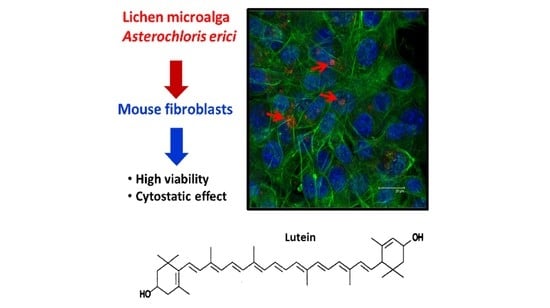
Chemical Characterization of the Lichen-Symbiont Microalga Asterochloris erici and Study of Its Cytostatic Effect on the L929 Murine Fibrosarcoma Cell Line
Abstract
:1. Introduction
2. Experimental Section
2.1. Instruments
2.2. Chemicals and Biochemicals
2.3. Strain Maintenance, Culture Conditions and Preparation of Microalgae Homogenates
2.4. Proximate Chemical Composition Determination
2.5. Antioxidant Activity and Total Phenolic Compounds
2.5.1. Extraction Procedure
2.5.2. Radical Scavenging Activity (RSA)
2.5.3. Total Phenolic Content
2.6. Carotenoids and Chlorophylls Extraction and Chromatographic Analysis
2.7. Cell Culture for the In Vitro Biocompatibility Studies
2.8. Cell Morphology Studies
2.8.1. Optical Microscopy
2.8.2. Confocal Microscopy
2.9. Cell Cycle Analysis and Apoptosis Detection
2.10. Intracellular Reactive Oxygen Species (ROS) Content and Cell Viability
2.11. Statistics
3. Results
3.1. Proximate Composition, Antioxidant Activity and Total Phenolic Compounds
3.2. Carotenoid and Chlorophyll Content
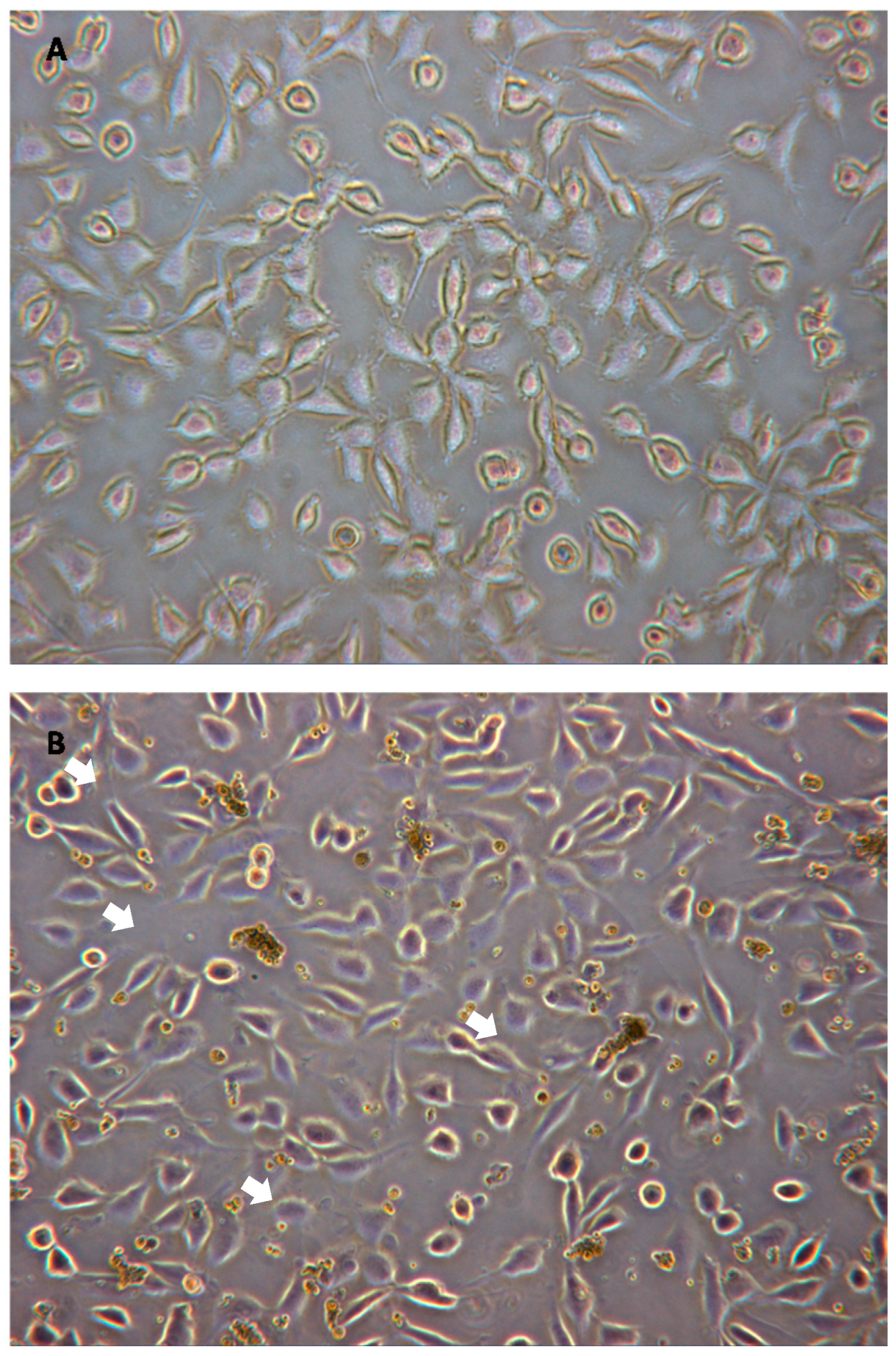
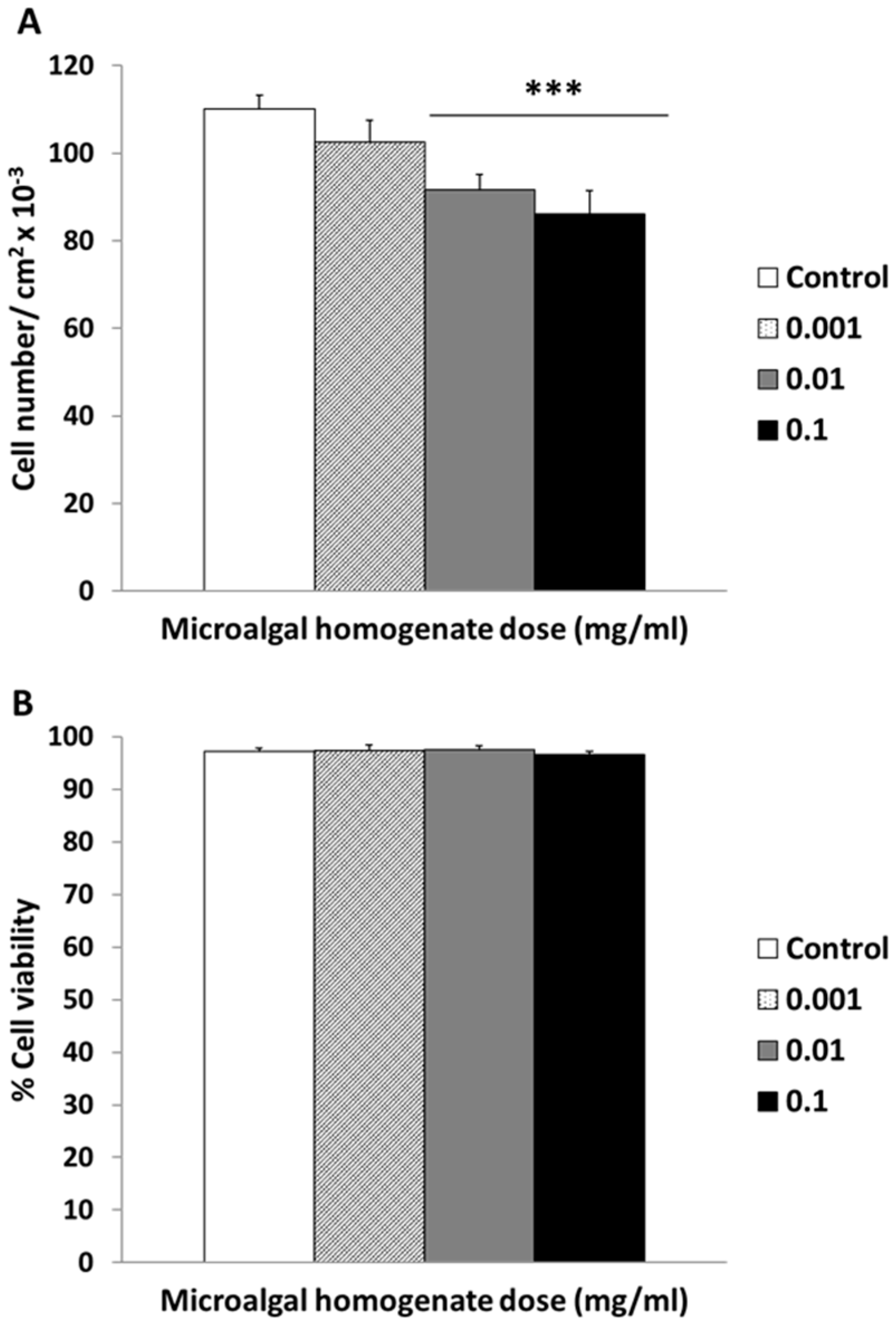
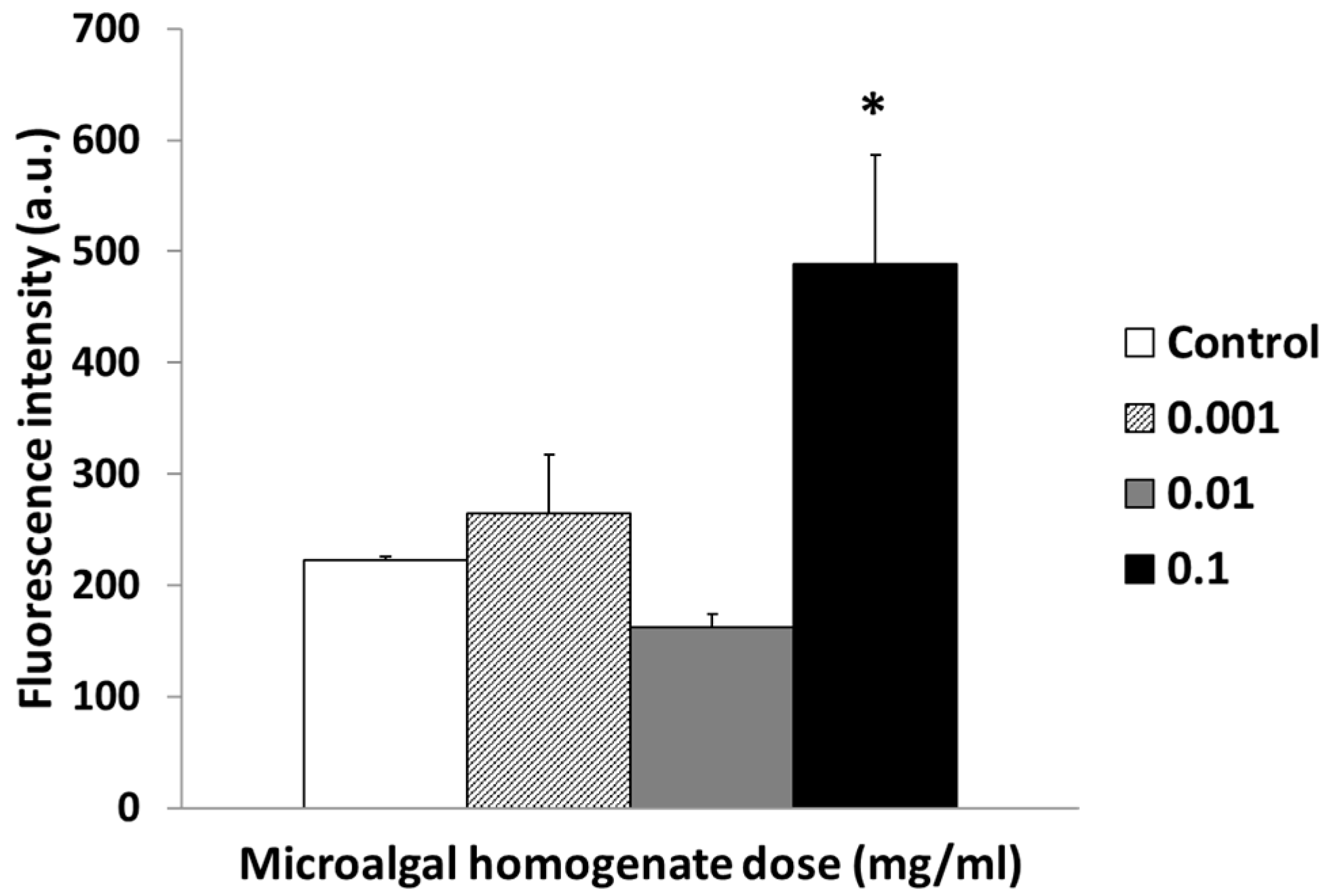
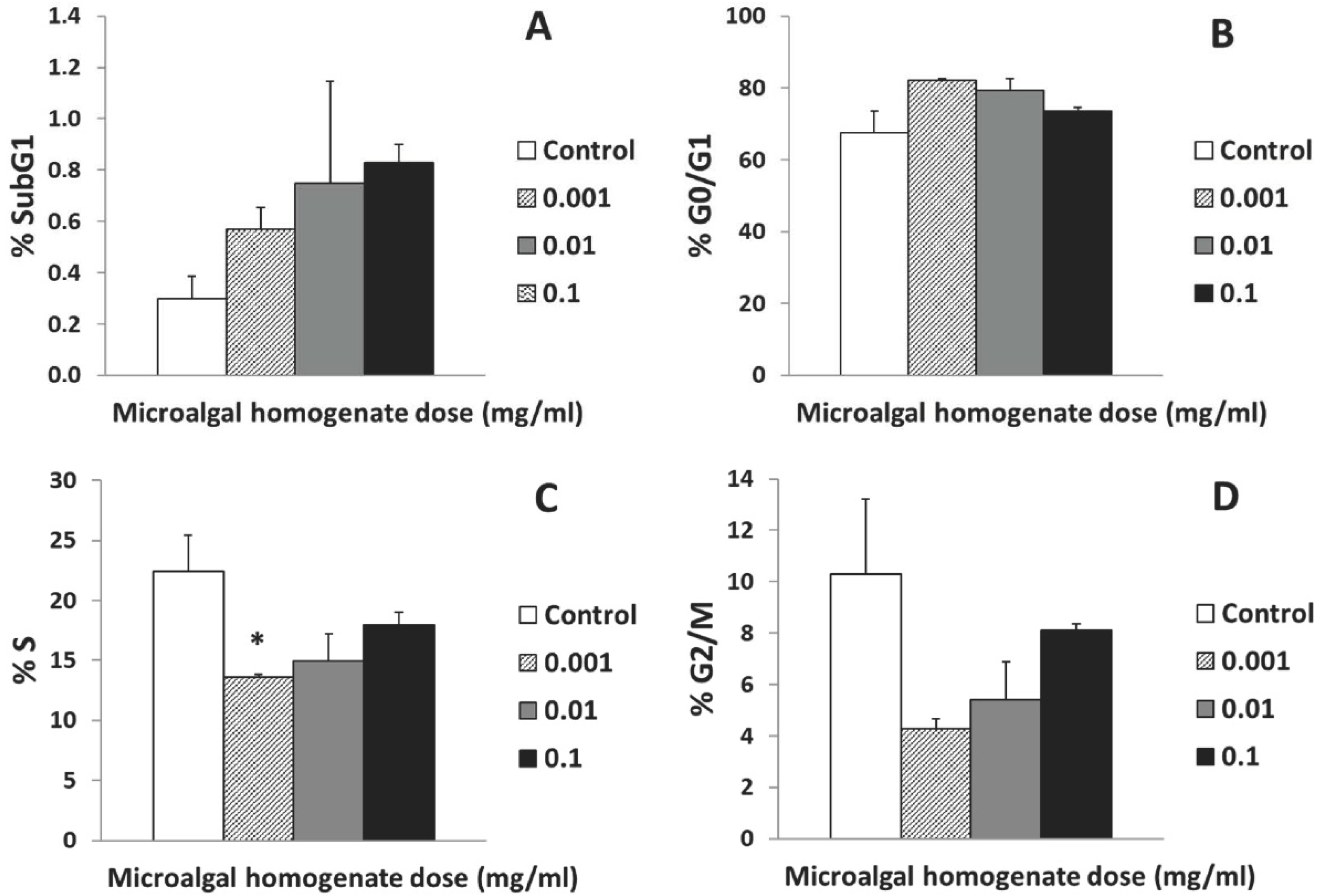
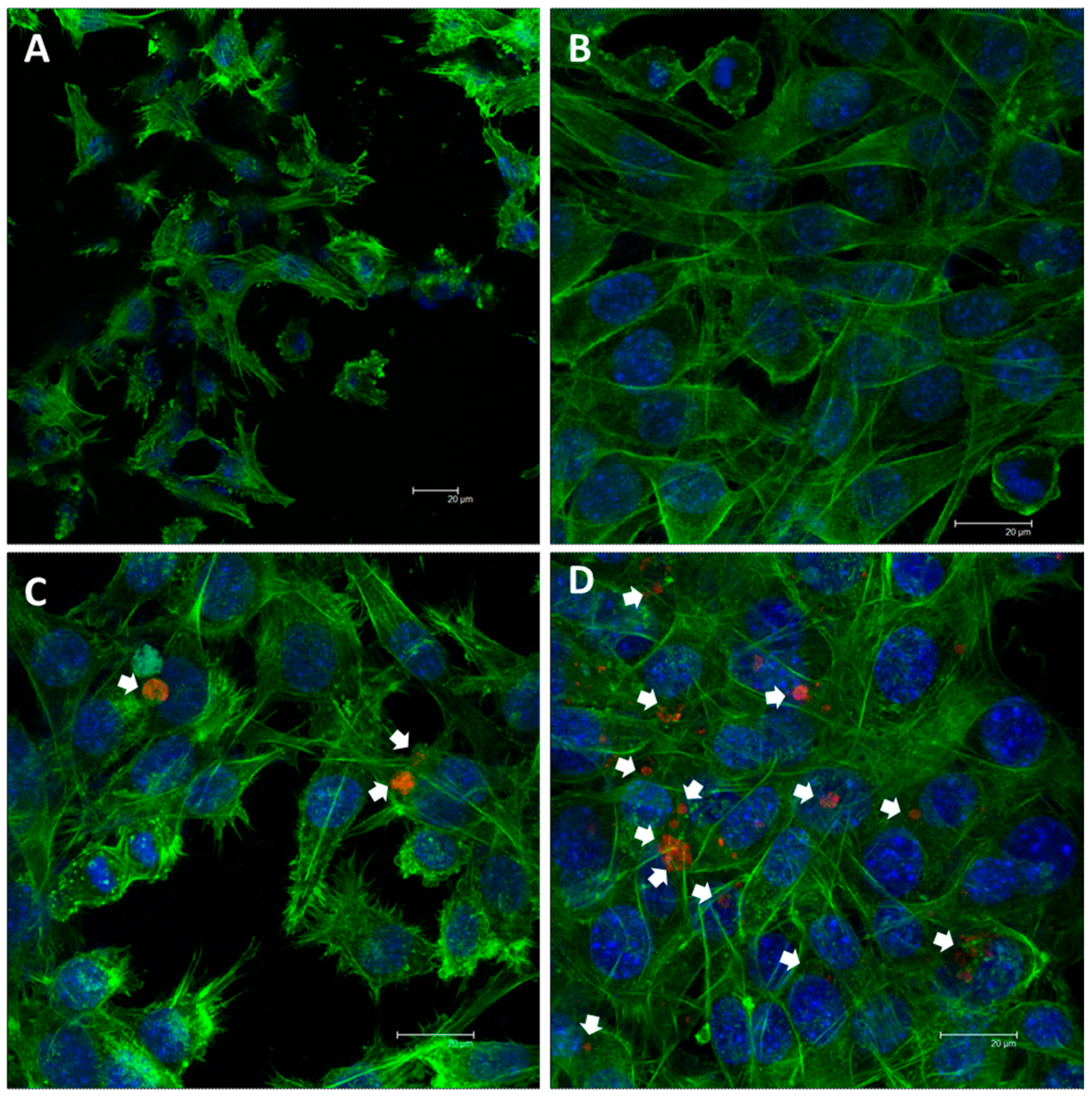
3.3. Cellular Cytocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Margulis, L.; Barreno, E. Looking at lichens. Bioscience 2003, 53, 776. [Google Scholar] [CrossRef]
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding microbial multi-species symbioses. Front. Microbiol. 2016, 18, 180. [Google Scholar] [CrossRef]
- Seckbach, J.; Grube, M. Die Hard: Lichens Stress and developmental strategies in lichens. In Symbioses and Stress: Joint Ventures in Biology; Seckbach, J., Grube, M., Eds.; Springer: Dordrecht, Germany, 2010; pp. 509–523. [Google Scholar]
- del Campo, E.M.; Catalá, S.; Gimeno, J.; del Hoyo, A.; Martínez-Alberola, F.; Casano, L.M.; Grube, M.; Barreno, E. The genetic structure of the cosmopolitan three-partner lichen Ramalina farinacea evidences the concerted diversification of symbionts. FEMS Microbiol. Ecol. 2013, 83, 310–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrović, T.; Stamenković, S.; Cvetković, V.; Nikolic, M.; Tosic, S.; Stojicic, D. Lichens as source of versatile bioactive compounds A brief history of lichen utilization. Biol. Nyssana 2011, 2, 1–6. [Google Scholar]
- Gasulla, F.; Guera, A.; Barreno, E.; Guéra, A.; Barreno, E. A simple and rapid method for isolating lichen photobionts. Symbiosis 2010, 51, 175–179. [Google Scholar] [CrossRef]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. Lichens as micro-ecosystems: Novel approaches to efficiently reveal the hidden diversity of phycobionts in a single thallus. In Book of Abstracts, Proceedings: 8th Congress of the International Symbiosis Society (Symbiotic Lifestyle), Oral Com., Lisbon, Portugal, 12–18 July 2015; Munzi, S., Ulm, F., Eds.; Faculdade de Ciências, Universidade de Lisboa: Lisbon, Portugal, 2015; p. 205. [Google Scholar]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. A multi-tool approach to assess microalgal diversity in lichens: Isolation, Sanger sequencing, HTS and ultrastructural correlations. Lichenologist 2018, 50, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Carballo-Cárdenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- García-Casal, M.N.; Ramírez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2009, 101, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Guedes, A.C.; Giao, M.S.; Seabra, R.; Ferreira, A.C.; Tamagnini, P.; Moradas-Ferreira, P.; Malcata, F.X. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [Green Version]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, I.V.; Bratkovskaia, L.B.; Plekhanov, S.E. Extracellular carbohydrates and polysaccharides of the algae Chlorella pyrenoidosa Chick S-39. Biol. Bull. 2004, 312, 217–224. [Google Scholar]
- Rumin, J.; Gonçalves de Oliveira Junior, R.; Bérard, J.B.; Picot, L. Improving Microalgae Research and Marketing in the European Atlantic Area: Analysis of Major Gaps and Barriers Limiting Sector Development. Mar. Drugs 2021, 19, 319. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Dikshit, A. Lichens: Fungal symbionts and their secondary metabolites. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 107–115. [Google Scholar]
- Muggia, B.L.; Schmitt, I.; Grube, M. Lichens as treasure chests of natural products. SIM News 2009, 59, 85–97. [Google Scholar]
- Amaro, H.; Guedes, A.; Malcata, F. Antimicrobial activities of microalgae: An invited review. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Microbiology Book Series; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1272–1280. [Google Scholar]
- Ranković, B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319133744. [Google Scholar]
- Shrestha, G.; St. Clair, L.L.; O’Neill, K.L. The immunostimulating role of lichen polysaccharides: A review. Phyther. Res. 2015, 29, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Pierce, G.N. The toxicity of dietary trans fats. Food Chem. Toxicol. 2015, 78, 170–176. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; Gonçalves de Oliveira Junior, R.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef]
- Petersen, G.; Rasmussen, D.; Gustavson, K. Study on Enhancing the Endocrine Disrupter Priority List with A Focus on Low Production Volume Chemicals; DG Environment (European Commision): Hørsholm, Denmark, 2007. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products from marine invertebrates and microbes as modulators of antitumor targets. Curr. Drug Targets 2006, 7, 279–304. [Google Scholar] [CrossRef]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Casano, L.M.; Braga, M.R.; Álvarez, R.; del Campo, E.M.; Barreno, E. Differences in the cell walls and extracellular polymers of the two Trebouxia microalgae coexisting in the lichen Ramalina farinacea are consistent with their distinct capacity to immobilize extracellular Pb. Plant Sci. 2015, 236, 195–204. [Google Scholar] [CrossRef]
- del Hoyo, A.; Álvarez, R.; del Campo, E.M.; Gasulla, F.; Barreno, E.; Casano, L.M.; Alvarez, R.; del Campo, E.M.; Gasulla, F.; Barreno, E.; et al. Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Ann. Bot. 2011, 107, 109–118. [Google Scholar] [CrossRef]
- Álvarez, R.; del Hoyo, A.; García-Breijo, F.; Reig-Armiñana, J.; del Campo, E.M.; Guéra, A.; Barreno, E.; Casano, L.M. Different strategies to achieve Pb-tolerance by the two Trebouxia algae coexisting in the lichen Ramalina farinacea. J. Plant Physiol. 2012, 169, 1797–1806. [Google Scholar] [CrossRef]
- Expósito, J.R.; Coello, A.J.; Barreno, E.; Casano, L.M.; Catalá, M. Endogenous NO Is Involved in Dissimilar Responses to Rehydration and Pb(NO3)2 in Ramalina farinacea Thalli and Its Isolated Phycobionts. Microb. Ecol. 2019, 72, 604–616. [Google Scholar] [CrossRef]
- Muggia, L.; Leavitt, S.; Barreno, E. The hidden diversity of lichenised Trebouxiophyceae (Chlorophyta). Phycologia 2018, 57, 503–524. [Google Scholar] [CrossRef] [Green Version]
- Cadoret, J.P.; Garnier, M.; Saint-Jean, B. Microalgae. Functional genomics and biotechnology. Adv. Bot. Res. 2012, 64, 285–341. [Google Scholar]
- Skaloud, P.; Peksa, O. Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta). Mol. Phylogenet. Evol. 2010, 54, 36–46. [Google Scholar] [CrossRef]
- Krewski, D.; Andersen, M.E.; Tyshenko, M.G.; Krishnan, K.; Hartung, T.; Boekelheide, K.; Wambaugh, J.F.; Jones, D.; Whelan, M.; Thomas, R.; et al. Toxicity testing in the 21st century: Progress in the past decade and future perspectives. Arch. Toxicol. 2019, 94, 1–58. [Google Scholar] [CrossRef]
- Goldsmith, S.J.; Thomas, M.A.; Gries, C. A new technique for photobiont culturing and manipulation. Lichenologist 1997, 29, 559–569. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International. In Association of Official Analysis Chemists International; AOAC International: Washington, DC, USA, 1995; ISBN 0935584544. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- García-Plazaola, J.I.; Becerril, J.M. A rapid high-performance liquid chromatography method to measure lipophilic antioxidants in stressed plants: Simultaneous determination of carotenoids and tocopherols. Phytochem. Anal. 1999, 10, 307–313. [Google Scholar] [CrossRef]
- Matesanz, M.-C.; Vila, M.; Feito, M.-J.; Linares, J.; Gonçalves, G.; Vallet-Regi, M.; Marques, P.-A.A.P.; Portolés, M.-T. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials 2013, 34, 1562–1569. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P. Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Herbreteau, F.; Coiffard, L.J.M.; Derrien, A.; De Roeck-Holtzhauer, Y. The Fatty Acid Composition of Five Species of Macroalgae. Bot. Mar. 1997, 40, 25–28. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kimura, F.; Nakashima, Y.; Maruyama, I.; Higuchi, O.; Miyazawa, T. Chlorella is an effective dietary source of lutein for human erythrocytes. J. Oleo Sci. 2013, 62, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.-G.; Peng, H.-Y.; Chu, Y.-C.; Hsieh, Y.-M.; Wang, S.-D.; Chou, S.-T. Anti-invasion and apoptosis induction of chlorella (Chlorella sorokiniana) in Hep G2 human hepatocellular carcinoma cells. J. Funct. Foods 2012, 4, 302–310. [Google Scholar] [CrossRef]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R.A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef]
- Perrone, S.; Tei, M.; Longini, M.; Buonocore, G. The Multiple Facets of Lutein: A Call for Further Investigation in the Perinatal Period. Oxidative Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, I. Ecological and biotechnological aspects of lichens. Appl. Microbiol. Biotechnol. 2006, 73, 723–734. [Google Scholar] [CrossRef]
- Culberson, C.F.; Armaleo, D. Induction of a complete secondary-product pathway in a cultured lichen fungus. Exp. Mycol. 1992, 16, 52–63. [Google Scholar] [CrossRef]
- Shrestha, G.; St. Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Takahashi, M.; Tatsumi, M.; Ohizumi, Y.; Yasumoto, T. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. J. Biol. Chem. 1983, 258, 10944–10949. [Google Scholar] [CrossRef]
- Ohizumi, Y.; Yasumoto, T. Contractile response of the rabbit aorta to maitotoxin, the most potent marine toxin. J. Physiol. 1983, 337, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Rho, M.-C.; Nakahata, N.; Nakamura, H.; Murai, A.; Ohizumi, Y. Activation of rabbit platelets by Ca2+ influx and thromboxane A2 release in an external Ca2+-dependent manner by zooxanthellatoxin-A, a novel polyol. Br. J. Pharmacol. 1995, 115, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Moriya, T.; Ishida, Y.; Nakamura, H.; Asari, T.; Murai, A.; Ohizumi, Y. Vasoconstriction induced by zooxanthellatoxin-B, a polyoxygenated long-chain product from a marine alga. Eur. J. Pharmacol. 1998, 350, 59–65. [Google Scholar] [CrossRef]
- Meilhoc, E.; Boscari, A.; Bruand, C.; Puppo, A.; Brouquisse, R. Nitric oxide in legume-rhizobium symbiosis. Plant Sci. 2011, 181, 573–581. [Google Scholar] [CrossRef]
- Gordon, B.R.; Leggat, W. Symbiodinium-invertebrate symbioses and the role of metabolomics. Mar. Drugs 2010, 8, 2546–2568. [Google Scholar] [CrossRef] [Green Version]
- Amaro, H.M.; Barros, R.; Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Microalgal compounds modulate carcinogenesis in the gastrointestinal tract. Trends Biotechnol. 2013, 31, 92–98. [Google Scholar] [CrossRef]
- Tanaka, K.; Konishi, F.; Himeno, K.; Taniguchi, K.; Nomoto, K. Augmentation of antitumor resistance by a strain of unicellular green algae, Chlorella vulgaris. Cancer Immunol. Immunother. 1984, 17, 90–94. [Google Scholar] [CrossRef]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- Vichi, S.; Lavorini, P.; Funari, E.; Scardala, S.; Testai, E. Contamination by Microcystis and microcystins of blue-green algae food supplements (BGAS) on the Italian market and possible risk for the exposed population. Food Chem. Toxicol. 2012, 50, 4493–4499. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [Green Version]
| Composition | Concentration (%) * |
|---|---|
| Moisture | 75 ± 1 |
| Ash | 5 ± 1 |
| Protein | 37 ± 1 |
| Lipids | 10 ± 2 |
| Pigment | Concentration (µg/g Microalga Dry Weight) |
|---|---|
| Chlorophyll a | 507 ± 41 |
| Chlorophyll b | 6105 ± 580 |
| β-Carotene | 268 ± 20 |
| Neoxanthin | 165 ± 12 |
| Fucoxanthin | n.d. (<0.058) |
| Violaxanthin | 267 ± 16 |
| Antheraxanthin | 57 ± 6 |
| Astaxanthin | 106 ± 9 |
| Zeaxanthin | n.d. (<0.079) |
| Lutein | 1211 ± 119 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matesanz, M.C.; Villa-Carvajal, M.; Linares, J.; Morante-Zarcero, S.; Sierra, I.; Barreno, E.; Catalá, M.; Portolés, M.T. Chemical Characterization of the Lichen-Symbiont Microalga Asterochloris erici and Study of Its Cytostatic Effect on the L929 Murine Fibrosarcoma Cell Line. Processes 2021, 9, 1509. https://doi.org/10.3390/pr9091509
Matesanz MC, Villa-Carvajal M, Linares J, Morante-Zarcero S, Sierra I, Barreno E, Catalá M, Portolés MT. Chemical Characterization of the Lichen-Symbiont Microalga Asterochloris erici and Study of Its Cytostatic Effect on the L929 Murine Fibrosarcoma Cell Line. Processes. 2021; 9(9):1509. https://doi.org/10.3390/pr9091509
Chicago/Turabian StyleMatesanz, M. Concepción, Mercedes Villa-Carvajal, Javier Linares, Sonia Morante-Zarcero, Isabel Sierra, Eva Barreno, Myriam Catalá, and M. Teresa Portolés. 2021. "Chemical Characterization of the Lichen-Symbiont Microalga Asterochloris erici and Study of Its Cytostatic Effect on the L929 Murine Fibrosarcoma Cell Line" Processes 9, no. 9: 1509. https://doi.org/10.3390/pr9091509
APA StyleMatesanz, M. C., Villa-Carvajal, M., Linares, J., Morante-Zarcero, S., Sierra, I., Barreno, E., Catalá, M., & Portolés, M. T. (2021). Chemical Characterization of the Lichen-Symbiont Microalga Asterochloris erici and Study of Its Cytostatic Effect on the L929 Murine Fibrosarcoma Cell Line. Processes, 9(9), 1509. https://doi.org/10.3390/pr9091509