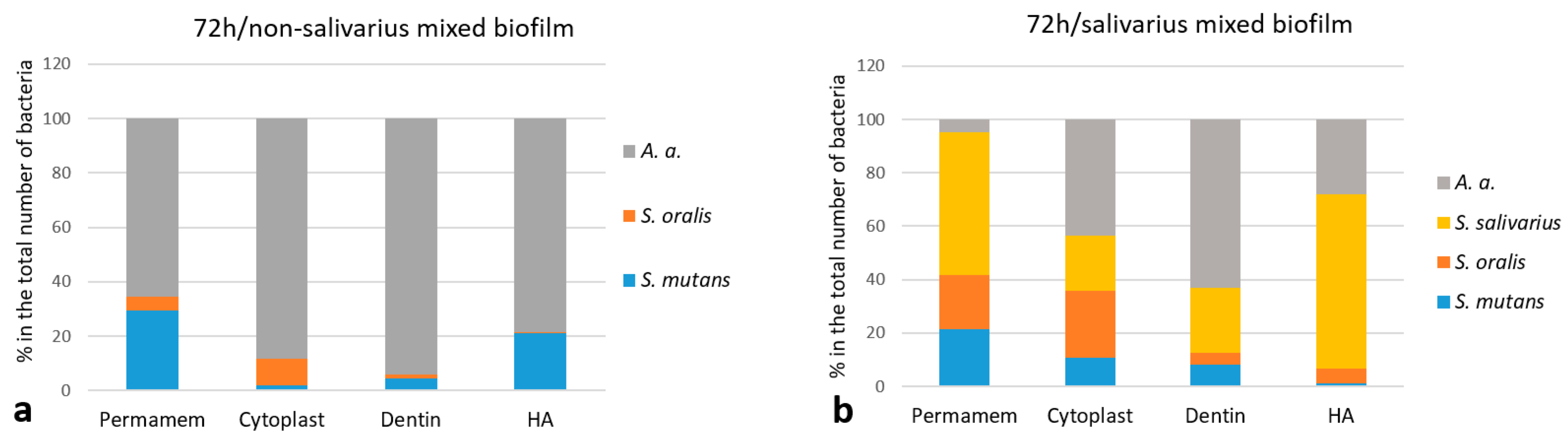
2.1.3. The Proportion of Individual Bacterial Species
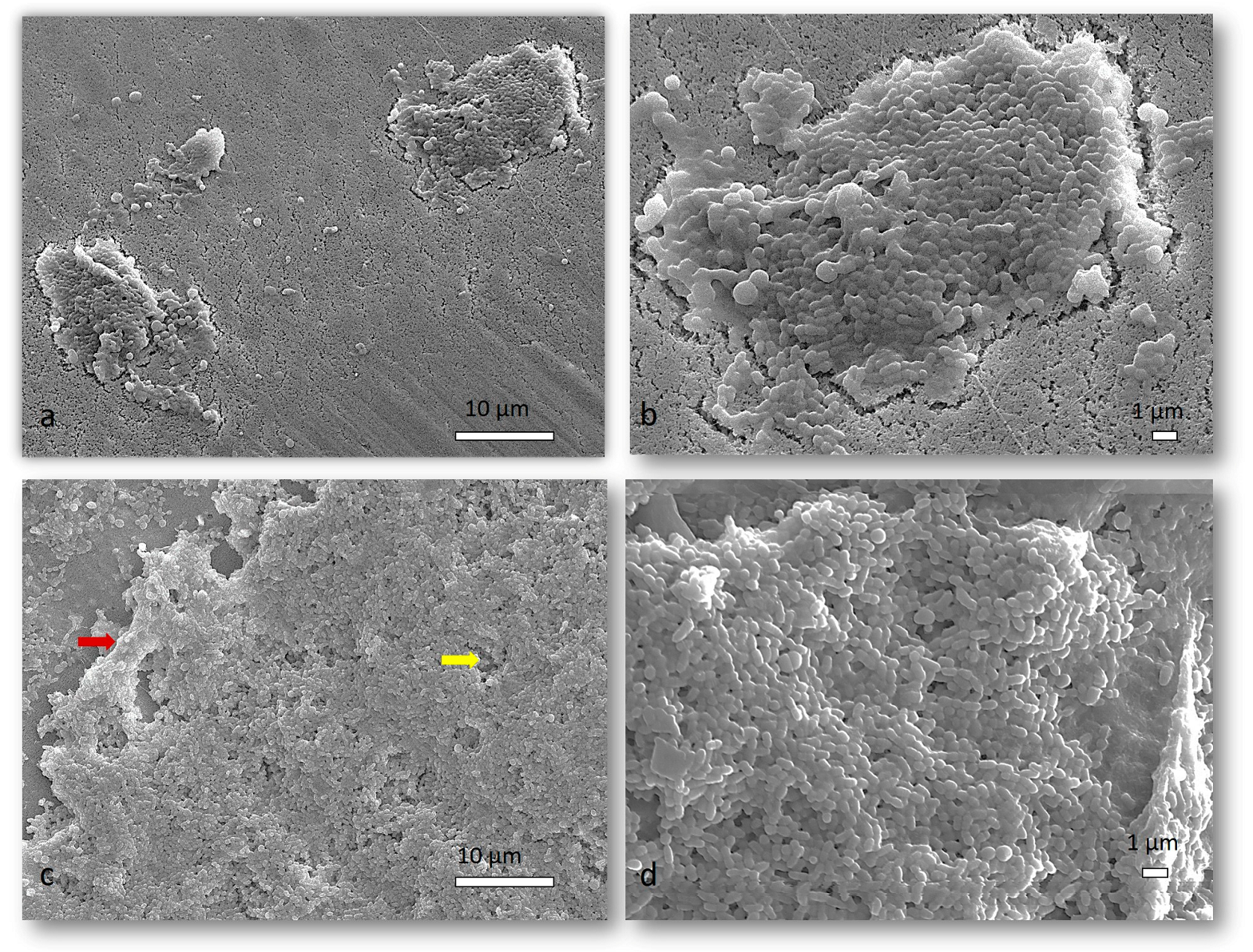
2.1.4. SEM Analysis of Mixed Biofilm
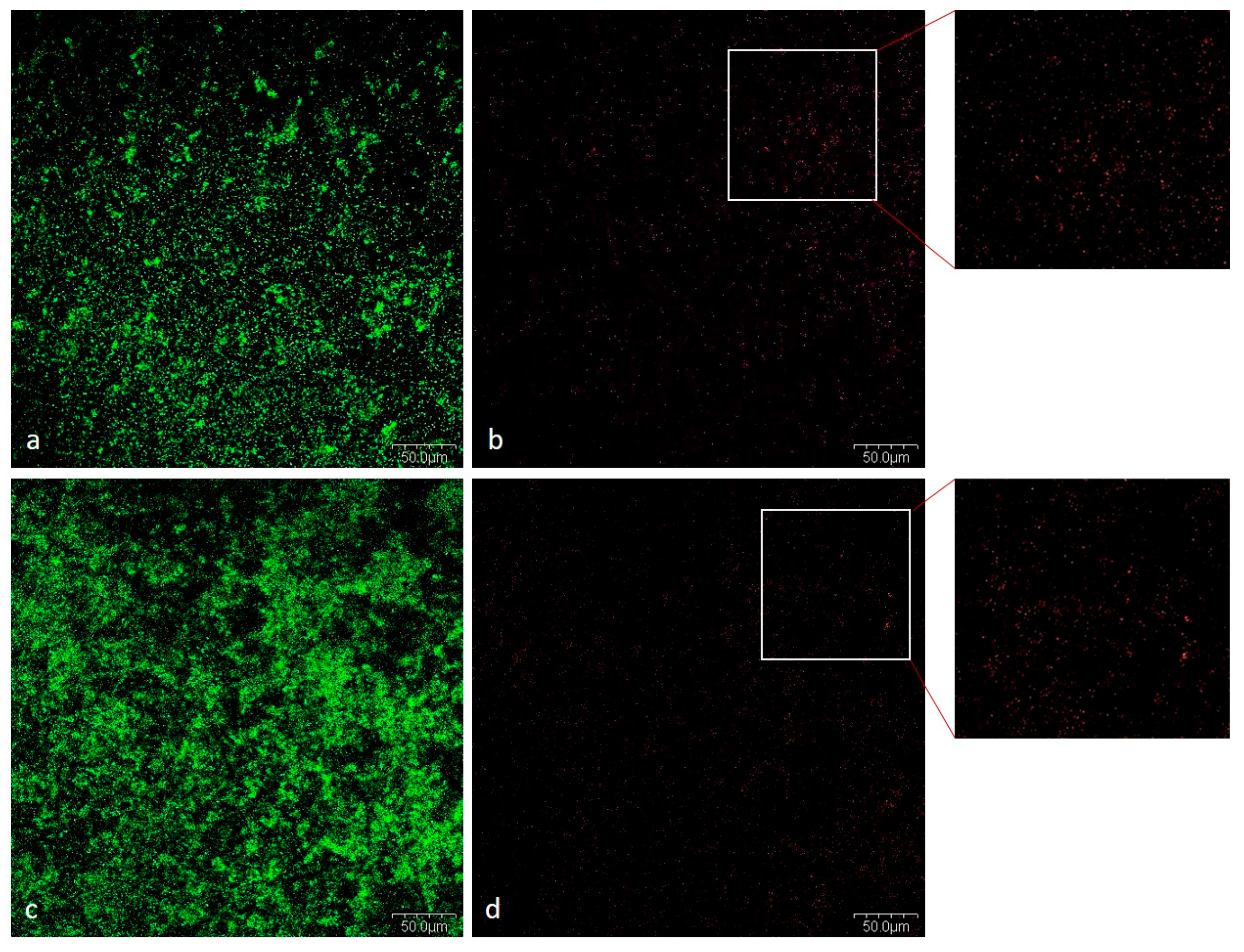
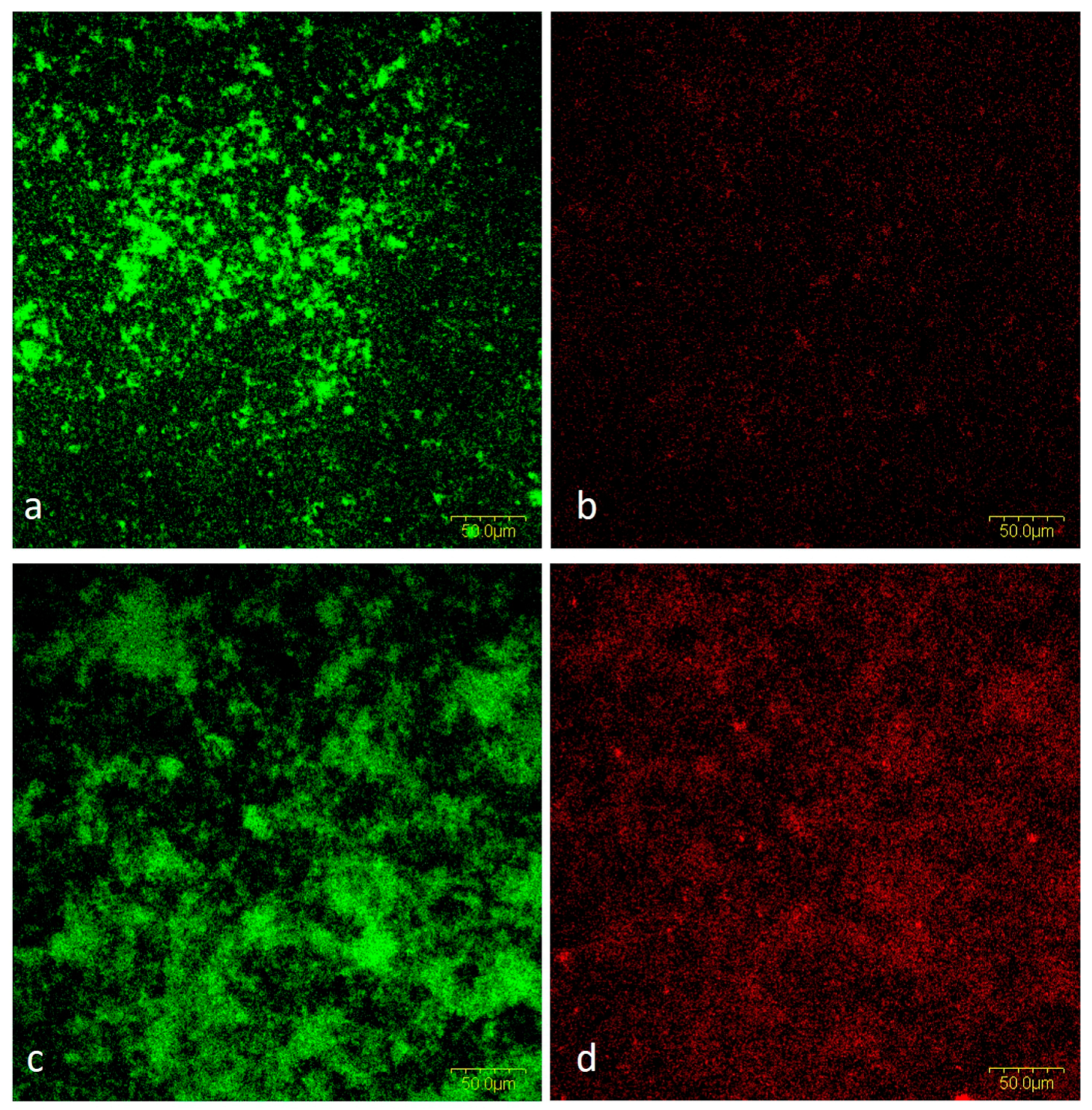
2.1.5. CLSM Analysis
Viability of Bacteria in Mixed Biofilm
Cells and Biofilm Matrix in Mixed Biofilm
3. Discussion
4. Materials and Methods
4.1. Tested Materials
4.2. Bacterial Strains and Cultivation Conditions
4.3. Biofilm Development
4.4. Biofilm Quantification
4.5. Emission Scanning Electron Microscope (SEM) Analysis
4.6. Confocal Laser Scanning Microscope (CLSM) Analysis
4.6.1. Testing the Viability of Bacteria in Biofilm
4.6.2. Simultaneous Staining of Cells and Biofilm Matrix
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Lamont, R.J. Oral Microbial Communities in Sickness and in Health. Trends Microbiol. 2005, 13, 589–595. [Google Scholar] [CrossRef]
- Belstrøm, D.; Holmstrup, P.; Bardow, A.; Kokaras, A.; Fiehn, N.-E.; Paster, B.J. Comparative Analysis of Bacterial Profiles in Unstimulated and Stimulated Saliva Samples. J. Oral Microbiol. 2016, 8, 30112. [Google Scholar] [CrossRef]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between Periodontal Infections and Systemic Disease. Clin. Microbiol. Infect. 2007, 13, 3–10. [Google Scholar] [CrossRef]
- Berbari, E.F.; Cockerill, F.R.; Steckelberg, J.M. Infective Endocarditis Due to Unusual or Fastidious Microorganisms. Mayo Clin. Proc. 1997, 72, 532–542. [Google Scholar] [CrossRef]
- Awano, S.; Ansai, T.; Takata, Y.; Soh, I.; Akifusa, S.; Hamasaki, T.; Yoshida, A.; Sonoki, K.; Fujisawa, K.; Takehara, T. Oral Health and Mortality Risk from Pneumonia in the Elderly. J. Dent. Res. 2008, 87, 334–339. [Google Scholar] [CrossRef]
- Michaud, D.S.; Liu, Y.; Meyer, M.; Giovannucci, E.; Joshipura, K. Periodontal Disease, Tooth Loss, and Cancer Risk in Male Health Professionals: A Prospective Cohort Study. Lancet Oncol. 2008, 9, 550–558. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Flemmig, T.F.; Beikler, T. Control of Oral Biofilms: Control of Oral Biofilms. Periodontol. 2000 2011, 55, 9–15. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental Plaque as a Biofilm and a Microbial Community—Implications for Health and Disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef]
- Heersema, L.A.; Smyth, H.D.C. A Multispecies Biofilm In Vitro Screening Model of Dental Caries for High-Throughput Susceptibility Testing. High-Throughput 2019, 8, 14. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Nyvad, B.; Kilian, M. Microbiology of the Early Colonization of Human Enamel and Root Surfaces In Vivo. Eur. J. Oral Sci. 1987, 95, 369–380. [Google Scholar] [CrossRef]
- Caufield, P.W.; Dasanayake, A.P.; Li, Y. The Antimicrobial Approach to Caries Management. J. Dent. Educ. 2001, 65, 1091–1095. [Google Scholar] [CrossRef]
- Mhaske, A.R.; Shetty, P.C.; Sham Bhat, N.; Ramachandra, C.S.; Laxmikanth, S.M.; Nagarahalli, K.; Tekale, P.D. Antiadherent and Antibacterial Properties of Stainless Steel and NiTi Orthodontic Wires Coated with Silver against Lactobacillus Acidophilus—An In Vitro Study. Prog. Orthod. 2015, 16, 40. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrillo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and Antimicrobial Properties of Silver Nanoparticles against Streptococcus Mutans on Brackets and Wires Used for Orthodontic Treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 68–81. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B.; Walsh, L. Beyond Streptococcus Mutans: Clinical Implications of the Evolving Dental Caries Aetiological Paradigms and Its Associated Microbiome. Br. Dent. J. 2018, 224, 219–225. [Google Scholar] [CrossRef]
- Zupancic, K.; Kriksic, V.; Kovacevic, I.; Kovacevic, D. Influence of Oral Probiotic Streptococcus Salivarius K12 on Ear and Oral Cavity Health in Humans: Systematic Review. Probiotics Antimicrob. Proteins 2017, 9, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, J.M.; Kurasz, A.B.; Clive, J. Competitive Displacement of Mutans Streptococci and Inhibition of Tooth Decay by Streptococcus Salivarius TOVE-R. Infect. Immun. 1985, 48, 44–50. [Google Scholar] [CrossRef]
- Di Pierro, F.; Colombo, M.; Zanvit, A.; Risso, P.; Rottoli, A. Use of Streptococcus Salivarius K12 in the Prevention of Streptococcal and Viral Pharyngotonsillitis in Children. Drug Healthc. Patient Saf. 2014, 6, 15–20. [Google Scholar] [CrossRef]
- Iebba, V.; Zanotta, N.; Campisciano, G.; Zerbato, V.; Di Bella, S.; Cason, C.; Luzzati, R.; Confalonieri, M.; Palamara, A.T.; Comar, M. Profiling of Oral Microbiota and Cytokines in COVID-19 Patients. Front. Microbiol. 2021, 12, 671813. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Mumtaz, S.U.; Bertuccioli, A.; Recchia, M.; Zerbinati, N.; Khan, A. Clinical Effects of Streptococcus Salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study. Microorganisms 2022, 10, 1926. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Llama-Palacios, A.; Blanc, V.; León, R.; Herrera, D.; Sanz, M. Structure, Viability and Bacterial Kinetics of an in Vitro Biofilm Model Using Six Bacteria from the Subgingival Microbiota: An in Vitro Subgingival Biofilm Model. J. Periodontal Res. 2011, 46, 252–260. [Google Scholar] [CrossRef]
- Lemos, J.A.; Burne, R.A. A Model of Efficiency: Stress Tolerance by Streptococcus Mutans. Microbiology 2008, 154, 3247–3255. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus Mutans. Microbiol. Spectr. 2019, 7, 7.1.03. [Google Scholar] [CrossRef]
- Okahashi, N.; Nakata, M.; Kuwata, H.; Kawabata, S. Streptococcus Oralis Induces Lysosomal Impairment of Macrophages via Bacterial Hydrogen Peroxide. Infect. Immun. 2016, 84, 2042–2050. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral Multispecies Biofilm Development and the Key Role of Cell–Cell Distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. The Pathogenic Persona of Community-Associated Oral Streptococci: Pathogenic Streptococcal Communities. Mol. Microbiol. 2011, 81, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Claesson, N.-L.; Jensen, B.; Åberg; Aggregatibacter, H. Actinomycetemcomitans: Clinical Significance of a Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens 2019, 8, 243. [Google Scholar] [CrossRef]
- van Essche, M.; Loozen, G.; Godts, C.; Boon, N.; Pauwels, M.; Quirynen, M.; Teughels, W. Bacterial Antagonism Against Periodontopathogens. J. Periodontol. 2013, 84, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Koll-Klais, P.; Mandar, R.; Leibur, E.; Marcotte, H.; Hammarstrom, L.; Mikelsaar, M. Oral Lactobacilli in Chronic Periodontitis and Periodontal Health: Species Composition and Antimicrobial Activity. Oral Microbiol. Immunol. 2005, 20, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verma, K.K. Skin and Soft Tissue Infection. Indian J. Pediatr. 2001, 68 (Suppl. S3), S46–S50. [Google Scholar] [PubMed]
- Di Pierro, F.; Adami, T.; Rapacioli, G.; Giardini, N.; Streitberger, C. Clinical Evaluation of the Oral Probiotic Streptococcus Salivarius K12 in the Prevention of Recurrent Pharyngitis and/or Tonsillitis Caused by Streptococcus Pyogenes in Adults. Expert Opin. Biol. Ther. 2013, 13, 339–343. [Google Scholar] [CrossRef]
- Masdea, L.; Kulik, E.M.; Hauser-Gerspach, I.; Ramseier, A.M.; Filippi, A.; Waltimo, T. Antimicrobial Activity of Streptococcus Salivarius K12 on Bacteria Involved in Oral Malodour. Arch. Oral Biol. 2012, 57, 1041–1047. [Google Scholar] [CrossRef]
- Burton, J.P.; Chilcott, C.N.; Moore, C.J.; Speiser, G.; Tagg, J.R. A Preliminary Study of the Effect of Probiotic Streptococcus Salivarius K12 on Oral Malodour Parameters. J. Appl. Microbiol. 2006, 100, 754–764. [Google Scholar] [CrossRef]
- Burton, J.; Chilcott, C.; Tagg, J. The Rationale and Potential for the Reduction of Oral Malodour Using Streptococcus Salivarius Probiotics. Oral Dis. 2005, 11, 29–31. [Google Scholar] [CrossRef]
- Jansen, P.M.; Abdelbary, M.M.H.; Conrads, G. A Concerted Probiotic Activity to Inhibit Periodontitis-Associated Bacteria. PLoS ONE 2021, 16, e0248308. [Google Scholar] [CrossRef]
- Van Hoogmoed, C.G.; Geertsema-doornbusch, G.I.; Teughels, W.; Quirynen, M.; Busscher, H.J.; Van der Mei, H.C. Reduction of Periodontal Pathogens Adhesion by Antagonistic Strains: Reduction of Periodontopathogens. Oral Microbiol. Immunol. 2007, 23, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, I.; Essche, M.V.; Loozen, G.; Eldere, J.V.; Quirynen, M.; Teughels, W. Interference with Aggregatibacter Actinomycetemcomitans: Colonization of Epithelial Cells. J. Dent. Res. 2007, 86, 611–617. [Google Scholar]
- Inhibition of Ecological Emergence of Mutans Streptococci Naturally Transmitted between Rats and Consequent Caries Inhibition by Streptococcus Salivarius TOVE-R Infection—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC262061/ (accessed on 7 February 2023).
- Lazzini, G.; Lutey, A.H.A.; Romoli, L.; Fuso, F. Molecular Dynamics Model for the Antibactericity of Textured Surfaces. Colloids Surf. B Biointerfaces 2021, 199, 111504. [Google Scholar] [CrossRef] [PubMed]
- Begić, G.; Petković Didović, M.; Lučić Blagojević, S.; Jelovica Badovinac, I.; Žigon, J.; Perčić, M.; Cvijanović Peloza, O.; Gobin, I. Adhesion of Oral Bacteria to Commercial D-PTFE Membranes: Polymer Microstructure Makes a Difference. Int. J. Mol. Sci. 2022, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Quantitatively Predicting Bacterial Adhesion Using Surface Free Energy Determined with a Spectrophotometric Method. Environ. Sci. Technol. 2015, 49, 6164–6171. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Surface Free Energy Activated High-Throughput Cell Sorting. Anal. Chem. 2014, 86, 9350–9355. [Google Scholar] [CrossRef]
- Okulus, Z.; Strzemiecka, B.; Czarnecka, B.; Buchwald, T.; Voelkel, A. Surface Energy of Bovine Dentin and Enamel by Means of Inverse Gas Chromatography. Mater. Sci. Eng. C 2015, 49, 382–389. [Google Scholar] [CrossRef]
- Lei, L.; Long, L.; Yang, X.; Qiu, Y.; Zeng, Y.; Hu, T.; Wang, S.; Li, Y. The VicRK Two-Component System Regulates Streptococcus Mutans Virulence. Curr. Issues Mol. Biol. 2019, 32, 167–200. [Google Scholar] [CrossRef]
- Steinberg, D.; Beeman, D.; Bowen, W.H. Kinetic Properties of Glucosyltransferase Adsorbed Onto Saliva-Coated Hydroxyapatite. Artif. Cells Blood Substit. Biotechnol. 1996, 24, 553–566. [Google Scholar] [CrossRef]
- Venkitaraman, A.R.; Vacca-Smith, A.M.; Kopec, L.K.; Bowen, W.H. Characterization of GlucosyltransferaseB, GtfC, and GtfD in Solution and on the Surface of Hydroxyapatite. J. Dent. Res. 1995, 74, 1695–1701. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Ansai, T.; Takehara, T.; Kuramitsu, H.K. LuxS-Based Signaling Affects Streptococcus Mutans Biofilm Formation. Appl. Environ. Microbiol. 2005, 71, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides Produced by Streptococcus Mutans Glucosyltransferases Modulate the Establishment of Microcolonies within Multispecies Biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef]
- Vaniabella, R.; Joenoes, H.; Bachtiar, B.M. Analysis on the Inhibition of Aggregatibacter Actinomycetemcomitans by Streptococcus Salivarius Isolated from Saliva and Tongue Dorsum of Adults. Asian J. Pharm. Clin. Res. 2017, 10, 6. [Google Scholar] [CrossRef]
- Staat, R.H.; Gawronski, T.H.; Cressey, D.E.; Harris, R.S.; Folke, L.E.A. Effects of Dietary Sucrose Levels on the Quantity and Microbial Composition of Human Dental Plaque. J. Dent. Res. 1975, 54, 872–880. [Google Scholar] [CrossRef]
- Brown, S.A.; Whiteley, M. A Novel Exclusion Mechanism for Carbon Resource Partitioning in Aggregatibacter Actinomycetemcomitans. J. Bacteriol. 2007, 189, 6407–6414. [Google Scholar] [CrossRef]
- Sissons, C.H.; Hancock, E.M. Urease Activity in Streptococcus Salivarius at Low PH. Arch. Oral Biol. 1993, 38, 507–516. [Google Scholar] [CrossRef]
- Burne, R.A.; Marquis, R.E. Alkali Production by Oral Bacteria and Protection against Dental Caries. FEMS Microbiol. Lett. 2000, 193, 1–6. [Google Scholar] [CrossRef]
- Jakubovics, N.S. Intermicrobial Interactions as a Driver for Community Composition and Stratification of Oral Biofilms. J. Mol. Biol. 2015, 427, 3662–3675. [Google Scholar] [CrossRef]
- Wescombe, P.A.; Heng, N.C.; Burton, J.P.; Chilcott, C.N.; Tagg, J.R. Streptococcal Bacteriocins and the Case for Streptococcus Salivarius as Model Oral Probiotics. Future Microbiol. 2009, 4, 819–835. [Google Scholar] [CrossRef]
- Wescombe, P.A.; Upton, M.; Renault, P.; Wirawan, R.E.; Power, D.; Burton, J.P.; Chilcott, C.N.; Tagg, J.R. Salivaricin 9, a New Lantibiotic Produced by Streptococcus Salivarius. Microbiology 2011, 157, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Lantibiotics: Structure, Biosynthesis and Mode of Action|FEMS Microbiology Reviews|Oxford Academic. Available online: https://academic.oup.com/femsre/article/25/3/285/624045 (accessed on 28 February 2023).
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed]
- Stanojević-Nikolić, S.; Dimić, G.R.; Mojović, L.; Pejin, J.D.; Djukić-Vuković, A.; Kocić-Tanackov, S.D. Antimicrobial Activity of Lactic Acid against Pathogen and Spoilage Microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Pratten, J.; Smith, A.W.; Wilson, M. Response of Single Species Biofilms and Microcosm Dental Plaques to Pulsing with Chlorhexidine. J. Antimicrob. Chemother. 1998, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]


| 72 h | p * | ||
|---|---|---|---|
| Non-Salivarius Mixed Biofilm | Salivarius Mixed Biofilm | ||
| Median (95% CI for Median) | |||
| d-PTFE-Permamem | 6.70 (6.12–6.94) | 7.09 (6.95–7.15) | 0.005 |
| d-PTFE-Cytoplast | 6.19 (5.73–7.13) | 6.45 (6.29–6.53) | 0.27 |
| Dentin | 6.89 (6.48–8.16) | 5.75 (5.47–6.01) | <0.001 |
| Hydroxyapatite | 7.29 (5.39–7.83) | 6.39 (5.95–6.77) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begić, G.; Badovinac, I.J.; Karleuša, L.; Kralik, K.; Cvijanovic Peloza, O.; Kuiš, D.; Gobin, I. Streptococcus salivarius as an Important Factor in Dental Biofilm Homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in Mixed Biofilm. Int. J. Mol. Sci. 2023, 24, 7249. https://doi.org/10.3390/ijms24087249
Begić G, Badovinac IJ, Karleuša L, Kralik K, Cvijanovic Peloza O, Kuiš D, Gobin I. Streptococcus salivarius as an Important Factor in Dental Biofilm Homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in Mixed Biofilm. International Journal of Molecular Sciences. 2023; 24(8):7249. https://doi.org/10.3390/ijms24087249
Chicago/Turabian StyleBegić, Gabrijela, Ivana Jelovica Badovinac, Ljerka Karleuša, Kristina Kralik, Olga Cvijanovic Peloza, Davor Kuiš, and Ivana Gobin. 2023. "Streptococcus salivarius as an Important Factor in Dental Biofilm Homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in Mixed Biofilm" International Journal of Molecular Sciences 24, no. 8: 7249. https://doi.org/10.3390/ijms24087249
APA StyleBegić, G., Badovinac, I. J., Karleuša, L., Kralik, K., Cvijanovic Peloza, O., Kuiš, D., & Gobin, I. (2023). Streptococcus salivarius as an Important Factor in Dental Biofilm Homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in Mixed Biofilm. International Journal of Molecular Sciences, 24(8), 7249. https://doi.org/10.3390/ijms24087249







