Decomposition and Nutrient Releasing of Biochar Compound Materials in Soil with Different Textures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Source and Preparation
2.2. Soil Collection for the Experiment
2.3. Calcium Carbonate Analysis
2.4. Compost Source
2.5. Biochar Compound Material Preparation
2.6. Indoor Incubation Experiment for Evaluating Nutrient Releasing and Decomposition of pBCMs
2.7. The Pot Experiment for Crop Production
2.8. Calculation and Statistical Analysis of the Nutrient Utility Rate
3. Results and Discussion
3.1. Decomposition Rate of pBCMs in Soil
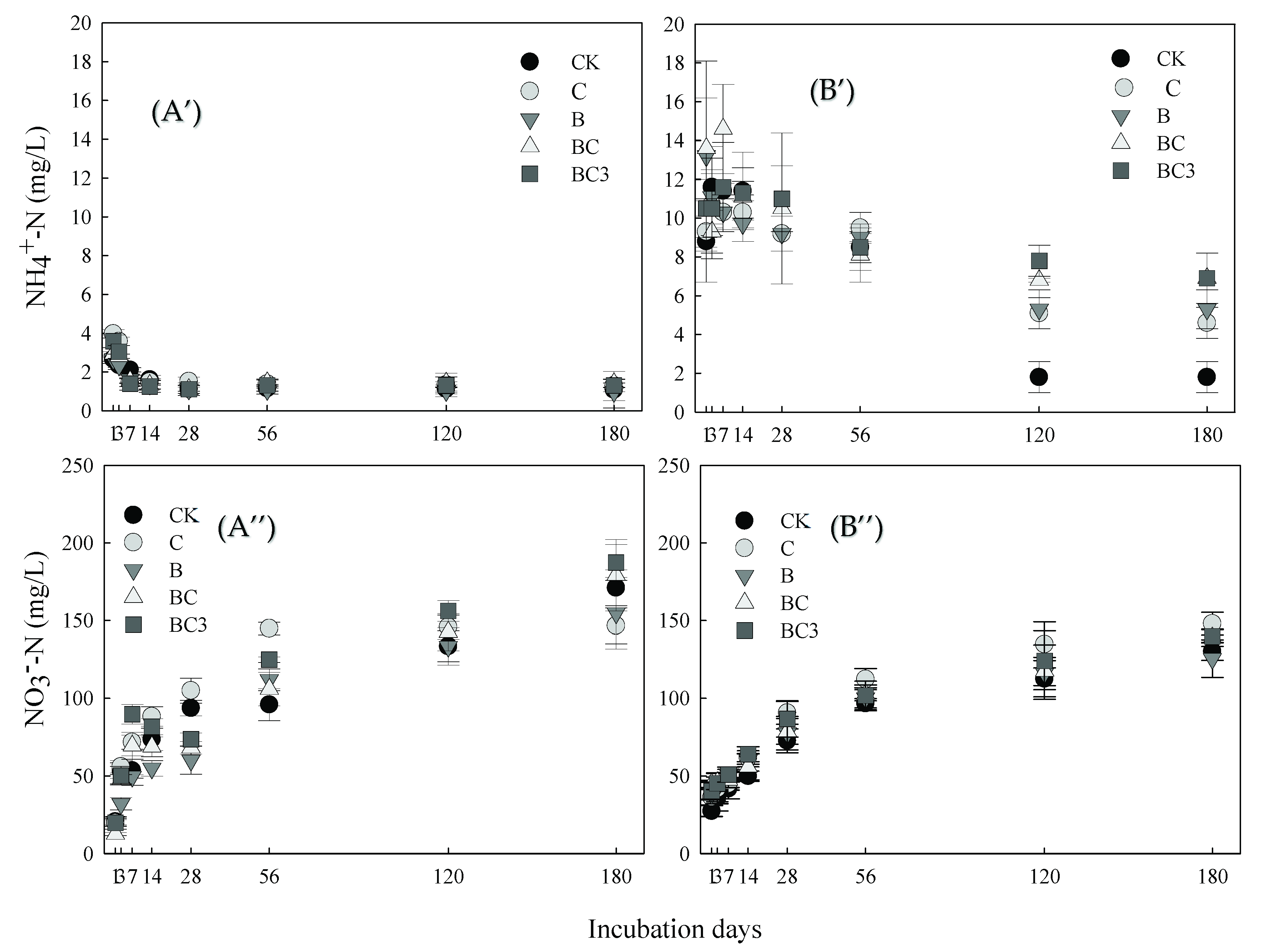
3.2. Effect of pBCMs on Inorganic N in Soil
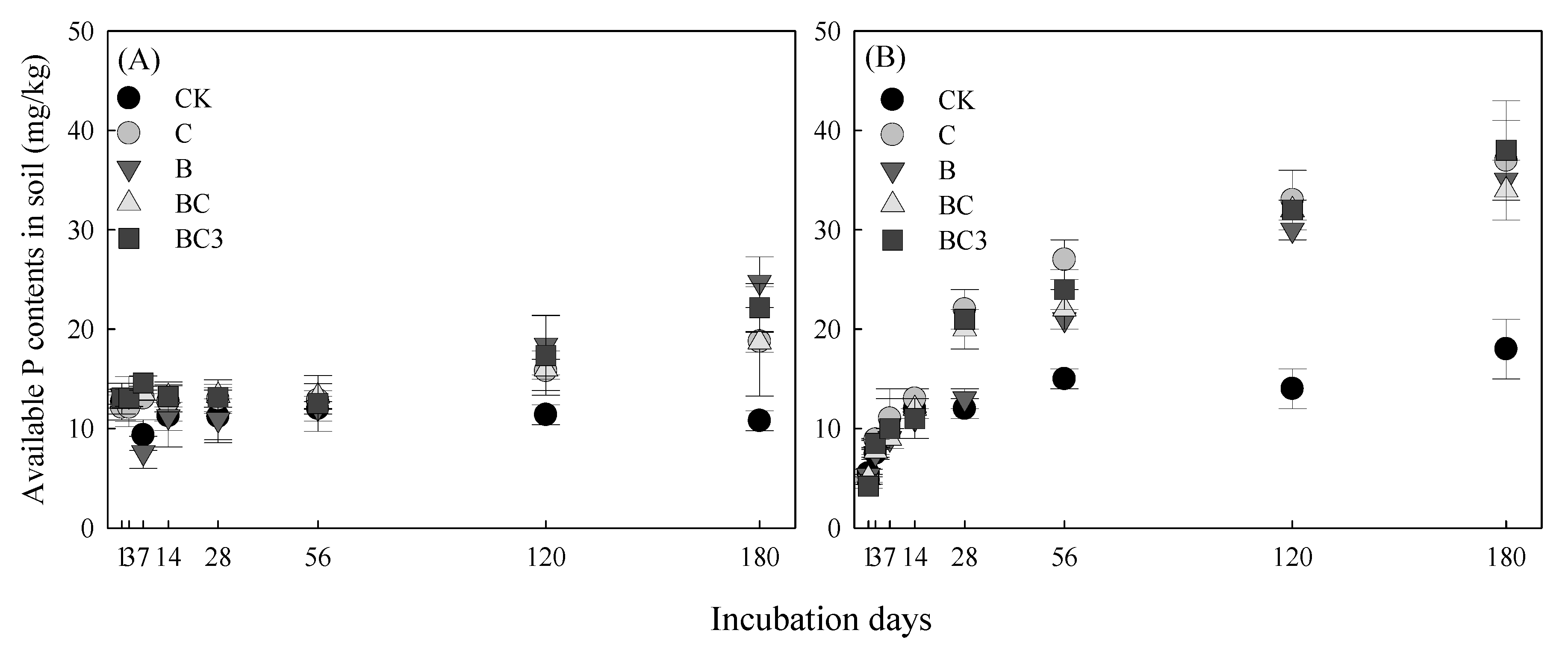
3.3. Effect of pBCMs on Available P in Soil
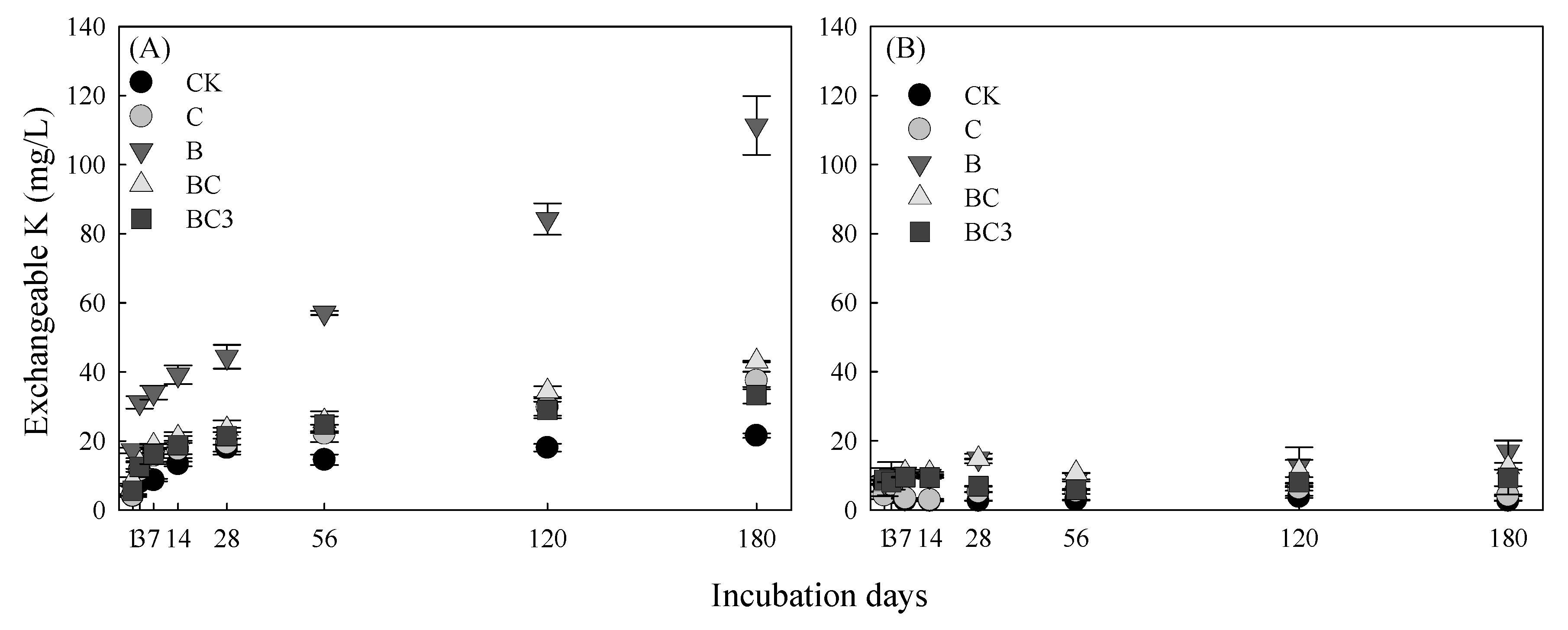
3.4. Effect of pBCMs on Exchangeable K Content
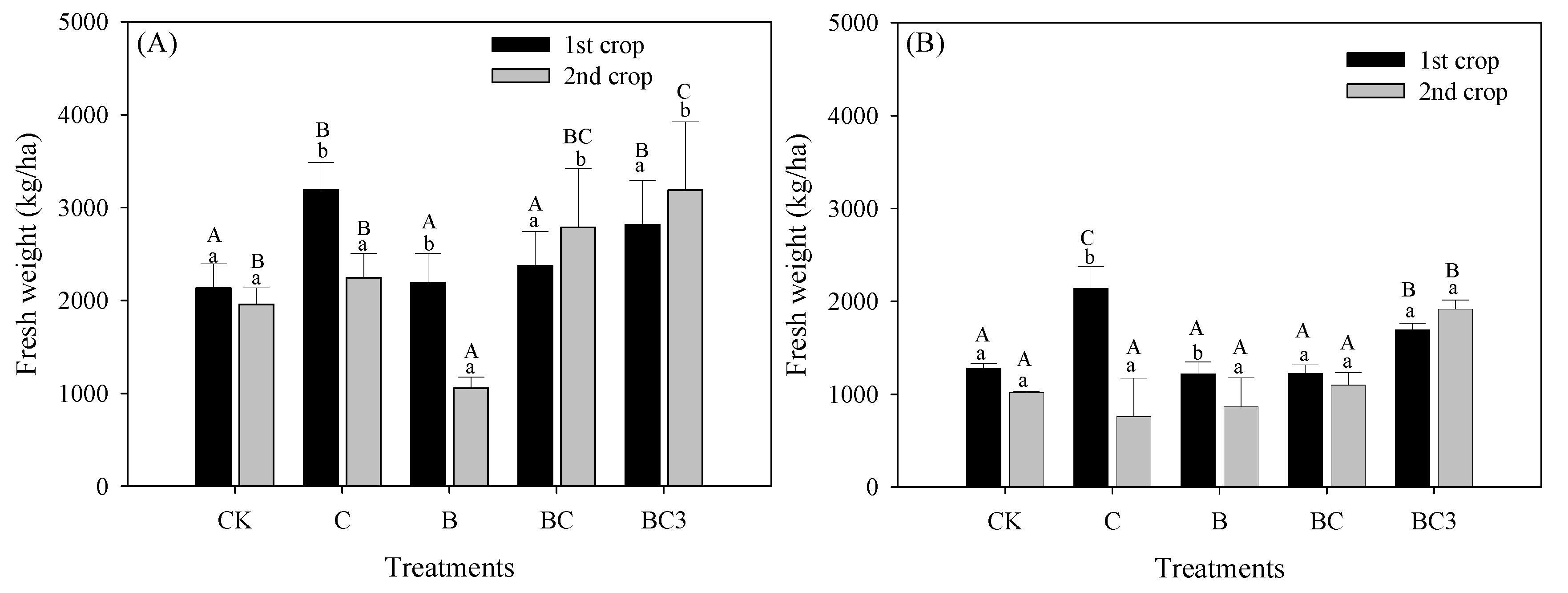
3.5. Effect of pBCMs on Crop Yield
3.6. Effect of pBCMs on Nutrient Use Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-.O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., Eds.; IPCC: Genève, Switzerland, 2019; in press. [Google Scholar]
- Massah, J.; Zadegan, B. Effect of Chemical Fertilizers on Soil Compaction and Degradation. Agric. Mech. Asia Afr. Lat. Am. 2016, 47, 44–50. [Google Scholar]
- Peltre, C.; Christensen, B.T.; Dragon, S.; Icard, C.; Kätterer, T.; Houot, S. RothC simulation of carbon accumulation in soil after repeated application of widely different organic amendments. Soil Biol. Biochem. 2012, 52, 46–60. [Google Scholar] [CrossRef]
- Nobile, C.M.; Bravin, M.N.; Becquer, T.; Paillat, J.M. Phosphorus sorption and availability in an andosol after a decade of organic or mineral fertilizer applications: Importance of pH and organic carbon modifications in soil as compared to phosphorus accumulation. Chemosphere 2020, 239, 124709. [Google Scholar] [CrossRef] [PubMed]
- Jien, S.H.; Wang, C.S. Effects of Biochar on Soil Properties and Erosion Potential in a Highly Weathered Soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Jien, S.H.; Wang, C.C.; Lee, C.H.; Lee, T.Y. Stabilization of organic matter by biochar application in compost-amended soils with contrasting pH values and textures. Sustainability 2015, 10, 13317–13333. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Wang, C.C.; Lin, H.H.; Lee, S.S.; Tsang, D.C.W.; Jien, S.H.; Ok, Y.S. In-situ biochar application conserves nutrients while simultaneously mitigating runoff and erosion of an Fe-oxide-enriched tropical soil. Sci. Total Environ. 2018, 619, 665–671. [Google Scholar] [CrossRef]
- Jien, S.H.; Kuo, Y.L.; Liao, C.S.; Wu, Y.T.; Igalavithana, A.D.; Tsang, D.C.W.; Ok, Y.S. Effects of field scale in situ biochar incorporation on soil environment in a tropical highly weathered soil. Environ. Pollut. 2021, 272, 116009. [Google Scholar] [CrossRef]
- Lehmann, J.; de Silva, J.P., Jr.; Steiner, C.; Nehl, S.T.; Zech, W.; Glaser, B. Nutrient ava ilab ilityand leaching in an archaeological Anthrosol anda Ferralsol of the central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Robert, B.; ol Varga, Y.K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]
- Novak, J.; Lima, I.; Xing, B.; Gaskin, J.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Kallenbach, C.; Grandy, S. Controls over soil microbial biomass responses to carbon amendments in agricultural systems: A meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 241–252. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Kubheka, B.; Laing, M.D.; Yobo, K.W. Combinations of a biofertilizer with micro-dosed chemical fertilizers increased yield of maize in a high acid saturated soil. Rhizosphere 2020, 13, 100189. [Google Scholar] [CrossRef]
- Zheng, J.; Han, J.; Liu, Z.; Xia, W.; Zhang, X.; Li, L.; Liu, X.; Bian, R.; Cheng, K.; Zheng, J.; et al. Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric. Ecosyst. Environ. 2017, 241, 70–78. [Google Scholar] [CrossRef]
- Qian, L.; Chen, L.; Joseph, S.D.; Cheng, K.; Li, L.Q.; Zheng, J.W.; Zheng, X.H.; Zheng, J.F.; Yu, X.Y.; Wang, J.F. Biochar compound fertilizer as an option to reach high productivity but low carbon intensity in rice agriculture of China. Carbon Manag. 2014, 5, 145–154. [Google Scholar] [CrossRef]
- Schulz1, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422. [Google Scholar] [CrossRef]
- Rahmaniana, N.; Naderia, S.; Supukb, E.; Abbasc, R.; Hassanpourc, A. Urea Finishing Process: Prilling versus Granulation. Proc. Eng. 2015, 102, 174–181. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wu, J.; Shu, Y.; Zhou, P.; Deng, Q. High-yielding Cultural Techniques of Super Hybrid Rice YLY 900 Yielded 15.40 t/hm2 on a 6.84 hm2 Scale. China Rice 2014, 20, 1–4. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; p. 199. [Google Scholar]
- Gee, G.W.; Bauder, J.W.; Klute, A. Particle-size analysis. In Methods of soil Analysis. Part 1. Physical and Mineralogical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1986; p. 383. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and exchange Coefficients. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.; Page, A.; Helmke, P.; Loeppert, R.; Soltanpour, P.; Tabatabai, M.; Johnston, C.; Sumner, M. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; p. 961. [Google Scholar]
- Bray, R.H.; Kurtz, L. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Leoppert, R.H.; Hallmark, C.T.; Koshy, M.M. Routine Procedure for Rapid Determination of Soil Carbonates. Soil Sci. Soc. Am. J. 1984, 48, 1030–1033. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Bationo, K.A.; Anand Kumar, K. Phosphorus use efficiency as related to sources of P fertilizers, rainfall, soil, crop management, and genotypes in the West African semi-arid tropics. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities. Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2002; p. 145. [Google Scholar]
- Bruun, S.; Thomsen, I.K.; Christensen, B.T.; Jensen, L.S. In search of stable soil organic carbon fractions: A comparison of methods applied to soils labelled with 14C for 40 days or 40 years. Eur. J. Soil Sci. 2008, 59, 247–256. [Google Scholar] [CrossRef]
- Nguyen, B.; Lehmann, J.; Hockaday, W.C.; Joseph, S.; Masiello, C.A. Temperature sensitivity of black carbon decomposition and oxidation. Environ. Sci. Technol. 2010, 44, 3324–3331. [Google Scholar] [CrossRef]
- Curtin, D.; Campbell, C.A.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Shangguan, Z. Combined biochar and nitrogen fertilization at appropriate rates could balance the leaching and availability of soil inorganic nitrogen. Agric. Ecosyst. Environ. 2019, 276, 21–23. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Velloso, A.C.X. Eletroquímica de solos modais e de sua matéria orgânicaem ambientes tropicais. Rev. Ceres 2014, 61, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Alovisi, A.M.T.; Cassol, C.J.; Nascimento, S.J.; Soares, N.B.; de Silva, I.R., Jr.; de Silva, R.S.; de Silva, J.A.M. Soil factors affecting phosphorus adsorption in soils of the Cerrado, Brazil. Geoderma Reg. 2020, 22, e00298. [Google Scholar] [CrossRef]
- Morales, M.M.; Comerford, N.; Guerrini, I.A.; Falcão, N.P.S.; Reeves, J.B. Sorption and desorption of phosphate on biochar and biochar–soil mixtures. Soil Use Manag. 2013, 29, 306–314. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Maienza, A.; Miglietta, F.; Baronti, S.; Lonardo, S.D.; Giagnoni, L.; Lagomarsino, A.; Pozzi, A.; Pusceddu, E.; Ranieri, R.; et al. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Manolikaki, I.I.; Mangolis, A.; Diamadopoulos, E. The impact of biochars prepared from agricultural residues on phosphorus release and availability in two fertile soils. J. Environ. Manag. 2016, 181, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.S. Taiwan Crop Fertilization Handbook; Chinese Fertilizer Association: Taichung, Taiwan, 2015. [Google Scholar]
- Dominguez, E.L.; Uttran, A.; Loh, S.K.; Manero, M.H.; Upperton, R.; Tanimu, M.I.; Bachmann, R.T. Characterisation of industrially produced oil palm kernel shell biochar and its potential as slow-release nitrogen-phosphate fertilizer and carbon sink. Mater. Today Proc. 2020, 31, 221–227. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Liu, Y.; Lua, H.; Yang, S.; Wang, Y. Impacts of biochar addition on rice yield and soil properties in a cold water logged paddy for two crop seasons. Field Crop. Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Dong, X.; Li, G.; Lin, Q.; Zhao, X. Quantity and quality changes of biochar aged for 5 years in soil under field conditions. Catena 2017, 159, 136–143. [Google Scholar] [CrossRef]
- Orama, N.J.; van de Voorde, T.F.J.; Ouwehanda, G.J.; Bezemer, T.M.; Mommer, L.; Jefferya, S.; Van Groenigena, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]

| Items | Loamy Sand | Clay Loam |
|---|---|---|
| Sand (%) | 80 | 32 |
| Silt (%) | 14 | 35 |
| Clay (%) | 6.0 | 33 |
| pH | 6.9 ± 0.1 | 5.3 ± 0.1 |
| Organic carbon (%) | 0.99 ± 1.39 | 0.82 ± 0.12 |
| Total N (mg/kg) | 642 ± 235 | 1004 ± 277 |
| Total P (mg/kg) | 424 ± 101 | 212 ± 80 |
| NH4+-N(mg/kg) | 106 ± 16 | 22 ± 2.1 |
| NO3−-N (mg/kg) | 181 ± 92 | 67 ± 4.3 |
| Av. P (mg/kg) | 13 ± 5.1 | 14 ± 1.6 |
| Exc. K (mg/kg) | 59 ± 3.2 | 105 ± 6.9 |
| CaCO3 (%) | 3.0 ± 0.3 | ND |
| Fed (%) | 1.18 ± 0.27 | 5.12 ± 0.66 |
| Ald (%) | 0.09 ± 0.02 | 0.62 ± 0.17 |
| Properties | B | C | BC | BC3 |
|---|---|---|---|---|
| pH | 10.0 ± 0.1 | 6.9 ± 0.1 | 8.3 ± 0.1 | 7.0 ± 0.1 |
| Organic carbon (%) | 2.25 ± 0.9 | 28.9 ± 6.6 | 24.8 ± 1.3 | 28.5 ± 2.2 |
| Total carbon (%) | 80 ± 0.1 | 41 ± 0.1 | 51 ± 0.1 | 64 ± 0.1 |
| Total N (mg/kg) | 1537 ± 232 | 12,041 ± 288 | 6661 ± 86 | 9412 ± 432 |
| Total P (mg/kg) | 940 ± 41 | 2125 ± 204 | 1113 ± 30 | 1738 ± 57 |
| NH4+-N(mg/kg) | 58 ± 12 | 211 ± 7.1 | 132 ± 8.3 | 194 ± 9.2 |
| NO3--N (mg/kg) | 94 ± 19 | 212 ± 5.5 | 113 ± 7.2 | 185 ± 9.1 |
| Av. P (mg/kg) | 42 ± 6.0 | 142 ± 41 | 85 ± 8.0 | 92 ± 14 |
| Ex. K (mg/kg) | 2008 ± 101 | 892 ± 76 | 1538 ± 14 | 1025 ± 100 |
| CaCO3 (%) | 14.4 ± 1.02 | 5.60 ± 0.37 | 9.47 ± 1.16 | 7.84 ± 0.22 |
| Treatments | Loamy Sand | Clay Loam | ||
|---|---|---|---|---|
| 1 Day | 180 Day | 1 Day | 180 Day | |
| CK | 7.6 ± 0.1 bB | 6.6 ± 0.1 aA | 4.3 ± 0.1 aA | 4.1 ± 0.1 aA |
| C | 7.3 ± 0.1 aA | 6.8 ± 0.1 aAB | 4.7 ± 0.1 aB | 4.2 ± 0.1 aA |
| B | 7.7 ± 0.1 bB | 7.3 ± 0.1 aB | 5.0 ± 0.1 aB | 4.6 ± 0.2 abA |
| BC | 7.3 ± 0.1 aA | 6.9 ± 0.1 aAB | 4.9 ± 0.1 aB | 4.6 ± 0.1 abA |
| BC3 | 7.3 ± 0.1 aA | 7.0 ± 0.1 aB | 4.8 ± 0.2 aB | 4.5 ± 0.1 abA |
| Loamy Sand | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Available P (mg/L) | |||||||
| 1 | 3 | 7 | 14 | 28 | 56 | 120 | 180 | |
| CK | 18 ± 0.6 d | 13 ± 2.0 a | 12 ± 1.3 a | 9 ± 2.0 a | 7 ± 1.4 a | 15 ± 1.1 a | 16 ± 4.0 a | 18 ± 4.0 ab |
| B | 19 ± 2.0 d | 18 ± 0.9 c | 20 ± 1.1 c | 15 ± 3.0 b | 11 ± 2.3 b | 16 ± 2.0 ab | 15 ± 6.0 a | 14 ± 5.6 a |
| C | 6.2 ± 0.6 a | 15 ± 1.0 b | 15 ± 2.0 b | 15 ± 1.0 b | 14 ± 1.8 c | 19 ± 0.6 d | 22 ± 7.0 ab | 34 ± 9.0 d |
| BC | 9.5 ± 1.0 b | 14 ± 0.7 ab | 14 ± 0.9 ab | 16 ± 4.0 b | 19 ± 1.8 d | 17 ± 2.0 cd | 19 ± 1.0 ab | 21 ± 1.8 b |
| BC3 | 14 ± 2.0 c | 17 ± 2.0 c | 14 ± 0.7 ab | 17 ± 4.0 b | 20 ± 2.1 d | 17 ± 1.0 bc | 22 ± 2.0 b | 27 ± 3.9 c |
| Treatments | Loamy Sand | Clay Loam | |||
|---|---|---|---|---|---|
| 1st | 2nd | 1st | 2nd | ||
| CK | 11.3 ± 0.6 aA | 11.0 ± 0.6 aB | CK | 6.1 ± 0.2 aA | 4.9 ± 0.1 bA |
| C | 17.5 ± 1.2 aC | 12.5 ± 0.8 aB | C | 9.9 ± 0.7 aC | 3.9 ± 1.7 bA |
| B | 12.0 ± 1.4 bA | 5.9 ± 0.3 aA | B | 5.8 ± 0.6 aA | 4.6 ± 0.7 aA |
| BC | 14.4 ± 1.0 aB | 15.9 ± 1.2 aC | BC | 5.8 ± 0.4 aA | 5.3 ± 0.5 aA |
| BC3 | 16.4 ± 0.7 aC | 19.5 ± 1.8 bD | BC3 | 8.0 ± 0.2 aB | 9.1 ± 0.2 bB |
| Treatments | Loamy Sand | Clay Loam | |||
|---|---|---|---|---|---|
| 1st | 2nd | 1st | 2nd | ||
| CK | 5.0 ± 1.3 bB | 5.9 ± 2.9 bA | CK | 4.9 ± 1.1 aA | 2.7 ± 1.2 bA |
| C | 11 ± 2.8 bC | 8.3 ± 3.1 bAB | C | 4.1 ± 0.8 aAB | 1.9 ± 0.9 bA |
| B | 3.6 ± 0.4 aAB | 9.6 ± 7.0 bAB | B | 2.5 ± 1.1 aB | 2.6 ± 0.5 aA |
| BC | 6.3 ± 1.7 aBC | 12 ± 4.4 bB | BC | 3.4 ± 1.0 aB | 4.8 ± 1.4 abAB |
| BC3 | 11 ± 1.6 aD | 29 ± 11 bC | BC3 | 4.1 ± 3.3 aAB | 5.0 ± 0.8 bB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-S.; Xie, Z.-H.; Jien, S.-H. Decomposition and Nutrient Releasing of Biochar Compound Materials in Soil with Different Textures. Processes 2021, 9, 1521. https://doi.org/10.3390/pr9091521
Liao C-S, Xie Z-H, Jien S-H. Decomposition and Nutrient Releasing of Biochar Compound Materials in Soil with Different Textures. Processes. 2021; 9(9):1521. https://doi.org/10.3390/pr9091521
Chicago/Turabian StyleLiao, Chien-Sen, Zheng-Han Xie, and Shih-Hao Jien. 2021. "Decomposition and Nutrient Releasing of Biochar Compound Materials in Soil with Different Textures" Processes 9, no. 9: 1521. https://doi.org/10.3390/pr9091521
APA StyleLiao, C.-S., Xie, Z.-H., & Jien, S.-H. (2021). Decomposition and Nutrient Releasing of Biochar Compound Materials in Soil with Different Textures. Processes, 9(9), 1521. https://doi.org/10.3390/pr9091521








