Smart Determination of Gold Content in PCBs of Waste Mobile Phones by Coupling of XRF and AAS Techniques
Abstract
:1. Introduction
- (1)
- The fundamental parameter approach (FPA) and theoretical influence coefficients [33] with some variations [34,35]. This approach consists of an algorithm that solves a set of non-linear equations describing the dependence of the measured intensities and the thickness of the samples with the element concentrations that have to be determined. This method lacks accuracy for a set of samples that contain the elements in a wide range of concentrations. Moreover, a model that is suitable for soils and sediments may not be applicable for sludge and industrial waste. The concentration of one element analyzed could be affected by the presence of the other elements; as a consequence, an accurate analysis requires the identification and quantification of all the elements present in the sample, even if not of interest, to eliminate their influence. A variant of this method is the empirical influence coefficient method [36] with filtered and unfiltered spectra [37]. This method transforms the non-linear equations into a set of linear ones. As pointed out by Rousseau [34], the accuracy of the results is dependent on the nature of the sample and on the element concentration range.
- (2)
- The multivariate statistical analysis (MVA) that demonstrates the interactions among elements with statistical methods and makes the needed corrections.The present work is the first to examine waste from printed circuit boards (PCBs) by using FPXRF to measure gold. The purpose of the study is to objectively determine whether gold can be detected by FPXRF and how the measurements can be compared with those obtainable by the more accurate AAS technique, which is a laborious and expensive procedure. The goal is to preliminarily evaluate whether FPXRF is suitable for fast analysis of gold in waste in order to determine whether it is worth purchasing waste to recover the precious metal.
2. Materials and Methods
2.1. Apparatus
2.2. Calibration Curve Construction by Using Real Matrices
2.3. Evaluation of the Calibration Curve
2.4. Statistical Analysis
3. Results
3.1. Calibration Curve Construction Using Real Matrices
3.2. Evaluation of the Calibration Curve
3.3. Statistical Analysis and FPXRF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilbride, C.; Poole, J.; Hutchings, T.R. A comparison of Cu, Pb, As, Cd, Zn, Fe, Ni and Mn determined by acid extraction/ICP-OES and ex situ field portable X-ray fluorescence analyses. Environ. Pollut. 2006, 143, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kalnicky, D.J.; Singhvi, R. Field portable XRF analysis of environmental samples. J. Hazard. Mater. 2001, 83, 93–122. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; He, Y.; Jones, B.T. Recent advances in portable X-ray fluorescence spectrometry. Appl. Spectrosc. Rev. 2004, 39, 1–25. [Google Scholar] [CrossRef]
- Drake, P.L.; Lawryk, N.J.; Ashley, K.; Sussell, A.L.; Hazelwood, K.J.; Song, R. Evaluation of two portable lead-monitoring methods at mining sites. J. Hazard. Mater. 2003, 102, 29–38. [Google Scholar] [CrossRef]
- Hall, G.E.M.; Bonham-Carter, G.F.; Buchar, A. Evaluation of portable X-ray fluorescence (pXRF) in exploration and mining. Geochem. Explor. Environ. Anal. 2014, 14, 99–123. [Google Scholar] [CrossRef] [Green Version]
- Simandl, G.J.; Stone, R.S.; Paradis, S.; Fajber, R.; Reid, H.M.; Grattan, K. An assessment of a handheld X-ray fluorescence instrument for use in exploration and development with an emphasis on REEs and related speciality metals. Miner. Deposita 2014, 49, 999–1012. [Google Scholar] [CrossRef]
- Young, K.E.; Evans, C.A.; Hodges, K.V.; Bleacher, J.E.; Graff, T.G. A review of the handheld X-ray fluorescence spectrometer as a tool for field geologic investigations on Earth and in a planetary surface exploration. Appl Geochem. 2016, 72, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.G.; Shevais, J.W.; Li, Y.; Reagan, M.K.; Li, H.Y.; Heaton, D.; Godard, M.; Kirchenbaur, M.; Whattam, S.A.; Pearce, J.A.; et al. Application of a handheld X-ray fluorescence spectrometer for real-time, high-density quantitative analysis of drilled igneous rocks and sediments during IODP Expedition 352. Chem. Geol. 2017, 451, 55–56. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Field Portable X-Ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment; Method 6200; US EPA: Washington, DC, USA, 1998. [Google Scholar]
- US EPA. Innovative Technology Verification Report: XRF Technologies for Measuring Trace Elements in Soil and Sediment; Office of Research and Development; US EPA: Washington, DC, USA, 2006. [Google Scholar]
- US EPA. Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment; SW-846 Test Method 6200; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- Radu, T.; Diamond, D. Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. J. Hazard. Mater. 2009, 171, 1168–1171. [Google Scholar] [CrossRef]
- Parsons, C.; Grabulosa, E.M.; Pili, E.; Floor, G.H.; Roman-Ross, G.; Charlet, L. Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: Considerations for sample preparation and measurement conditions. J. Hazard. Mater. 2013, 262, 1213–1222. [Google Scholar] [CrossRef]
- Mejía-Piña, G.; Huerta-Diaz, M.A.; González-Yajimovich, O. Calibration of handheld X-ray fluorescence (XRF) equipment for optimum determination of elemental concentrations in sediment samples. Talanta 2016, 161, 359–367. [Google Scholar] [CrossRef]
- Brent, R.N.; Wines, H.; Luther, J.; Irving, N.; Collins, J.; Drake, B.L. Validation of handheld X-ray fluorescence for in situ measurement of mercury in soils. J. Environ. Chem. Eng. 2017, 5, 768–776. [Google Scholar] [CrossRef]
- Marguì, E.; Queralt, I.; Hidalgo, M. Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. Trac. Trend. Anal. Chem. 2009, 28, 362–372. [Google Scholar] [CrossRef]
- Chou, J.; Clement, G.; Bursavich, B.; Elbers, D.; Cao, B.; Zhou, W. Rapid detection of toxic metals in non-crushed oyster shells by portable X-ray fluorescence spectrometry. Environ. Pollut. 2010, 158, 2230–2234. [Google Scholar] [CrossRef]
- Weindorf, D.C.; Zhu, Y.; Chakraborty, S.; Bakr, N.; Huang, B. Use of portable X-ray fluorescence spectrometry for environmental quality assessment of peri-urban agriculture. Environ. Monit. Assess. 2012, 184, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Solman, K.R. Analysis of the elemental composition of marine litter by field-portable-XRF. Talanta 2016, 159, 262–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, A.; Brown, M.T.; Turner, A. Novel use of field-portable-XRF for the direct analysis of trace elements in marine macroalgae. Environ. Pollut. 2017, 200, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Turner, A. In situ elemental characterization of marine micro-plastics by portable XRF. Mar. Pollut. Bull. 2017, 124, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Filella, M. Field-portable-XRF reveals the ubiquity of antimony in plastic consumer products. Sci. Total Environ. 2017, 584–584, 982–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higueras, P.; Oyarzun, R.; Iraizoz, J.M.; Lorenzo, S.; Esbrí, J.M.; Martínez-Coronado, A. Low-cost geochemical surveys for environmental studies in developing countries: Testing a field portable XRF instrument under quasi-realistic conditions. J. Geochem. Explor. 2012, 113, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Rowe, H.; Hughes, N.; Robinson, K. The quantification and application of handheld energy-dispersive x-ray fluorescence (ED-XRF) in mudrock chemostratigraphy and geochemistry. Chem. Geol. 2012, 324–325, 122–131. [Google Scholar] [CrossRef]
- Cohen, D.R.; Cohen, E.J.; Graham, I.T.; Soares, G.G.; Hand, S.J.; Archer, M. Geochemical exploration for vertebrate fossils using field portable XRF. J. Geochem. Explor. 2017, 181, 1–9. [Google Scholar] [CrossRef]
- Figi, R.; Nagel, O.; Tuchschmid, M.; Lienemanna, P.; Gfeller, U.; Bukowiecki, N. Quantitative analysis of heavy metals in automotive brake linings: A comparison between wet-chemistry based analysis and in-situ screening with a handheld X-ray fluorescence spectrometer. Anal. Chim. Acta 2010, 676, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kloos, D. Underkarat jewelry: The perfect crime? Investigations and analysis of jewelry using XRF. ICCD Adv. X-Ray Anal. 2003, 46. [Google Scholar]
- Su, Q.; Yin, Y. ED-XRF determination on the content of cadmium and lead in polymeric materials. Chem. Res. Appl. 2015.
- Adami, K.Z. Variational Methods in Bayesian Deconvolution; PHYSTAT2003; SLAC: Stanford, CA, USA, 8–11 September 2003. [Google Scholar]
- Curis, E.; Osan, J.; Falkenberg, G.; Benazeth, S.; Torok, S. Simulating systematic errors in X-ray absorption spectroscopy experiments: Sample and beam effects, Spectrochim. Acta Part. B 2005, 60, 841–849. [Google Scholar] [CrossRef]
- Elam, W.T.; Scruggs, B.; Eggert, F.; Nicolosi, J.A. F-13 Advantages and Disadvantages of Bayesian Methods for Obtaining XRF Net Intensities. Powder Diffr. 2010, 25, 215. [Google Scholar] [CrossRef] [Green Version]
- Manninen, S.; Pitkanen, T.; Koikkalainen, S.; Pakkari, T. Study of the ratio of elastic to 333 inelastic scattering of photons. Int. J. Appl. Radiat. Isot. 1984, 35, 93–98. [Google Scholar] [CrossRef]
- Sherman, J. The theoretical derivation of fluorescent X-ray intensities from mixtures. Spectrochim. Acta. 1955, 7, 283–306. [Google Scholar] [CrossRef]
- Rousseau, R.M.; Boivin, J.A. The Fundamental Algorithm: A natural extension of the Sherman equation, part I: Theory. Rigaku J. 1998, 15, 13–15. [Google Scholar]
- Rousseau, R.M. The quest for a Fundamental Algorithm in X-ray fluorescence analysis and calibration. Open Spectrosc, J. 2009, 3, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Criss, J.W.; Birks, L.S. Calculation methods for fluorescent Xray spectrometry, empirical coefficients vs. fundamental parameters. Anal. Chem. 1968, 40, 1080–1087. [Google Scholar] [CrossRef]
- Conrey, R.M.; Goodman-Elgar, M.; Bettencourt, N.; Seyfarth, A.; Van Hoose, A.; Wolff, J.A. Calibration of a portable X-ray fluorescence spectrometer in the analysis of archaeological samples using influence coefficients. Geochem. Explor. Environ. A 2014, 14, 291–301. [Google Scholar] [CrossRef]
- Ravansari, R.; Wilson, S.C.; Tighe, M. Portable X-ray fluorescence for enviromental assessment of soils: Not just a point and shoot method. Environ. Int. 2020, 134, 105250. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Bando, A.; Komatani, S.; Wada, S.; Tsuji, K. Experimental parameters for XRF analysis of soils. Adv. X-ray Anal. 2010, 53, 248–255. [Google Scholar]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Measure of agreement. Perspect. Clin. Res. 2017, 8, 187–191. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Flame Atomic Absorption Spectrophotometry; Method 7000B; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- Havukainen, J.; Hiltunen, J.; Puro, L.; Horttanainen, M. Applicability of a field portable X-ray fluorescence for analyzing elemental concentration of waste samples. Waste Manag. 2019, 83, 6–13. [Google Scholar] [CrossRef]
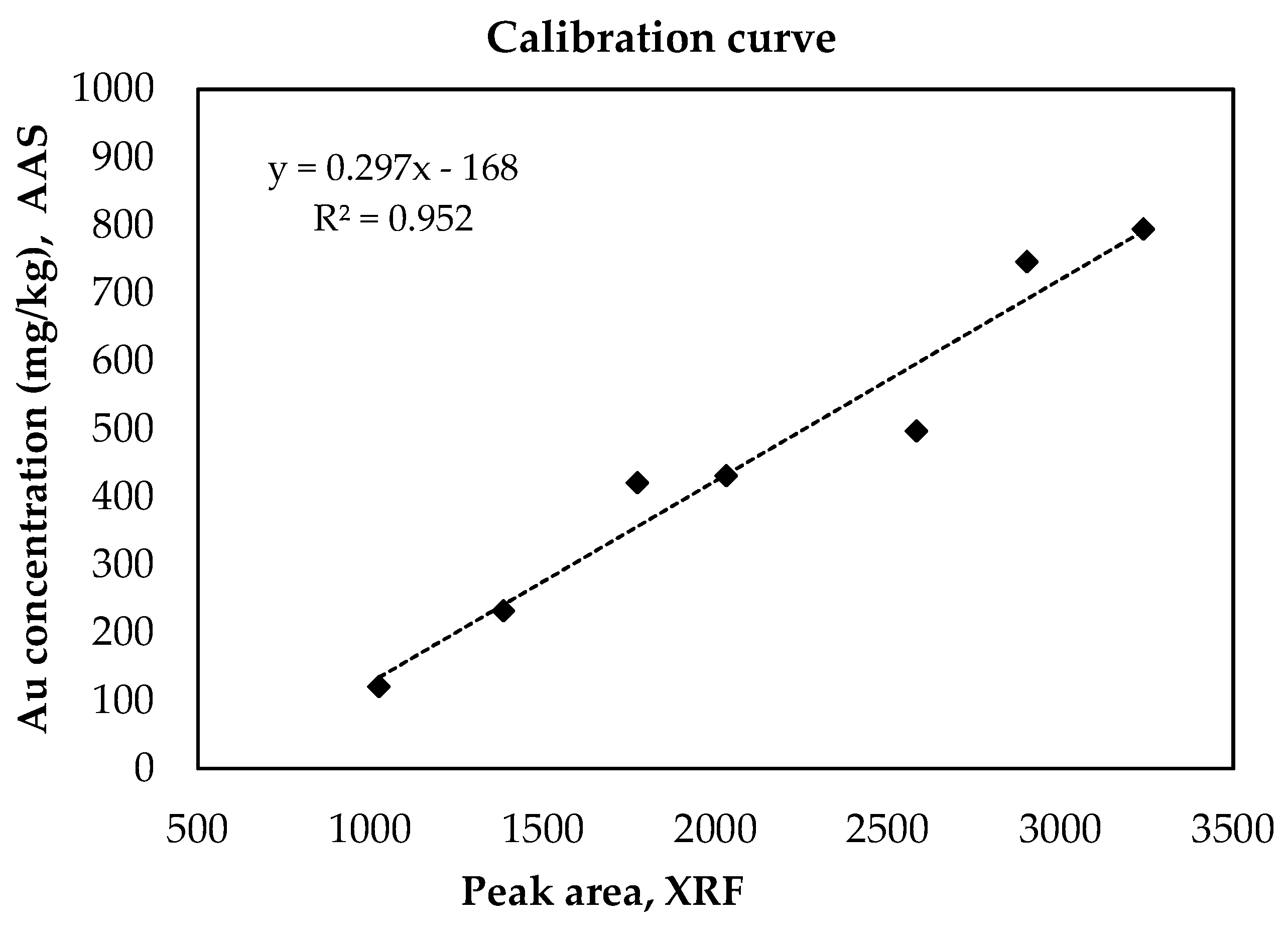

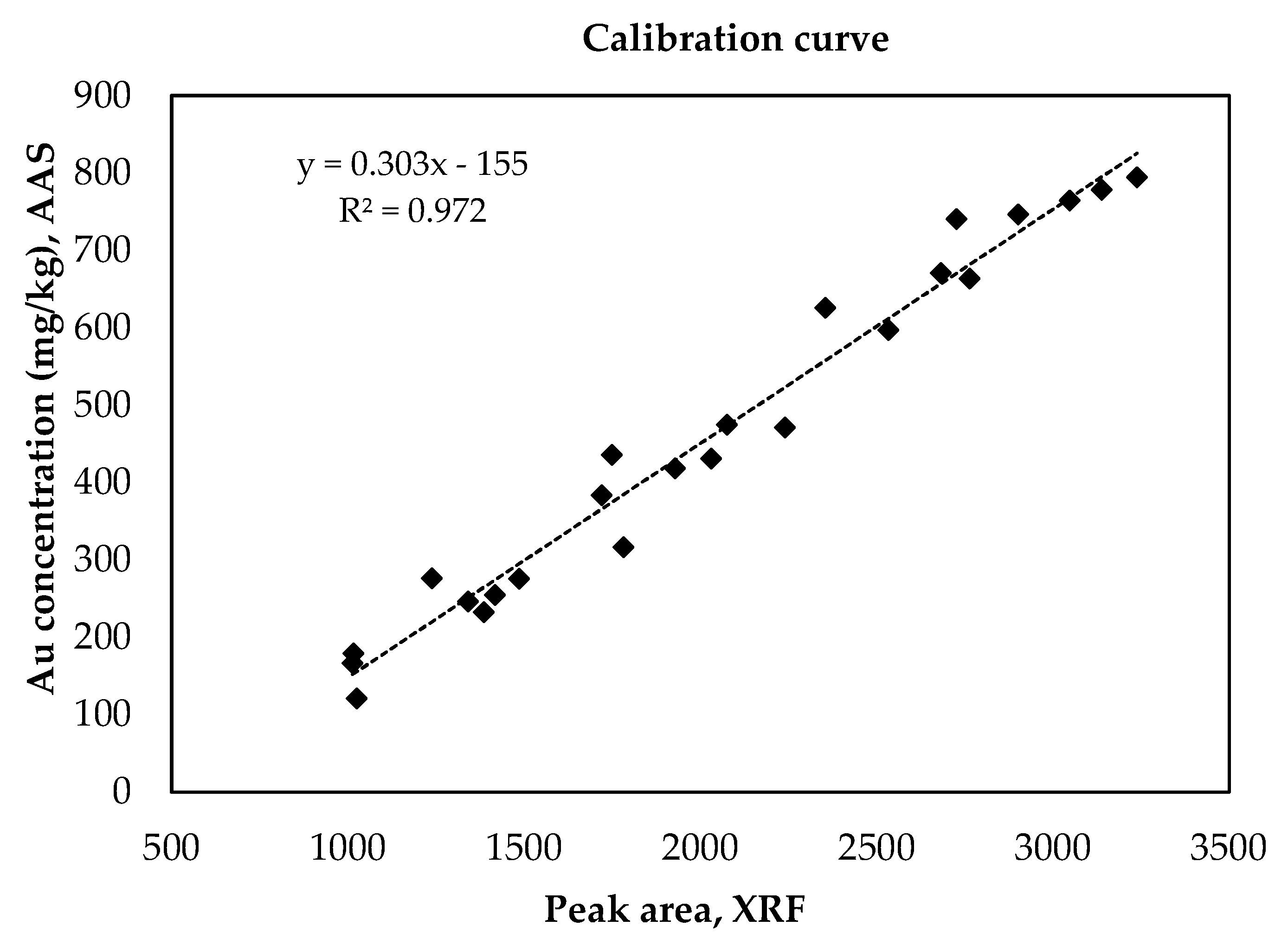


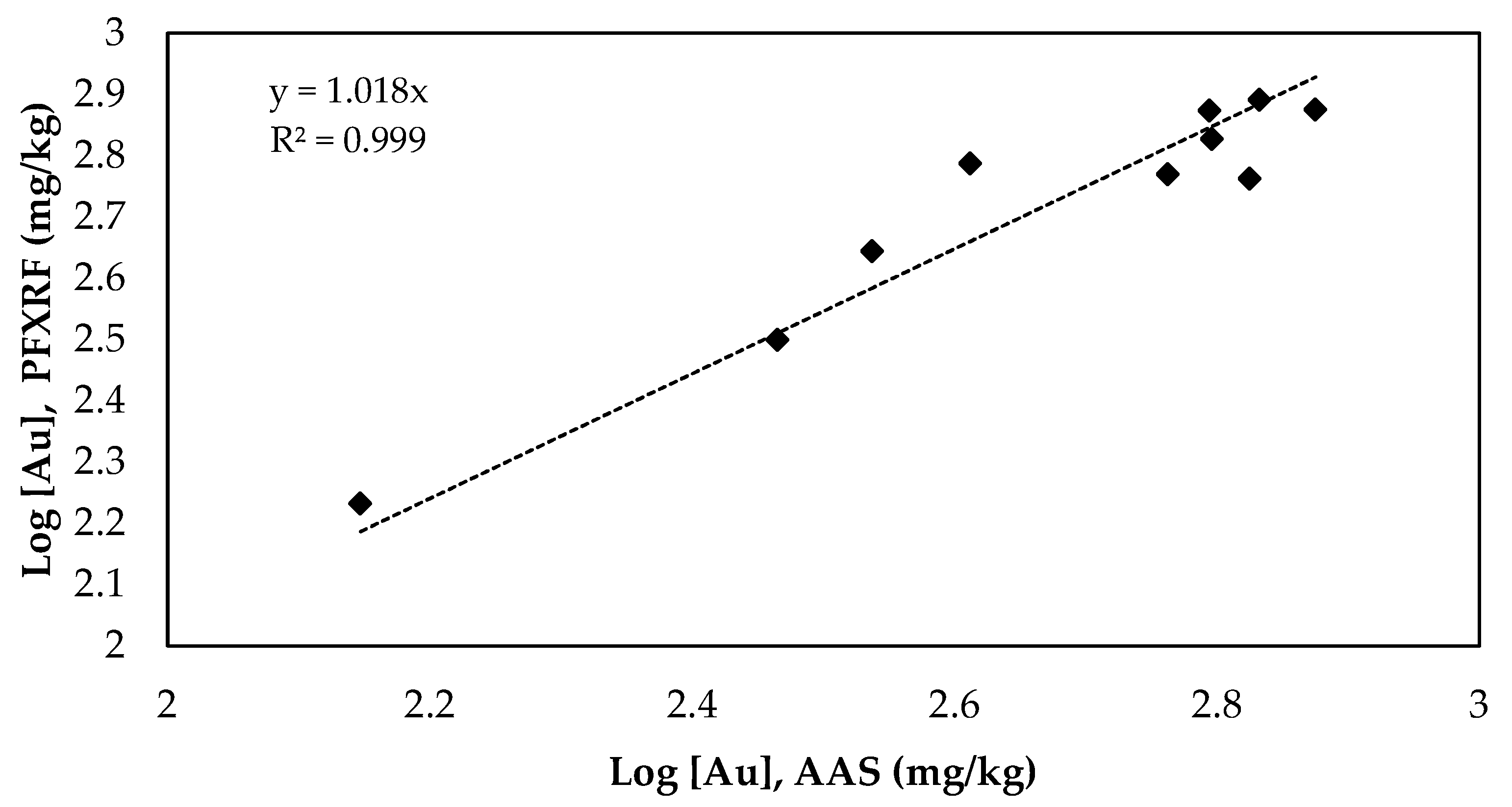

| PCB Samples | Au Peak Area—XRF Analysis | Au Concentration (mg/kg)—AAS Analysis |
|---|---|---|
| S1 | 1385.0 | 232.5 |
| S2 | 9451.4 | 999.8 |
| S3 | 2901.4 | 746.6 |
| S4 | 2581.8 | 497.0 |
| S5 | 1024.6 | 120.7 |
| S6 | 3238.8 | 794.5 |
| S7 | 1772.4 | 420.7 |
| S8 | 20,146.0 | 943.1 |
| S9 | 30,044.6 | 1000.2 |
| S10 | 2030.4 | 431.2 |
| Sample | Au Concentration—AAS (mg/kg) | Au Concentration—XRF (mg/kg) | Difference (mg/kg) | RPD (%) |
|---|---|---|---|---|
| 1 | 471.0 | 495.1 | 24.1 | 5.0 |
| 2 | 430.6 | 510.9 | 80.3 | 17.1 |
| 3 | 451.2 | 453.0 | 1.8 | 0.4 |
| 4 | 625.9 | 529.2 | −96.7 | 16.7 |
| 5 | 254.4 | 251.0 | −3.4 | 1.3 |
| 6 | 418.5 | 402.6 | −15.9 | 3.9 |
| 7 | 764.3 | 745.7 | −18.6 | 2.5 |
| 8 | 166.5 | 133.8 | −32.7 | 21.8 |
| 9 | 778.1 | 796.5 | 18.4 | 2.3 |
| 10 | 663.5 | 650.3 | −13.2 | 2.0 |
| 11 | 474.5 | 477.9 | 3.4 | 0.7 |
| 12 | 316.3 | 359.0 | 42.7 | 12.6 |
| 13 | 246.0 | 228.4 | −17.6 | 7.4 |
| average | ±28.4 | 7.2 |
| Sample | Au Concentration—AAS (mg/kg) | Au Concentration—XRF (mg/kg) | Difference (mg/kg) | RPD (%) |
|---|---|---|---|---|
| 14 | 650.4 | 487.5 | −162.9 | 28.6 |
| 15 | 597.4 | 601.0 | 3.6 | 0.6 |
| 16 | 275.6 | 278.1 | 2.5 | 0.9 |
| 17 | 383.8 | 350.2 | −33.6 | 9.2 |
| 18 | 671.0 | 646.9 | −24.1 | 3.7 |
| 19 | 179.0 | 133.2 | −45.8 | 29.3 |
| 20 | 276.2 | 201.8 | −74.4 | 31.1 |
| 21 | 435.6 | 359.3 | −76.3 | 19.2 |
| 22 | 566.5 | 380.5 | −186.0 | 39.3 |
| 23 | 740.3 | 660.8 | −79.5 | 11.3 |
| average | ± 68.9 | 17.3 |
| Sample | Au Concentration—AAS (mg/kg) | Au Concentration—XRF (mg/kg) | Difference (mg/kg) | RPD (%) |
|---|---|---|---|---|
| 24 | 578.8 | 588.4 | 9.6 | 1.6 |
| 25 | 668.4 | 579.5 | −88.9 | 14.2 |
| 26 | 625.7 | 672.5 | 46.8 | 7.2 |
| 27 | 291.8 | 316.3 | 24.5 | 8.1 |
| 28 | 680.0 | 779.0 | 99.0 | 13.6 |
| 29 | 409.3 | 612.7 | 203.4 | 39.8 |
| 30 | 750.2 | 751.5 | 1.3 | 0.2 |
| 31 | 140.3 | 170.7 | 30.4 | 19.3 |
| 32 | 622.5 | 747.7 | 125.2 | 18.3 |
| 33 | 344.5 | 441.3 | 96.8 | 24.6 |
| average | ± 72.6 | 14.7 |
| Bland–Altman Analysis (mg/kg) | |
|---|---|
| Mean of difference | 54.81 |
| SD of difference | 80.19 |
| Limits of agreement | |
| Lower | −102.38 |
| Upper | 212.00 |
| Au Concentration Range (mg/kg) AAS | Au Concentration Range (mg/kg) FPXRF | R2 | Gradient of Line | Y-Intercept | Variance | Standard Deviation | Confidential Interval | Data Quality Level |
|---|---|---|---|---|---|---|---|---|
| 140.3–750.2 | 170.7–779 | 0.999 | 1.018 | -- | 0.011 | 0.104 | Range 0.061–0.154 Average 0.080 | Definitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippolito, N.M.; Belardi, G.; Innocenzi, V.; Medici, F.; Pietrelli, L.; Piga, L. Smart Determination of Gold Content in PCBs of Waste Mobile Phones by Coupling of XRF and AAS Techniques. Processes 2021, 9, 1618. https://doi.org/10.3390/pr9091618
Ippolito NM, Belardi G, Innocenzi V, Medici F, Pietrelli L, Piga L. Smart Determination of Gold Content in PCBs of Waste Mobile Phones by Coupling of XRF and AAS Techniques. Processes. 2021; 9(9):1618. https://doi.org/10.3390/pr9091618
Chicago/Turabian StyleIppolito, Nicolò Maria, Gianmaria Belardi, Valentina Innocenzi, Franco Medici, Loris Pietrelli, and Luigi Piga. 2021. "Smart Determination of Gold Content in PCBs of Waste Mobile Phones by Coupling of XRF and AAS Techniques" Processes 9, no. 9: 1618. https://doi.org/10.3390/pr9091618
APA StyleIppolito, N. M., Belardi, G., Innocenzi, V., Medici, F., Pietrelli, L., & Piga, L. (2021). Smart Determination of Gold Content in PCBs of Waste Mobile Phones by Coupling of XRF and AAS Techniques. Processes, 9(9), 1618. https://doi.org/10.3390/pr9091618











