Effects of Boiling and Roasting Treatments on the Content of Total Phenolics and Flavonoids and the Antioxidant Activity of Peanut (Arachis hypogaea L.) Pod Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Boiling and Dry Roasting Processes
2.3. Crude Extract Preparation
2.4. Determination of the Total Phenolic Content
2.5. Determination of the Total Flavonoid Content
2.6. Antioxidant Activity of Peanut Shell
2.6.1. DPPH Scavenging Assay
2.6.2. Reducing Power
2.6.3. Hydrogen Peroxide Scavenging Assay
2.7. Statistical Analysis
3. Results and Discussion
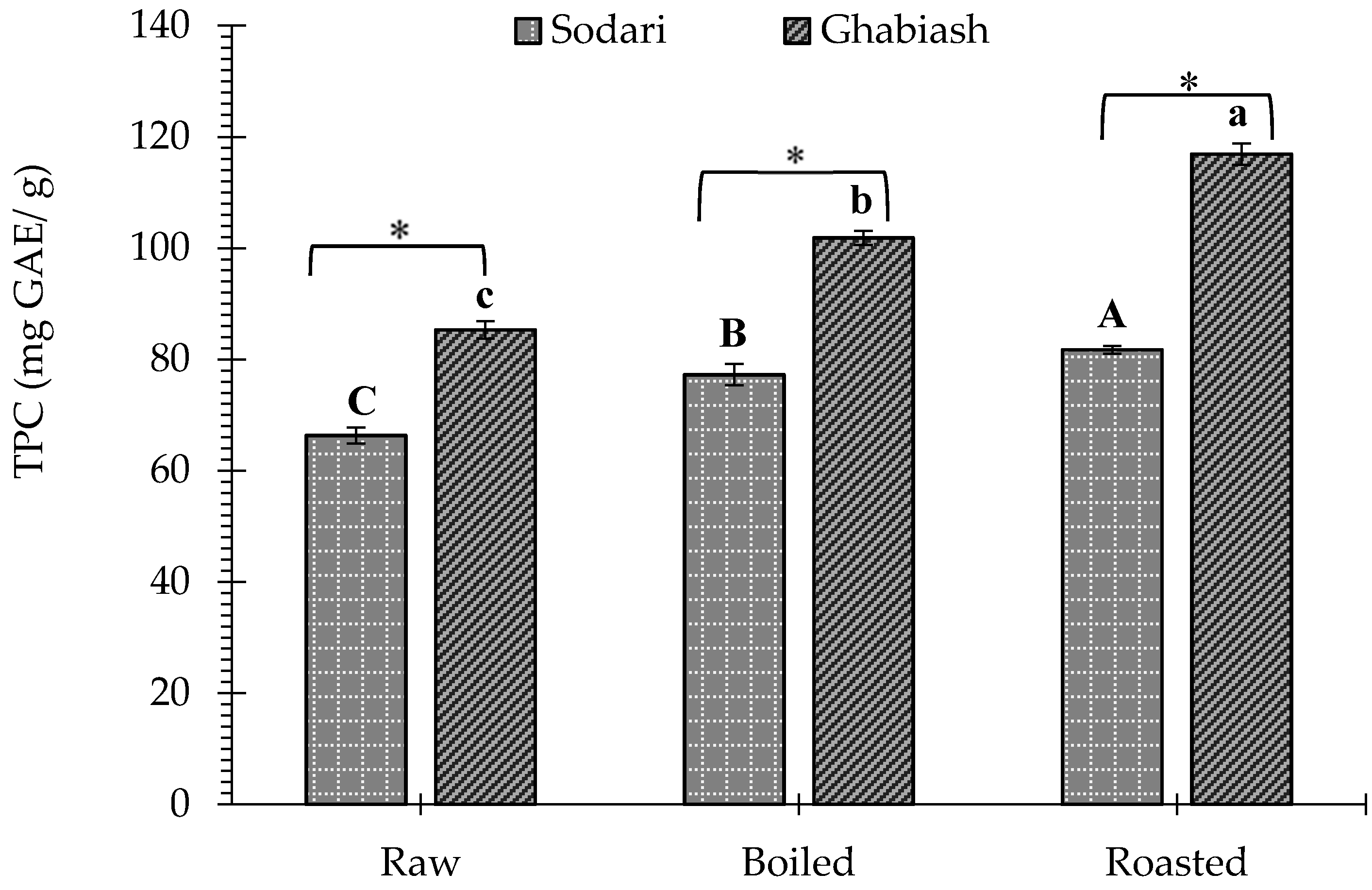
3.1. Effect of Boiling and Roasting on the Total Phenolic Content of Peanut Shell
3.2. Effect of Boiling and Roasting on the Total Flavonoid Content of Peanut Shell
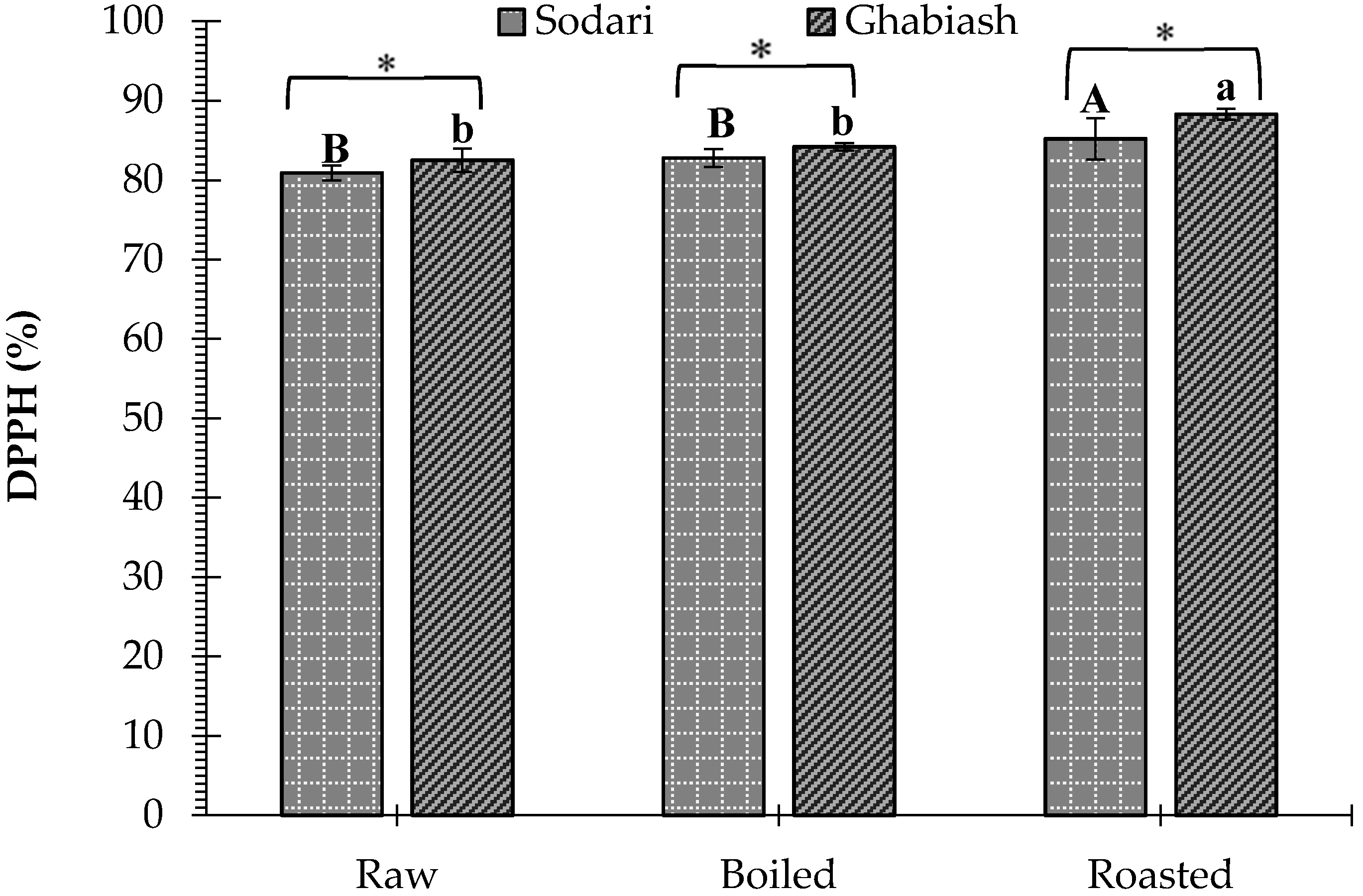
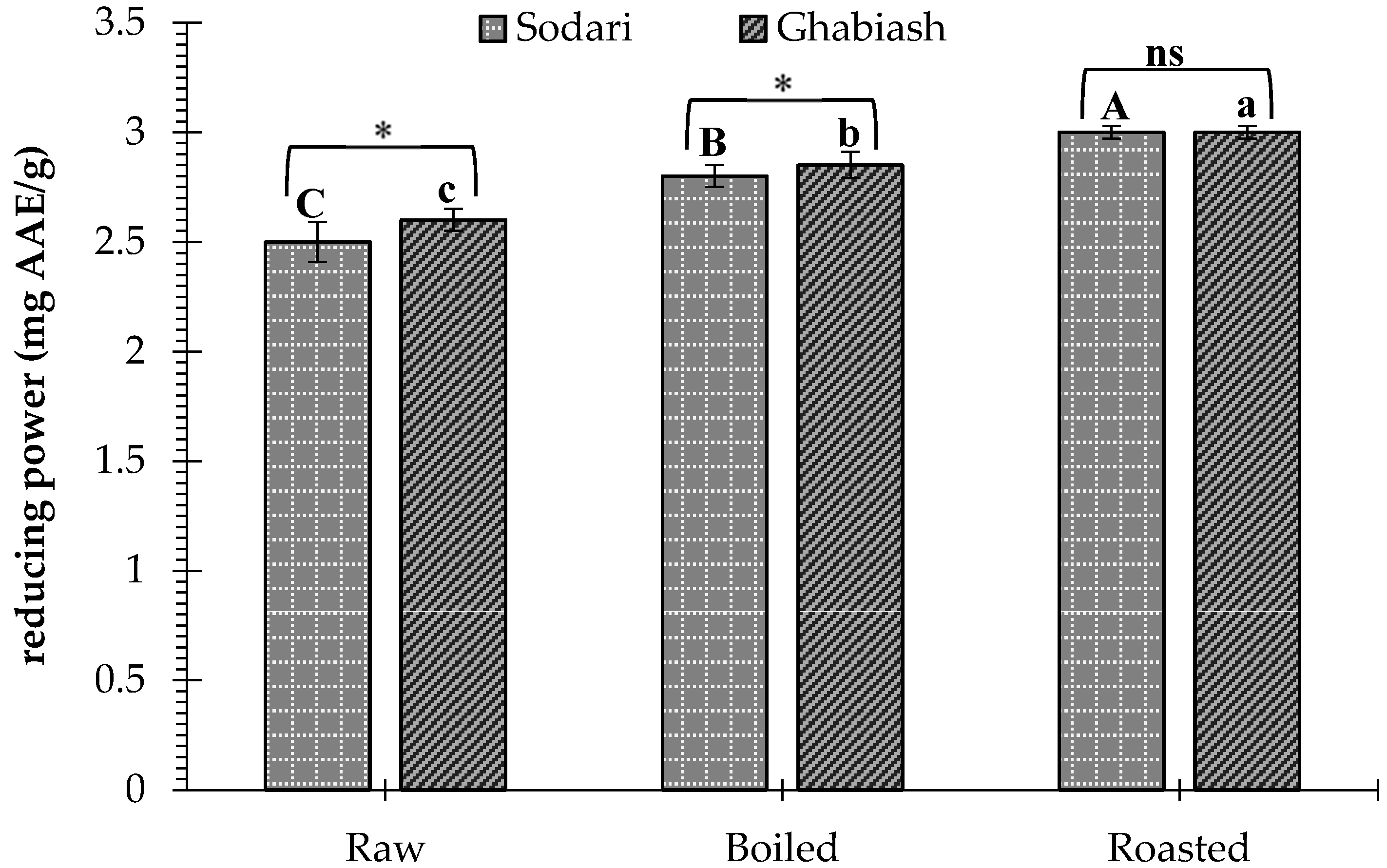
3.3. Effect of Boiling and Roasting on the Antioxidant Activity of Peanut Shell
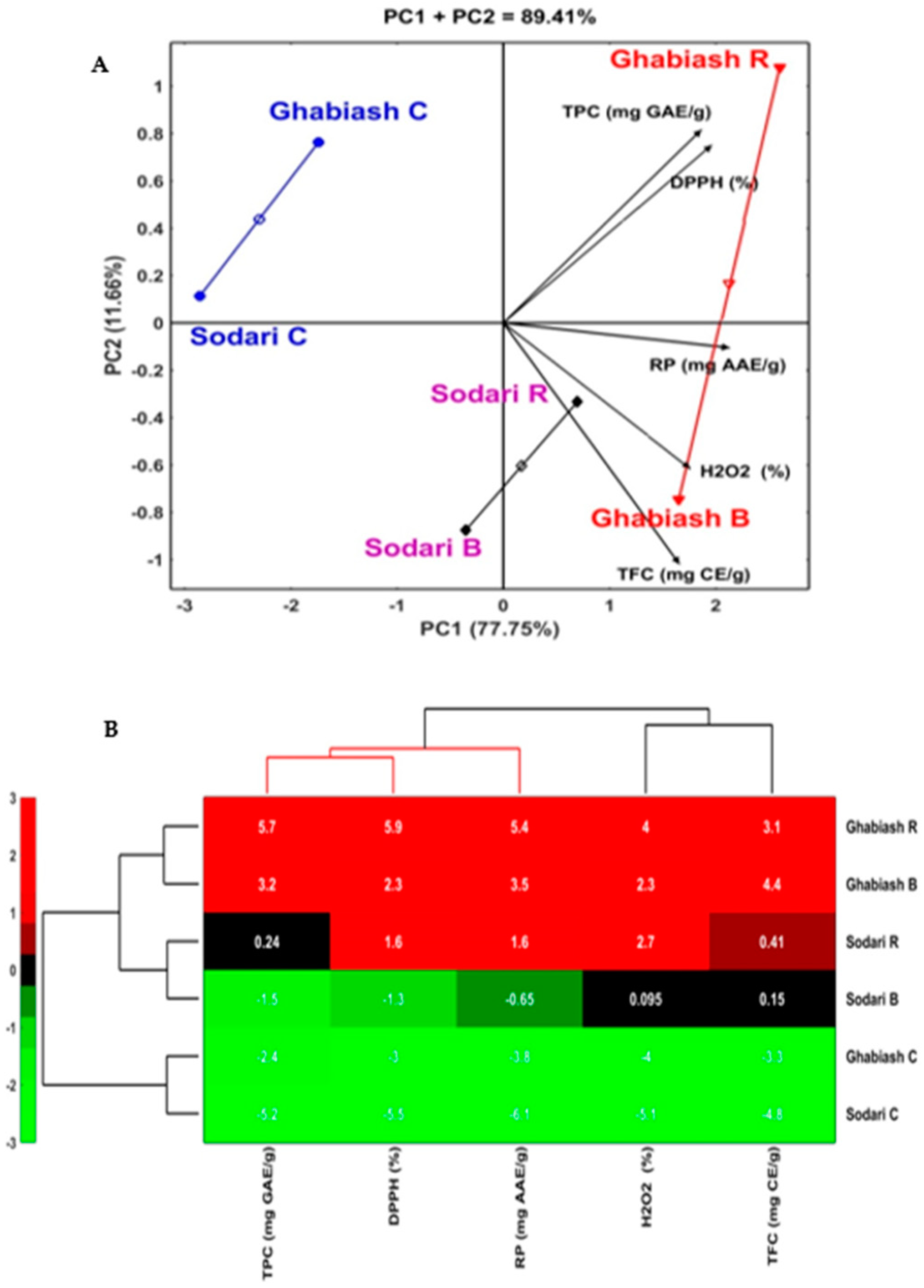
3.4. Principle Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertioli, D.J.; Seijo, G.; Freitas, F.O.; Valls, J.F.; Leal-Bertioli, S.C.; Moretzsohn, M.C. An overview of peanut and its wild relatives. Plant Genet. Res. 2011, 9, 134–149. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.B.; Fink, C.S. Peanuts as a source of B-sitosterol, a sterol with anticancer properties. Nutr. Cancer 2000, 130, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabaté, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J. Nutr. 2008, 138, 1746S–1751S. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.E.; Stampfer, M.J.; Liu, S.; Willett, W.C.; Hu, F.B. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002, 288, 2554–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Camargo, A.C.; Vieira, T.M.F.d.S.; Regitano-D’Arce, M.A.B.; De Alencar, S.M.; Calori-Domingues, M.A.; Canniatti-Brazaca, S.G. Gamma radiation induced oxidation and tocopherols decrease in in-shell, peeled and blanched peanuts. Int. J. Mol. Sci. 2012, 13, 2827–2845. [Google Scholar] [CrossRef] [PubMed]
- Rui-he, M. Study on comprehensive utilization of peanut hull. Modern Agric. Sci. Technol. 2010, 4. [Google Scholar]
- Ramırez-López, E.; Corona-Hernández, J.; Dendooven, L.; Rangel, P.; Thalasso, F. Characterization of five agricultural by-products as potential biofilter carriers. Bioresour. Technol. 2003, 88, 259–263. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H.; Jaafar, M. Effect of peanut shell powder content on the properties of recycled polypropylene (RPP)/peanut shell powder (PSP) composites. BioResources 2013, 8, 5826–5841. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, M.; Shah, A.U.R.; Rao, K.C.; Song, J.-I. Mechanical and thermal properties of epoxy composites reinforced with waste peanut shell powder as a bio-filler. Fibers Polym. 2015, 16, 1119–1124. [Google Scholar] [CrossRef]
- USDA. Full Report (All Nutrients): 45213163. In Shell Peanuts; 2017; UPC: 011161033875. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 22 June 2017).
- Rosales-Martínez, P.; Arellano-Cárdenas, S.; Dorantes-Álvarez, L.; García-Ochoa, F.; López-Cortez, M.d.S. Comparison between antioxidant activities of phenolic extracts from Mexican peanuts, peanuts skins, nuts and pistachios. J. Mex. Chem. Soc. 2014, 58, 185–193. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, M.; He, L.; Fu, P.; Wang, L.; Zhou, J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod. Process. 2013, 91, 158–168. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, X.; Gao, F. Inhibitory effect of polyphenols extracts from peanut shells on the activity of pancreatic lipase in vitro. Asian J. Chem. 2014, 26, 3401. [Google Scholar] [CrossRef]
- Wee, J.-H.; Park, K.-H. Isolation of 4-hydroxycinnamic acid, 3-methoxy-4-hydroxycinnamic acid, and 3, 4-dihydroxybenzoic acid with antioxidative and antimicrobial activity from peanut (Arachis hypogaea). Food Sci. Biotechnol. 2001, 10, 84–89. [Google Scholar]
- Qiu, J.; Chen, L.; Zhu, Q.; Wang, D.; Wang, W.; Sun, X.; Liu, X.; Du, F. Screening natural antioxidants in peanut shell using DPPH–HPLC–DAD–TOF/MS methods. Food Chem. 2012, 135, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Saari, N. Effects of roasting on phenolics composition and antioxidant activity of peanut (Arachis hypogaea L.) kernel flour. Eur. Food Res. Technol. 2011, 233, 599–608. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, S.K.; Ali, M.W.; Adhikari, A.; Kim, I.-D.; Shin, D.-H. Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J. Saudi Soc. Agric. Sci. 2019, 18, 437–442. [Google Scholar] [CrossRef]
- Lee, S.-C.; Jeong, S.-M.; Kim, S.-Y.; Park, H.-R.; Nam, K.; Ahn, D. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem. 2006, 94, 489–493. [Google Scholar] [CrossRef]
- Elsorady, M.; Ali, S. Antioxidant activity of roasted and unroasted peanut skin extracts. Int. Food Res. J. 2018, 25, 43–50. [Google Scholar]
- Radhakrishnan, R.; Pae, S.-B.; Lee, B.-K.; Baek, I.-Y. Evaluation of luteolin from shells of Korean peanut cultivars for industrial utilization. Afr. J. Biotechnol. 2013, 12, 4477–4480. [Google Scholar]
- Gao, F.; Ye, H.; Yu, Y.; Zhang, T.; Deng, X. Lack of toxicological effect through mutagenicity test of polyphenol extracts from peanut shells. Food Chem. 2011, 129, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations Statistical Database (FAOSTAT). 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 16 July 2020).
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley and Sons: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Chang, S.-T.; Wu, J.-H.; Wang, S.-Y.; Kang, P.-L.; Yang, N.-S.; Shyur, L.-F. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Oktay, M.; Küfrevioğlu, Ö.İ.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L.) Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorg. Med. Chem. 2004, 12, 5141–5146. [Google Scholar] [CrossRef]
- Kamalaja, T.; Prashanthi, M.; Rajeswari, K. Evaluation of antioxidant activity and bioactive compounds on domestic cooking method. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 4090–4097. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Kim, S.-Y.; Kim, D.-R.; Jo, S.-C.; Nam, K.; Ahn, D.; Lee, S.-C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem. 2004, 52, 3389–3393. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Zhang, G.; He, H.; Yang, T. Antioxidant activities and phenolic compositions of wheat germ as affected by the roasting process. J. Am. Oil Chem. Soc. 2015, 92, 1303–1312. [Google Scholar] [CrossRef]
- Dittrich, R.; El-Massry, F.; Kunz, K.; Rinaldi, F.; Peich, C.C.; Beckmann, M.W.; Pischetsrieder, M. Maillard reaction products inhibit oxidation of human low-density lipoproteins in vitro. J. Agric. Food Chem. 2003, 51, 3900–3904. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.M.; Al Juhaimi, F.Y.; Osman, M.A.; Al Maiman, S.A.; Hassan, A.B.; Alqah, H.A.; Babiker, E.E.; Ghafoor, K. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT 2020, 131, 109825. [Google Scholar] [CrossRef]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. Changes in the phytochemical composition and profile of raw, boiled, and roasted peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273. [Google Scholar] [CrossRef] [PubMed]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Flavonoid content in ethanolic extracts of selected raw and traditionally processed indigenous foods consumed by vulnerable groups of Kenya: Antioxidant and type II diabetes-related functional properties. Int. J. Food Sci. Nutr. 2011, 62, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Verghese, M.; Walker, L.; Ogutu, S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Ee, K.; Agboola, S.; Rehman, A.; Zhao, J. Characterisation of phenolic components present in raw and roasted wattle (Acacia victoriae Bentham) seeds. Food Chem. 2011, 129, 816–821. [Google Scholar] [CrossRef]
- Boligon, A.A.; Machado, M.M.; Athayde, M.L. Technical evaluation of antioxidant activity. Med. Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Fidrianny, I.; Puspitasari, N. Antioxidant activities, total flavonoid, phenolic, carotenoid of various shells extracts from four species of legumes. Asian J. Pharm. Clin. Res. 2014, 7, 42–46. [Google Scholar]
- Carciochi, R.A.; Galván, D.; Alessandro, L.; Manrique, G.D. Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int. J. Food Sci. Technol. 2016, 51, 1018–1025. [Google Scholar] [CrossRef]
- Wani, I.A.; Gani, A.; Tariq, A.; Sharma, P.; Masoodi, F.A.; Wani, H.M. Effect of roasting on physicochemical, functional and antioxidant properties of arrowhead (Sagittaria sagittifolia L.) flour. Food Chem. 2016, 197, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jogihalli, P.; Singh, L.; Sharanagat, V.S. Effect of microwave roasting parameters on functional and antioxidant properties of chickpea (Cicer arietinum). LWT 2017, 79, 223–233. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. 2011, 1, 106. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Mutwali, N.I.; Mustafa, A.I.; Gorafi, Y.S.; Mohamed Ahmed, I.A. Effect of environment and genotypes on the physicochemical quality of the grains of newly developed wheat inbred lines. Food Sci. Nutr. 2016, 4, 508–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Li, X.; Cao, Y.; Wang, C.; Xue, Y. Effect of roasting on the chemical components of peanut oil. LWT 2020, 125, 109249. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.B.; Al Maiman, S.A.; Alshammari, G.M.; Mohammed, M.A.; Alhuthayli, H.F.; Ahmed, I.A.M.; Alfawaz, M.A.; Yagoub, A.E.A.; Fickak, A.; Osman, M.A. Effects of Boiling and Roasting Treatments on the Content of Total Phenolics and Flavonoids and the Antioxidant Activity of Peanut (Arachis hypogaea L.) Pod Shells. Processes 2021, 9, 1542. https://doi.org/10.3390/pr9091542
Hassan AB, Al Maiman SA, Alshammari GM, Mohammed MA, Alhuthayli HF, Ahmed IAM, Alfawaz MA, Yagoub AEA, Fickak A, Osman MA. Effects of Boiling and Roasting Treatments on the Content of Total Phenolics and Flavonoids and the Antioxidant Activity of Peanut (Arachis hypogaea L.) Pod Shells. Processes. 2021; 9(9):1542. https://doi.org/10.3390/pr9091542
Chicago/Turabian StyleHassan, Amro B., Salah A. Al Maiman, Ghedeir M. Alshammari, Mohammed A. Mohammed, Haya F. Alhuthayli, Isam A. Mohamed Ahmed, Mohammed A. Alfawaz, Abu ElGasim A. Yagoub, Adil Fickak, and Magdi A. Osman. 2021. "Effects of Boiling and Roasting Treatments on the Content of Total Phenolics and Flavonoids and the Antioxidant Activity of Peanut (Arachis hypogaea L.) Pod Shells" Processes 9, no. 9: 1542. https://doi.org/10.3390/pr9091542
APA StyleHassan, A. B., Al Maiman, S. A., Alshammari, G. M., Mohammed, M. A., Alhuthayli, H. F., Ahmed, I. A. M., Alfawaz, M. A., Yagoub, A. E. A., Fickak, A., & Osman, M. A. (2021). Effects of Boiling and Roasting Treatments on the Content of Total Phenolics and Flavonoids and the Antioxidant Activity of Peanut (Arachis hypogaea L.) Pod Shells. Processes, 9(9), 1542. https://doi.org/10.3390/pr9091542






