Synthesis of Ti Powder from the Reduction of TiCl4 with Metal Hydrides in the H2 Atmosphere: Thermodynamic and Techno-Economic Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermodynamic Calculation
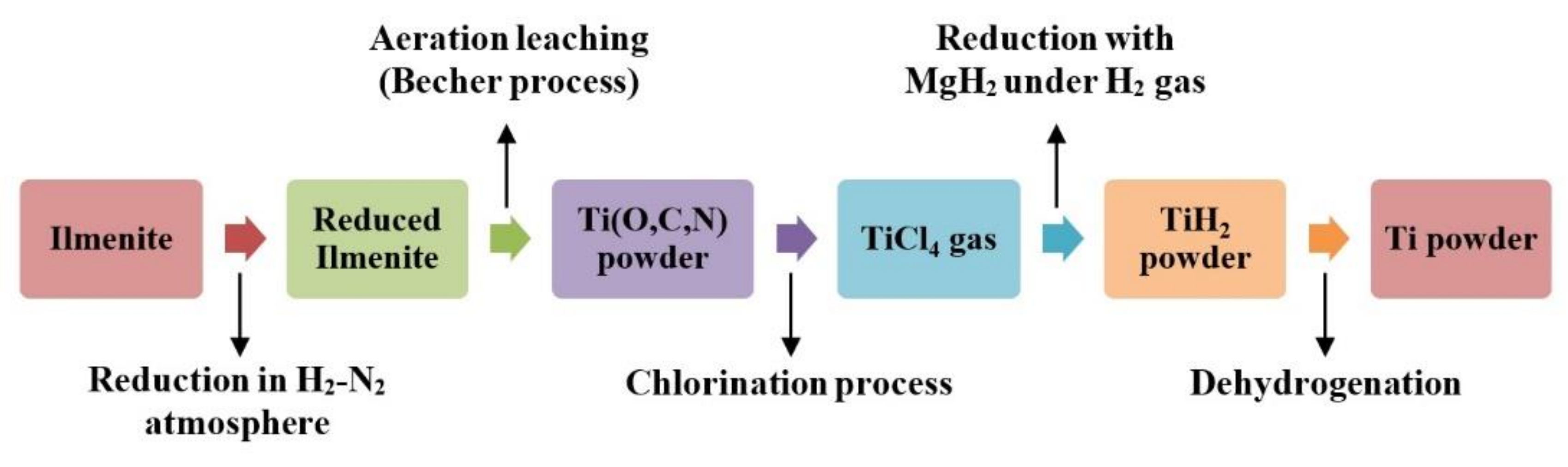
2.2. Synthesis Process
2.3. Economic Evaluation
3. Results
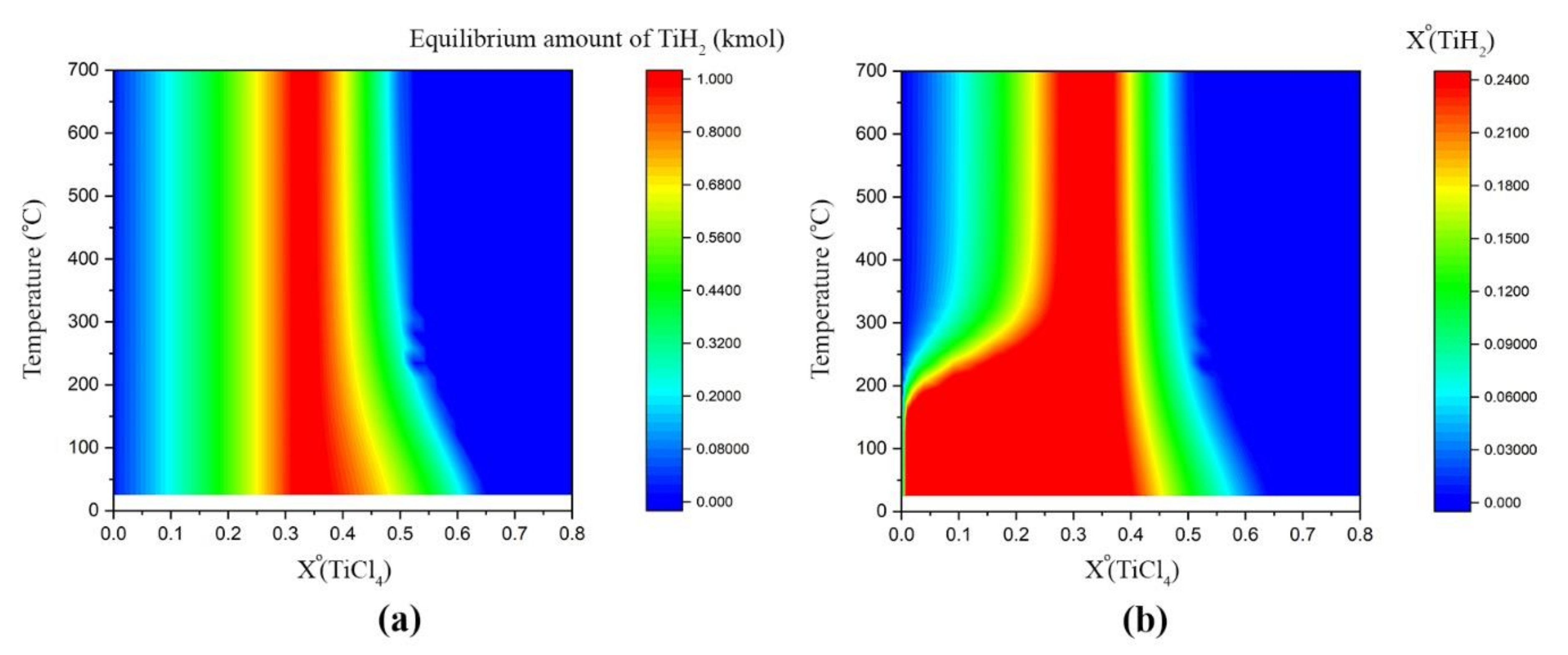
3.1. Thermodynamic Assessment
Phase Composition Diagrams
3.2. Phase Analysis
3.3. Process Simulation and Economics Evaluation
3.3.1. Raw Material and Installed Equipment Costs
3.3.2. Economic Analysis
3.3.3. Unit Production Cost of Ti Metal Powder
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Process Simulation Setting
| Procedure | Operation | Process Time a (h) | Start Time (h) | End Time (h) |
|---|---|---|---|---|
| P-1 | Grinding ilmenite below 75 µm | 1.25 | 0 | 1.25 |
| P-1/2 | Mixing ilmenite with coal | 1.25 | 1.25 | 2.50 |
| P-2 | Ilmenite reduction with H2-N2 gases mixture at 1250 °C | 12.31 | 2.50 | 14.81 |
| P-3 | TiN with Fe aeration leaching at 90 °C to remove Fe particles | 9.00 | 14.81 | 23.81 |
| P-4 | Filtration to separate TiN from Fe particles | 4.25 | 23.81 | 28.06 |
| P-4/1 | Filtration to separate Fe2O3 from NH4Cl solution | 4.25 | 23.81 | 28.06 |
| P-5 | Acid washing at room temperature to remove any excess Fe particles from TiN | 7.43 | 28.06 | 35.49 |
| P-5/1 | FeCl2 Storage for sewage treatment industries | 7.32 | 32.85 | 40.17 |
| P-6 | TiN chlorination process at 350 °C | 7.64 | 32.85 | 40.49 |
| P-7 | TiCl4 Reduction process in H2 atmosphere at 500 °C | 14.00 | 40.49 | 54.49 |
| P-8 | Water washing at room temperature to remove impurities from TiH2 | 1.25 | 54.49 | 55.74 |
| P-8/1 | MgCl2 electrolysis to produce Mg metal | 3.50 | 55.74 | 59.24 |
| P-9 | TiH2 dehydrogenation to Ti powder at 700 °C | 4.50 | 55.74 | 60.24 |
| P-10 | H2 gas Storage | 3.50 | 56.24 | 59.24 |
| Process | Description |
|---|---|
| Reduction reactor at P-2 | heating for 3 h at 1250 °C, extent of reaction 100% |
| Aeration leaching at P-3 | leaching at 90 °C for 7 h, extent of leaching 95% |
| Microfiltration at P-4 | filtration for 2 h with recovery yield of 90% |
| Microfiltration at P-4/1 | filtration for 4 h with recovery yield of 90% |
| Acid washing in P-5 | washing for 30 min and separation for 1 h with 100% recovery at room temperature |
| Chlorination reaction at P-6 | heating for 5 h at 350 °C, extent of chlorination 95% |
| Reduction reaction in P-7 | heating for 12 h at 500 °C, extent of reaction 95% |
| Acid washing and separation in P-8 | washing for 1 h with 100% recovery after filtration |
| Electrolysis in P-8/1 | reaction duration for 1 h at room temperature, extent of separation 100% |
| Dehydrogenation in P-9 | heating for 3 h at 700 °C, extent of reaction 100% |
| Name | Type | Production Scale | ||
|---|---|---|---|---|
| 1-t/Batch | 5-t/Batch | 10-t/Batch | ||
| R-101 | Stirred Reactor | 1.21 m3 | 1.52 m3 | 1.73 m3 |
| AFR-101 | Air-Lift Fermentor | 124,952.11 L | 624,760.53 L | 1,249,521.06 L |
| GR-101 | Grinder | 3519.05 kg/h | 17,595.23 kg/h | 35,190.46 kg/h |
| MF-101 | Microfilter | 2.15 m2 | 10.74 m2 | 21.48 m2 |
| MF-102 | Microfilter | 2.80 m2 | 14.00 m2 | 28.01 m2 |
| SFR-101 | Shake FlaskRack | 2224.00 L | 11,120.00 L | 22,240.00 L |
| MX-101 | Mixer | 4632.32 kg/h | 23,161.60 kg/h | 46,323.21 kg/h |
| R-102 | Stirred Reactor | 277.62 L | 1388.08 L | 2776.15 L |
| V-101 | Horizontal Tank | 1946.24 L | 9731.18 L | 19,462.35 L |
| R-103 | Stirred Reactor | 2,312.99 L | 11,564.93 L | 23,129.86 L |
| WSH-101 | SWasher (Bulk Flow) | 5149.38 kg/h | 12,873.45 kg/h | 12,873.45 kg/h |
| R-104 | Stirred Reactor | 2361.94 L | 11,809.69 L | 23,619.38 L |
| R-105 | Stirred Reactor | 308.76 L | 1543.78 L | 3087.57 L |
| V-102 | Horizontal Tank | 3214.24 L | 16,071.20 L | 32,142.41 L |
Appendix B. Process Costs
| Name | 1-t/Batch | 5-t/Batch | 10-t/Batch | |||
|---|---|---|---|---|---|---|
| Unit Cost ($) | Number | Unit Cost ($) | Number | Unit Cost ($) | Number | |
| R-101 | 649,000 | 1 | 670,000 | 4 | 682,000 | 7 |
| AFR-101 | 436,000 | 1 | 1,106,000 | 1 | 1,700,000 | 1 |
| GR-101 | 105,000 | 1 | 174,000 | 1 | 260,000 | 1 |
| MF-101 | 30,000 | 1 | 48,000 | 1 | 73,000 | 1 |
| MF-102 | 30,000 | 1 | 56,000 | 1 | 85,000 | 1 |
| SFR-101 | 0 | 1 | 0 | 1 | 0 | 1 |
| MX-101 | 0 | 1 | 0 | 1 | 0 | 1 |
| R-102 | 531,000 | 1 | 661,000 | 1 | 728,000 | 1 |
| V-101 | 29,000 | 1 | 69,000 | 1 | 101,000 | 1 |
| R-103 | 710,000 | 1 | 844,000 | 1 | 984,000 | 1 |
| WSH-101 | 0 | 1 | 0 | 2 | 0 | 4 |
| R-104 | 712,000 | 1 | 848,000 | 1 | 989,000 | 1 |
| R-105 | 538,000 | 1 | 671,000 | 1 | 738,000 | 1 |
| V-102 | 60,000 | 1 | 134,000 | 1 | 229,000 | 1 |
| Material | 1 t/Batch | 5 t/Batch | 10 t/Batch |
|---|---|---|---|
| Hydrogen (m3) | 5,103,992 | 11,397,705 | 11,624,751 |
| Nitrogen (m3) | 309,694 | 691,576 | 705,352 |
| Ammonium chloride (kg) | 7,054,073 | 15,752,423 | 16,066,216 |
| Water (kg) | 0 | 0 | 0 |
| Oxygen (kg) | 0 | 0 | 0 |
| Ilmenite (kg) | 7,910,815 | 17,665,611 | 18,017,515 |
| Hydrochloric acid (kg) | 121,323 | 270,926 | 276,323 |
| Carbon (kg) | 625,660 | 1,397,160 | 1,424,992 |
| Chlorine (kg) | 2,306,088 | 5,149,716 | 5,252,300 |
| Magnesium hydride (kg) | 98,764,347 | 220,550,276 | 224,943,708 |
| Total price ($) | 122,195,993 | 272,875,393 | 278,311,158 |
| Parameter | Unit Cost ($) | 1 t/Batch | 5 t/Batch | 10 t/Batch | |||
|---|---|---|---|---|---|---|---|
| Amount | Cost ($) | Amount | Cost ($) | Amount | Cost ($) | ||
| Std Power (Kwh) | 0.100 | 249,994 | 24,999 | 558,261 | 55,826 | 569,382 | 56,938 |
| Steam (MT) | 4.200 | 7154 | 30,045 | 15,975 | 67,094 | 16,293 | 68,431 |
| Chilled Water (MT) | 0.400 | 4138 | 1655 | 9241 | 3696 | 9425 | 3770 |
| Steam (High P) (MT) | 20.000 | 10 | 197 | 22 | 441 | 22 | 450 |
| TOTAL ($) | 56,897 | 127,057 | 129,588 | ||||
Appendix C. Percentage Coefficient for Capital Cost
| Name | Percentage | Name | Percentage |
|---|---|---|---|
| Piping | 35% EPC 1 | Auxiliary facilities | 40% EPC |
| Instrumentation | 40% EPC | Engineering fee | 25% DC 2 |
| Insulation | 3% EPC | Construction | 35% DC |
| Electrical facilities | 10% EPC | Contractor fee | 5% (DC + IC 3) |
| Building | 45% EPC | Contingency fee | 10% (DC + IC) |
| Yard improvement | 15% EPC |
Appendix D. Sensitivity Analysis Assumptions
| Variable | Base Case Values | Distribution | Variation and Range |
|---|---|---|---|
| Reduction process in P-7 | 14 | Triangular | 12.60–15.40 |
| Dehydrogenation process in P-9 | 4.5 | Triangular | 4.05–4.95 |
| R-102 stirred reactor cost | 661,000 | Normal | S.D. = 0.1 |
| R-103 stirred reactor cost | 844,000 | Normal | S.D. = 0.1 |
| R-104 stirred reactor cost | 848,000 | Normal | S.D. = 0.1 |
| Hydrogen price | 11,397,705 | Normal | S.D. = 0.1 |
| Ammonium chloride price | 15,752,423 | Normal | S.D. = 0.1 |
| Ilmenite price | 17,665,611 | Normal | S.D. = 0.1 |
| MgH2 price | 220,550,276 | Normal | S.D. = 0.1 |
| Chlorine price | 5,149,716 | Normal | S.D. = 0.1 |
References
- Ezugwu, E.; Bonney, J.; Yamane, Y. An overview of the machinability of aeroengine alloys. J. Mater. Process. Technol. 2003, 134, 233–253. [Google Scholar] [CrossRef]
- Boyer, R.R. Attributes, characteristics, and applications of titanium and its alloys. JOM 2010, 62, 21–24. [Google Scholar] [CrossRef]
- Crowley, G. How to Extract Low-Cost Titanium. Adv. Mater. Process 2003, 161, 25–27. [Google Scholar]
- Fray, D.J. Novel methods for the production of titanium. Int. Mater. Rev. 2008, 53, 317–325. [Google Scholar] [CrossRef]
- Takeda, O.; Uda, T.; Okabe, T.H. Rare Earth, Titanium Group Metals, and Reactive Metals Production. In Treatise on Process Metallurgy, Volume 3: Industrial Processes, Part A; Seetharaman, S., Mclean, A., Guthrie, R., Sridhar, S., Eds.; Elsevier: Oxford, UK, 2014; pp. 995–1069. [Google Scholar]
- Van Vuuren, D.S. A Critical Evaluation of Processes to Produce Primary Titanium. J. S. Afr. Inst. Min. Metall. 2009, 109, 455–461. [Google Scholar]
- Kraft, E.H. Summary of Emerging Titanium Cost Reduction Technologies; Study for US DofE and ORNL/Subcontract 4000023694; EHK Technology: Vancouver, WA, USA, 2004. [Google Scholar]
- McCracken, C.G.; Motchenbacher, C.; Barbis, D.P. Review of titanium-powder-production methods. Int. J. Powder Metall. 2010, 46, 19–26. [Google Scholar]
- Van Vuuren, D.S.; Oosthuizen, S.J.; Heydenrych, M.D. Titanium production via metallothermic reduction of TiCl4 in molten salt: Problems and products. J. S. Afr. Inst. Min. Metall. 2011, 111, 141–148. [Google Scholar]
- Doblin, C.; Chryss, A.; Monch, A. Titanium Powder from the TiRO™ Process. Key Eng. Mater. 2012, 520, 95–100. [Google Scholar] [CrossRef]
- Oosthuizen, S.J. In search of low cost titanium: The Fray Farthing Chen (FFC) Cambridge process. J. S. Afr. Inst. Min. Metall. 2011, 111, 199–202. [Google Scholar]
- Chen, W.; Yamamoto, Y.; Peter, W.; Clark, M.; Nunn, S.; Kiggans, J.; Muth, T.; Blue, C.; Williams, J.; Akhtar, K. The investigation of die-pressing and sintering behavior of ITP CP-Ti and Ti-6Al-4V powders. J. Alloy. Compd. 2012, 541, 440–447. [Google Scholar] [CrossRef]
- Van Vuuren, D.S. Direct titanium powder production by metallothermic processes. In Titanium Powder Metallurgy: Science, Technology and Applications; Qian, M., Froes, F.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 69–93. [Google Scholar]
- Fang, Z.Z.; Middlemas, S.; Guo, J.; Fan, P. A New, Energy-Efficient Chemical Pathway for Extracting Ti Metal from Ti Minerals. J. Am. Chem. Soc. 2013, 135, 18248–18251. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Sun, P.; Devener, B.V.; Free, M.; Lefler, H.; Zheng, S. Hydrogen assisted magnesiothermic reduction of TiO2. Chem. Eng. J. 2017, 308, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, E.; Yashima, Y.; Suzuki, R.O.; Rezan, S. Formation of Titanium Sulfide from Titanium Oxycarbonitride by CS2 Gas. Metall. Mater. Trans. B 2018, 49, 1808–1821. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Ono, K.; Teranuma, K. Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall. Mater. Trans. B 2003, 34, 287–295. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Natsui, S.; Kikuchi, T. Recent Studies on Titanium Refining: 2017–2020. Mater. Trans. 2021, 62, 905–913. [Google Scholar] [CrossRef]
- U.S. Geological Survey, Mineral Commodity Summaries, February 2019. Titanium Statistics and Information. Available online: https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/mcs-2019-timin.pdf (accessed on 29 June 2021).
- Geetha, K.; Surender, G. Experimental and modelling studies on the aeration leaching process for metallic iron removal in the manufacture of synthetic rutile. Hydrometallurgy 2000, 56, 41–62. [Google Scholar] [CrossRef]
- Yaraghi, A.; Sapri, M.H.A.; Baharun, N.; Rezan, S.A.; Shoparwe, N.I.; Ramakrishnan, S.; Ariffin, K.S.; Fauzi, M.A.; Zabidi, H.B.; Ismail, H.; et al. Aeration Leaching of Iron from Nitrided Malaysian Ilmenite Reduced by Polystyrene-Coal Reductant. Procedia Chem. 2016, 19, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, E.; Rezan, S.A.; Baharun, N.; Ramakrishnan, S.; Fauzi, M.N.A.; Zhang, G. Chlorination Kinetics of Titanium Nitride for Production of Titanium Tetrachloride from Nitrided Ilmenite. Metall. Mater. Trans. B 2017, 48, 2354–2366. [Google Scholar] [CrossRef]
- Ahmadi, E.; Fauzi, M.N.A.; Hussin, H.; Baharun, N.; Ariffin, K.S.; Rezan, S.A. Synthesis of titanium oxycarbonitride by carbothermal reduction and nitridation of ilmenite with recycling of polyethylene terephthalate (PET). Int. J. Min. Met. Mater. 2017, 24, 444–454. [Google Scholar] [CrossRef]
- Ardani, M.R.; Al Janabi, A.S.M.; Udayakumar, S.; Rezan, S.A.; Mohamed, A.R.; Lee, H.L.; Ibrahim, I. Reduction of TiCl4 to TiH2 with CaH2 in presence of Ni powder. In Rare Metal Technology 2019; Azimi, G., Kim, H., Alam, S., Ouchi, T., Neelameggham, N.R., Baba, A.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 131–144. [Google Scholar]
- Ardani, M.R.; Fauzi, M.N.A.; Rezan, S.A.; Mohamed, A.R.; Lee, H.L.; Ibrahim, I. Thermodynamic Investigation of Titanium Hydride Formation from Reduction of Titanium (Ⅳ) Chloride with Magnesium Hydride in Presence of Hydrogen Atmosphere. MATEC Web Conf. 2020, 321, 07014. [Google Scholar] [CrossRef]
- Ardani, M.R.; Rezan, S.A.; Lee, H.L.; Mohamed, A.R.; Ibrahim, I. Low Temperature Synthesis Method of TiH2 Powder from TiCl4 Reduction with MgH2. In Proceedings of the TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings, San Diego, CA, USA, 23–27 February 2020; The Minerals, Metals & Materials Society, Ed.; Springer: Cham, Switzerland, 2020; pp. 1669–1679. [Google Scholar]
- Robertson, M.I.; Schaffer, G.B. Comparison of sintering of titanium and titanium hydride powders. Powder Metall. 2010, 53, 12–19. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Fan, P.; Middlemas, S.; Guo, J.; Zhang, Y.; Free, M.; Sathyapalan, A.; Xia, Y. Producing a Titanium Product. U.S. Patent US2016/0194733 A1, 7 July 2016. [Google Scholar]
- Granjo, J.F.O.; Oliveira, N.M.C. Process Simulation and Techno-Economic Analysis of the Production of Sodium Methoxide. Ind. Eng. Chem. Res. 2016, 55, 156–167. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.; Portugal, I.; Silva, C.M. Simulation and techno-economic optimization of the supercritical CO2 extraction of Eucalyptus globulus bark at industrial scale. J. Supercrit. Fluids 2019, 145, 169–180. [Google Scholar] [CrossRef]
- Larbi, F.; García, A.; del Valle, L.J.; Hamou, A.; Puiggalí, J.; Belgacem, N.; Bras, J. Simulation basis for a techno-economic evaluation of chitin nanomaterials production process using Aspen Plus® software. Data Brief 2018, 20, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Cardero, A.; Pagés-Díaz, J.; Contino, F.; Rajendran, K.; Lorenzo-Llanes, J. Process simulation and techno-economic assessment of vinasse-to-biogas in Cuba: Deterministic and uncertainty analysis. Chem. Eng. Res. Des. 2021, 169, 33–45. [Google Scholar] [CrossRef]
- Petrides, D.; Sapidou, E.; Calandranis, J. Computer-aided process analysis and economic evaluation for biosynthetic human insulin production—A case study. Biotechnol. Bioeng. 1995, 48, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Foo, D.C.Y.; Kumaresan, S.; Aziz, R.A. Debottlenecking of a Batch Pharmaceutical Cream Production. Pharm. Eng. 2006, 26, 72–84. [Google Scholar]
- Koulouris, A.; Calandranis, J.; Petrides, D.P. Throughput Analysis and Debottlenecking of Integrated Batch Chemical Processes. Comput. Chem. Eng. 2000, 24, 1387–1394. [Google Scholar] [CrossRef]
- Athimulam, A.; Kumaresan, S.; Foo, D.; Sarmidi, M.; Aziz, R. Modelling and Optimization of Eurycoma longifolia Water Extract Production. Food Bioprod. Process. 2006, 84, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Alshekhli, O.; Foo, D.C.Y.; Hii, C.L.; Law, C.L. Process Simulation and Debottlenecking for An Industrial Cocoa Manufacturing Process. Food Bioprod. Process. 2011, 89, 528–536. [Google Scholar] [CrossRef]
- Noor, N.Z.M.; Foo, D.C.Y.; Rahman, B.A.; Aziz, R.A. Modelling and Uncertainty Analysis for Pilot Scale Monoclonal Antibody Production. Pharm. Eng. 2014, 34, 40–54. [Google Scholar]
- Gebremariam, S.N.; Marchetti, J.M. Process simulation and techno-economic performance evaluation of alternative technologies for biodiesel production from low value non-edible oil. Biomass Bioenergy 2021, 149, 106102. [Google Scholar] [CrossRef]
- Lim, S.S.; Foo, D.C. Simulation and scale-up study for a chitosan–TiO2 nanotubes scaffold production. Food Bioprod. Process. 2017, 106, 108–116. [Google Scholar] [CrossRef]
- Sayar, N.A.; Şam, S.D.; Pinar, O.; Serper, D.; Akbulut, B.S.; Kazan, D.; Sayar, A.A. Techno-economic analysis of caffeine and catechins production from black tea waste. Food Bioprod. Process. 2019, 118, 1–12. [Google Scholar] [CrossRef]
- Pang, Y.X.; Yan, Y.; Foo, D.C.; Sharmin, N.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. The influence of lignocellulose on biomass pyrolysis product distribution and economics via steady state process simulation. J. Anal. Appl. Pyrolysis 2020, 158, 104968. [Google Scholar] [CrossRef]
- Udayakumar, S.; Baharun, N.; Rezan, S.A.; Ismail, A.F.; Takip, K.M. Economic evaluation of thorium oxide production from monazite using alkaline fusion method. Nucl. Eng. Technol. 2021, 53, 2418–2425. [Google Scholar] [CrossRef]
- Ardani, M.R.; Sheikh Abdul Hamid, S.A.R.; Lee, H.L.; Mohamed, A.R.; Ibrahim, I. Characterization of TiH2 Powders Produced from TiCl4-MgH2 Reactions under Hydrogen Atmosphere. J. Mater. Eng. Perform. 2021, 30, 3243–3257. [Google Scholar] [CrossRef]
- Ardani, M.R.; Sheikh Abdul Hamid, S.A.R.; Mohamed, A.R. Synthesis of TiH2 powder from ilmenite using MgH2 under H2 atmosphere. Mater. Lett. 2021, 298, 129997. [Google Scholar] [CrossRef]
- Ahmadi, E.; Shoparwe, N.I.; Ibrahim, N.; Sheikh Abdul Hamid, S.A.R.; Baharun, N.; Ariffin, K.S.; Hussin, H.; Fauzi, M.N.A. The Effects of Experimental Variables on Iron Removal from Nitrided Malaysian Ilmenite by Becher Process. In Extraction 2018; Davis, B., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Flores, G.R.A., Jak, E., Goodall, G., et al., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 1383–1396. [Google Scholar]
- Yaraghi, A.; Sapri, M.H.A.; Ahmadi, E.; Baharun, N.; Rezan, S.; Shoparwe, N.F.; Ramakrishan, S.; Ariffin, K.S.; Fauzi, M.N.A.; Zabidi, H.; et al. Microstructural Study of Leached Nitrided Malaysian Ilmenite with Coal-Polystyrene Reductant. Key Eng. Mater. 2016, 701, 132–137. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry, Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database; Finland Outokumpu Research Oy: Temper, Finland, 2006. [Google Scholar]
- Intelligen. SuperPro Designer User’s Guide; Intelligen, Inc.: Scotch Plains, NJ, USA, 2006. [Google Scholar]
- Stull, D.R.; Prophet, H. JANAF Thermochemical Tables, 2nd ed.; National Standard Reference Data System: USA, 1971. Available online: https://www.govinfo.gov/app/details/GOVPUB-C13-bfd606acc2525ccef2762b19002a6d4f (accessed on 30 August 2021).
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Rezan, S.; Zhang, G.; Ostrovski, O. Carbothermal Reduction and Nitridation of Titanium Dioxide in a H2-N2 Gas Mixture. J. Am. Ceram. Soc. 2011, 94, 3804–3811. [Google Scholar] [CrossRef]
- Hui, Y.J.; Shoparwe, N.I.; Rezan, S.A.; Baharun, N.; Sreekantan, S.; Fauzi, A.M.N. Effect of Carbon Reductant on the Formation of Copper Doped Titanium Oxycarbonitride by Carbothermal Reduction and Nitridation. In 8th International Symposium on High-Temperature Metallurgical Processing; Hwang, J.Y., Jiang, T., Kennedy, M.W., Yucel, O., Pistorius, P., Seshadri, V., Zhao, B., Gregurek, D., Keskinkilic, E., Eds.; The Minerals Metals & Materials Series; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Udayakumar, S.; Sadaqi, A.; Ibrahim, N.; Fauzi, M.N.A.; Ramakrishnan, S.; Rezan, S.A. Mathematical Modelling of Titanium Hydride Formation from Titanium Tetrachloride with Magnesium Hydride using Matlab. J. Phys. Conf. Ser. 2018, 1082, 012037–012043. [Google Scholar] [CrossRef]
- Udayakumar, S.; Sadaqi, A.; Ibrahim, N.; Fauzi, M.N.A.; Ramakrishnan, S.; Rezan, S.A. Formation of Titanium Hydride from the Reaction Between Magnesium Hydride and Titanium Tetrachloride. J. Phys.Conf. Ser. 2018, 1082, 012003. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Rezan, S.; Zhang, G.; Ostrovski, O. Carbothermal Reduction and Nitridation of Ilmenite Concentrates. ISIJ Int. 2012, 52, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Rezan, S.A.; Zhang, G.Q.; Ostrovski, O. Phase Development in Carbothermal Reduction and Nitridation of Ilmenite Concentrates. High Temp. Mater. Process. 2012, 31, 381–388. [Google Scholar] [CrossRef]
- Teyssandier, F.; Allendorf, M.D. Thermodynamics and Kinetics of Gas-Phase Reactions in the Ti-Cl-H System. J. Electrochem. Soc. 1998, 145, 2167–2178. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Liu, Y.; Liu, S.; Xiao, S.; Chen, Y. Surface Characterizations of TiH2 Powders before and after Dehydrogenation. Appl. Surf. Sci. 2017, 410, 177–185. [Google Scholar] [CrossRef]
- Couper, J.R.; Herts, D.W.; Smith, L. Process Economics. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Green, D., Perry, R., Eds.; McGraw-Hill: New York, NY, USA, 2007; pp. 9/1–9/56. [Google Scholar]
- Sanjith, U.; Choong, W.; Sivakumar, R.; Fauzi, M.; Rezan, S. Kinetic modelling of oxidation of metallic iron to iron sulfate from iron-titanium oxycarbonitride composite. Mater. Today Proc. 2019, 17, 525–533. [Google Scholar] [CrossRef]
- Becher, R.G.; Canning, R.G.; Goodheart, B.A.; Uusna, S. A new process for upgrading ilmenite mineral sands. Proc. Aust. Min. Metall. 1965, 214, 21–44. [Google Scholar]
- Liu, J.; He, K.; Tang, S.; Wang, T.; Zhang, Z. A comparative study of ferrous, ferric and ferrate pretreatment for ceramic membrane fouling alleviation in reclaimed water treatment. Sep. Purif. Technol. 2019, 217, 118–127. [Google Scholar] [CrossRef]
- Swaminathan, K.; Subramanian, C.; Rao, C.S. The pressure oxidation of acidic FeCl2 solution with oxygen. Hydrometallurgy 1981, 6, 339–346. [Google Scholar] [CrossRef]
- Ahmadi, E.; Sheikh Abdul Hamid, S.A.R.; Hussin, H.; Baharun, N.; Ramakrishnan, S.; Ariffin, K.S.; Fauzi, A.M.N. The Preparation of Iron-Free TiCl4 from Reduced and Nitrided Ilmenite by Polyethylene Terephthalate. INROADS 2016, 5, 11–16. [Google Scholar]
- Moxson, V.S.; Duz, V.A.; Klevtsov, A.G.; Sukhoplyuev, V.D.; Sopka, M.D.; Shuvalov, Y.V.; Matviychuk, M. Manufacturing Pure Titanium Hydride Powder and Alloyed Titanium Hydride Powders by Combined Hydrogen-Magnesium Reduction of Metal Halides. U.S. Patent 9067264, 30 June 2015. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Achilleos, E.C.; Calandranis, J.C.; Petrides, D.P. Quantifying the impact of uncertainty parameters in the batch manufacturing of active pharmaceutical ingredients. Pharm. Eng. 2006, 26, 1–6. [Google Scholar]
- Heinzle, E.; Biwer, A.P.; Cooney, C.L. Development of Sustainable Bioprocesses: Modelling and Assessment; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]









| Component | Standard Gibbs Energy of Formation | Reference |
|---|---|---|
| HCl (g) | = −93.084 − 0.008T | [50] |
| MgCl2 | = −638.230 + 0.157T | [50] |
| MgH2 | = −78.504 + 0.139T | [51] |
| TiCl2 | = −512.330 + 0.158T | [50] |
| TiCl2 (g) | = −238.000 − 0.022T | [50] |
| TiCl3 | = −716.800 + 0.212T | [50] |
| TiCl3 (g) | = −539.510 + 0.049T | [50] |
| TiCl4 (g) | = −762.400 + 0.119T | [50] |
| TiH2 | = −147.290 + 0.140T | [50] |
| Ti2Cl6 (g) | = −245.430 − 0.018T | [51] |
| Parameter | 1 t/batch | 5 t/batch | 10 t/batch |
|---|---|---|---|
| Utilized annual operation time (h/year) | 7914.24 | 7898.54 | 7894.90 |
| Recipe batch time (h) | 60.24 | 83.45 | 124.09 |
| Recipe cycle time (h) | 14.00 | 31.26 | 61.19 |
| Number of batches per year | 562 | 251 | 128 |
| Total amount of Ti produced (kg/year) | 562,000 | 1,255,000 | 1,280,000 |
| Raw Material | Unit Cost | Amount for Different Production Scales of Ti (kg/Batch) | ||
|---|---|---|---|---|
| 1-t/Batch | 5-t/Batch | 10-t/Batch | ||
| NH4Cl 3.2 M (11% NH4Cl and 89% water) | 0.11 $/kg | 114,106.65 | 570,533.24 | 1,141,066.48 |
| Carbon (C) | 1.00 $/kg | 1113.27 | 5566.37 | 11,132.74 |
| Chlorine gas (Cl2) | 1.25 $/kg | 3282.68 | 16,413.43 | 32,826.87 |
| HCl 1 M (9.7% HCl and 90.3% water) (P-5) | 0.097 $/kg | 1873.64 | 9368.19 | 18,736.37 |
| HCl 1 M (9.7% HCl and 90.3% water) (P-8) | 0.097 $/kg | 351.91 | 1759.52 | 3519.05 |
| H2 gas (P-2) | 8.60 $/m3 | 93.25 | 466.27 | 932.55 |
| H2 gas (P-7) | 8.60 $/m3 | 0.21 | 1.04 | 2.08 |
| Ilmenite (FeTiO3) | 4.0 $/kg | 3519.04 | 17,595.23 | 35,190.45 |
| Magnesium hydride (MgH2) | 143.00 $/kg | 1228.93 | 6144.66 | 12,289.32 |
| Nitrogen gas (N2) | 2.10 $/m3 | 323.75 | 1618.76 | 3237.52 |
| Oxygen gas (O2) (from air) | 0.0 | 529.32 | 2646.63 | 5293.26 |
| Name | 1-t/Batch | 5-t/Batch | 10-t/Batch | |||
|---|---|---|---|---|---|---|
| Number | Total Costs | Number | Total Costs | Number | Total Costs | |
| R-101 | 1 | 649,000 | 4 | 2,680,000 | 7 | 4,774,000 |
| AFR-101 | 1 | 436,000 | 1 | 1,106,000 | 1 | 1,700,000 |
| GR-101 | 1 | 105,000 | 1 | 174,000 | 1 | 260,000 |
| MF-101 | 1 | 30,000 | 1 | 48,000 | 1 | 73,000 |
| MF-102 | 1 | 30,000 | 1 | 56,000 | 1 | 85,000 |
| R-102 | 1 | 531,000 | 1 | 661,000 | 1 | 728,000 |
| V-101 | 1 | 29,000 | 1 | 69,000 | 1 | 101,000 |
| R-103 | 1 | 710,000 | 1 | 844,000 | 1 | 984,000 |
| R-104 | 1 | 712,000 | 1 | 848,000 | 1 | 989,000 |
| R-105 | 1 | 538,000 | 1 | 671,000 | 1 | 738,000 |
| V-102 | 1 | 60,000 | 1 | 134,000 | 1 | 229,000 |
| Total price ($) | 3,830,000 | 7,291,000 | 10,661,000 | |||
| Parameter | 1-t/Batch | 5-t/Batch | 10-t/Batch |
|---|---|---|---|
| Equipment Purchase Cost | 4,697,500 | 9,114,000 | 13,326,000 |
| Total Plant Direct Cost (TPDC) | 15,539,000 | 29,630,000 | 44,344,000 |
| Total Plant Indirect Cost (TPIC) | 9,323,000 | 17,778,000 | 26,007,000 |
| Contractor’s Fee and Contingency | 3,729,000 | 7,111,000 | 10,403,000 |
| Direct Fixed Capital Cost | 28,054,388 | 54,519,000 | 79,753,000 |
| Parameter | 1-t/Batch | 5-t/Batch | 10-t/Batch |
|---|---|---|---|
| Total Capital Investment ($) | 42,427,000 | 83,025,000 | 109,793,000 |
| Operating Cost ($/year) | 138,577,000 | 284,499,000 | 286,964,000 |
| Revenues ($/year) | 140,500,000 | 313,750,000 | 320,000,000 |
| Net Profit ($/year) | 4,523,000 | 32,675,000 | 38,630,000 |
| Cost Basis Annual Rate (kg MP/year) a | 562,000 | 1,255,000 | 1,280,000 |
| Unit Production Cost ($/kg MP) | 246.58 | 226.69 | 224.19 |
| Unit Production Revenue ($/kg MP) | 250.00 | 250.00 | 250.00 |
| Gross Margin (%) | 1.37 | 9.32 | 10.32 |
| Return on Investment (ROI) (%) | 10.66 | 39.36 | 35.18 |
| Payback Time (year) | 9.38 | 2.54 | 2.84 |
| Rate of Return (IRR) after taxes (%) | 9.77 | 63.20 | 53.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaei Ardani, M.; Sheikh Abdul Hamid, S.A.R.; Foo, D.C.Y.; Mohamed, A.R. Synthesis of Ti Powder from the Reduction of TiCl4 with Metal Hydrides in the H2 Atmosphere: Thermodynamic and Techno-Economic Analyses. Processes 2021, 9, 1567. https://doi.org/10.3390/pr9091567
Rezaei Ardani M, Sheikh Abdul Hamid SAR, Foo DCY, Mohamed AR. Synthesis of Ti Powder from the Reduction of TiCl4 with Metal Hydrides in the H2 Atmosphere: Thermodynamic and Techno-Economic Analyses. Processes. 2021; 9(9):1567. https://doi.org/10.3390/pr9091567
Chicago/Turabian StyleRezaei Ardani, Mohammad, Sheikh Abdul Rezan Sheikh Abdul Hamid, Dominic C. Y. Foo, and Abdul Rahman Mohamed. 2021. "Synthesis of Ti Powder from the Reduction of TiCl4 with Metal Hydrides in the H2 Atmosphere: Thermodynamic and Techno-Economic Analyses" Processes 9, no. 9: 1567. https://doi.org/10.3390/pr9091567
APA StyleRezaei Ardani, M., Sheikh Abdul Hamid, S. A. R., Foo, D. C. Y., & Mohamed, A. R. (2021). Synthesis of Ti Powder from the Reduction of TiCl4 with Metal Hydrides in the H2 Atmosphere: Thermodynamic and Techno-Economic Analyses. Processes, 9(9), 1567. https://doi.org/10.3390/pr9091567








