Assessment of the Effective Impact of Bisphenols on Mitochondrial Activity, Viability and Steroidogenesis in a Dose-Dependency in Human Adrenocortical Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. H295R Cell Culture and Treatment
2.2. Mitochondrial Activity Assay
2.3. Triple Assay
2.4. Evaluation of Testosterone and Progesterone Production
2.5. Statistical Analysis
3. Results
3.1. Mitochondrial Activity
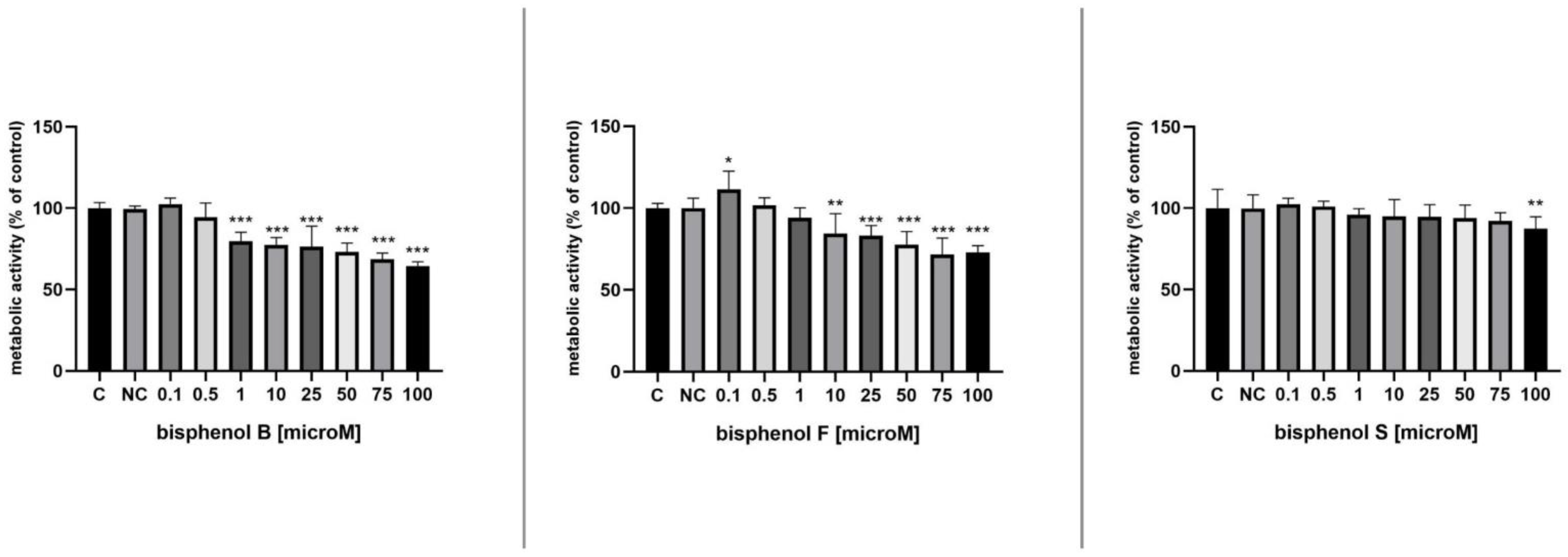
3.2. Metabolic Activity
3.3. Membrane Integrity
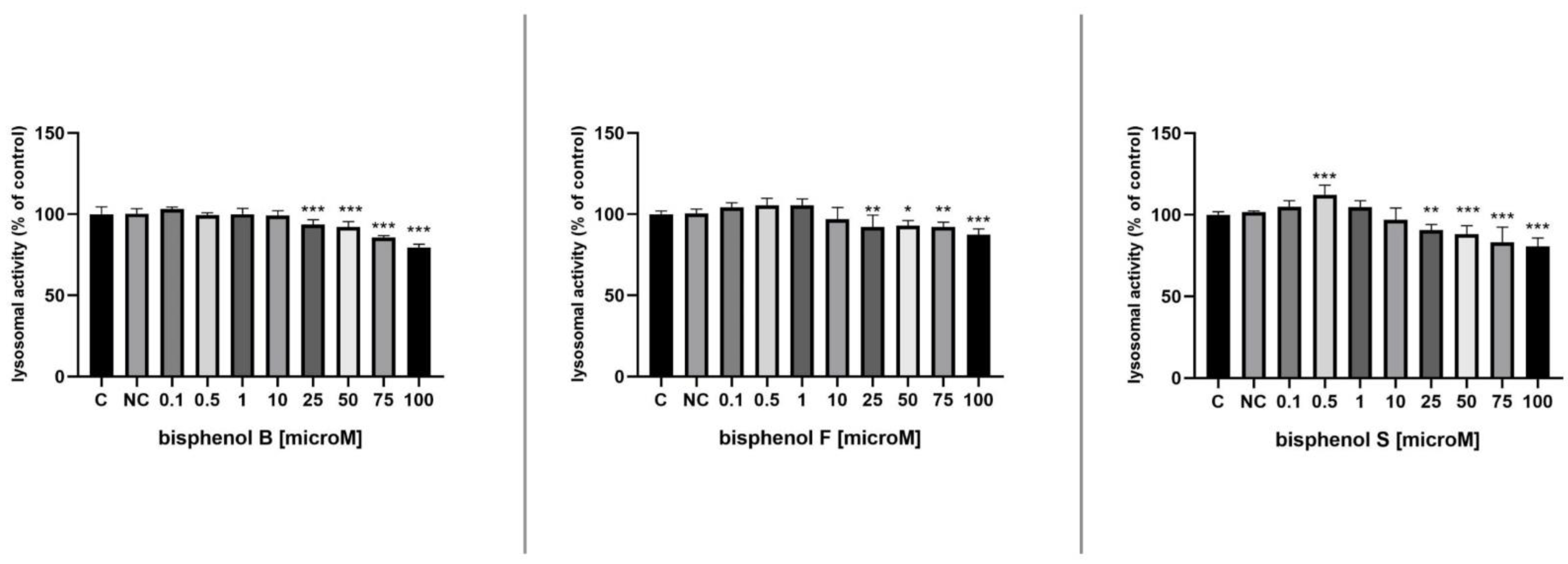
3.4. Lysosomal Activity
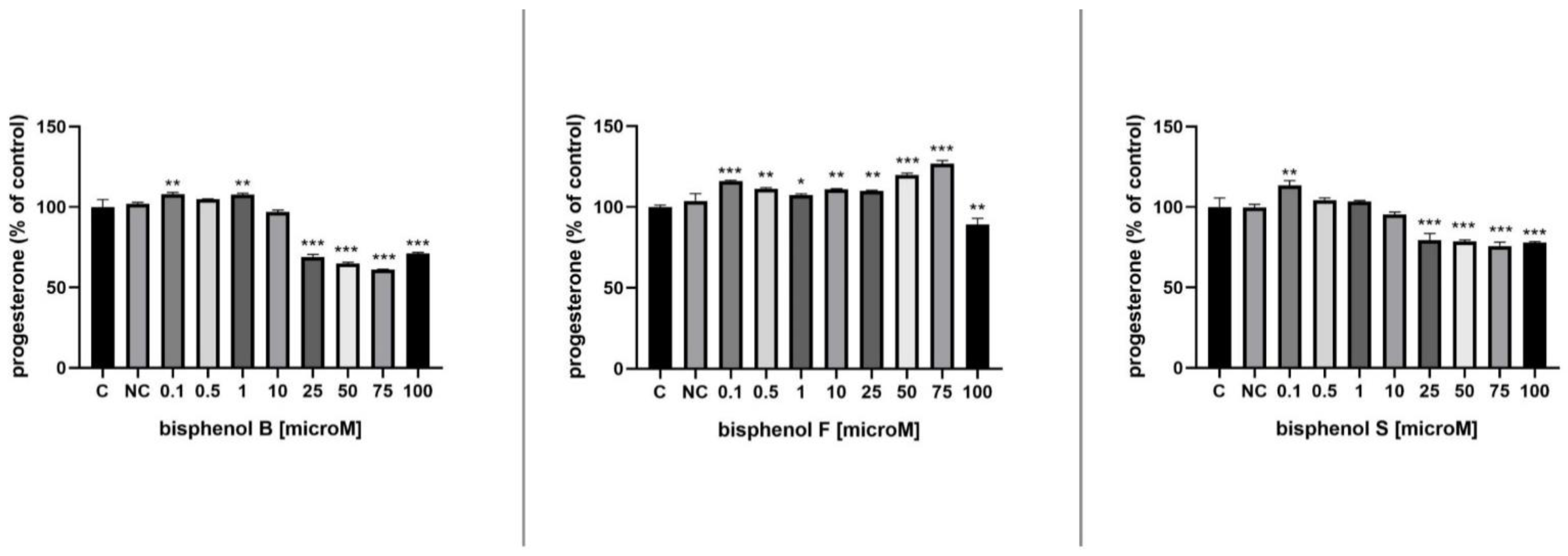
3.5. Progesterone Secretion
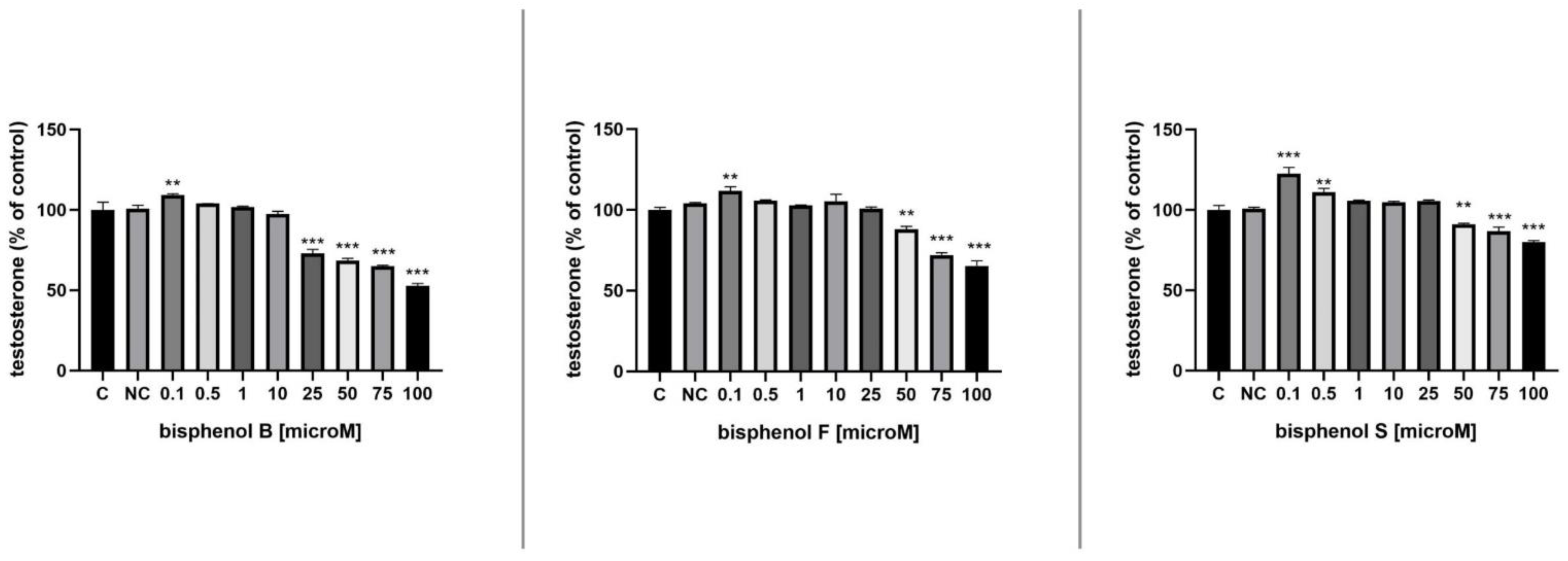
3.6. Testosterone Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanderson, J.T. The Steroid Hormone Biosynthesis Pathway as a Target for Endocrine-Disrupting Chemicals. Toxicol. Sci. 2006, 94, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Jambor, T.; Kovacikova, E.; Greifova, H.; Kovacik, A.; Libova, L.; Lukac, N. Assessment of the effective impact of bisphenols on mitochondrial activity and steroidogenesis in a dose-dependency in mice TM3 Leydig cells. Physiol. Res. 2019, 68, 689–693. [Google Scholar] [CrossRef]
- Reif, D.M.; Martin, M.T.; Tan, S.W.; Houck, K.A.; Judson, R.S.; Richard, A.M.; Knudsen, T.B.; Dix, D.J.; Kavlock, R.J. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ. Health Perspect. 2010, 118, 1714–1720. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Jiao, Z.; Shi, J.; Li, M.; Guo, Q.; Shao, B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere 2016, 147, 9–19. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, H.; Wiseman, S.; He, Y.; Higley, E.; Jones, P.; Wong, C.K.; Al-Khedhairy, A.; Giesy, J.P.; Hecker, M. Bisphenol A disrupts steroidogenesis in human H295R cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 121, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Alomirah, H.; Cho, H.S.; Li, Y.F.; Liao, C.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Ren, N.; Kannan, K. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ. Sci. Technol. 2011, 45, 7044–7050. [Google Scholar] [CrossRef]
- Gonçalves, G.D.; Semprebon, S.C.; Biazi, B.I.; Mantovani, M.S.; Fernandes, G. Bisphenol A reduces testosterone production in TM3 Leydig cells independently of its effects on cell death and mitochondrial membrane potential. Reprod. Toxicol. 2018, 76, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, D.; Pogrmic-Majkic, K.; Fa, S.; Stanic, B.; Jasnic, J.; Andric, N. Bisphenol A decreases progesterone synthesis by disrupting cholesterol homeostasis in rat granulosa cells. Mol. Cell. Endocrinol. 2018, 461, 55–63. [Google Scholar] [CrossRef]
- Moral, R.; Wang, R.; Russo, I.H.; Lamartiniere, C.A.; Pereira, J.; Russo, J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J. Endocrinol. 2008, 196, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007, 14, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Chapin, R.E.; Adams, J.; Boekelheide, K.; Gray, L.E.; Hayward, S.W., Jr.; Lees, P.S.; McIntyre, B.S.; Portier, K.M.; Schnorr, T.M.; Selevan, S.G.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2008, 83, 157–395. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Beronius, A.; Rudén, C.; Håkansson, H.; Hanberg, A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol A. Reprod. Toxicol. 2010, 29, 132–146. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Bucher, J.R.; Collman, G.W.; Zeldin, D.C.; Johnson, A.F.; Schug, T.T.; Heindel, J.J. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ. Health Perspect. 2012, 120, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Su, X.; Yan, H.; Sun, Z. Oral administration of low-dose bisphenol A promotes proliferation of ventral prostate and upregulates prostaglandin D2 synthase expression in adult rats. Toxicol. Ind. Health 2016, 32, 1848–1858. [Google Scholar] [CrossRef]
- Wang, F.; Hua, J.; Chen, M.; Xia, Y.; Zhang, Q.; Zhao, R.; Zhou, W.; Zhang, Z.; Wang, B. High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup. Environ. Med. 2012, 69, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Heinälä, M.; Ylinen, K.; Tuomi, T.; Santonen, T.; Porras, S.P. Assessment of Occupational Exposure to Bisphenol A in Five Different Production Companies in Finland. Ann. Work Expo. Health 2017, 61, 44–55. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Rosenschöld, J.M.A.; Honkela, N.; Hukkinen, J.I. Addressing the temporal fit of institutions: The regulation of endocrine-disrupting chemicals in Europe. Ecol. Soc. 2014, 19, 30. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [Green Version]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Analysis of bisphenols in soft drinks by on-line solid phase extraction fast liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2011, 683, 227–233. [Google Scholar] [CrossRef]
- Becerra, V.; Odermatt, J. Detection and quantification of traces of bisphenol A and bisphenol S in paper samples using analytical pyrolysis-GC/MS. Analyst 2012, 137, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Campillo, N.; Martínez-Castillo, N.; Hernández-Córdoba, M. Comparison of two derivatization-based methods for solid-phase microextraction-gas chromatography-mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal. Bioanal. Chem. 2010, 397, 115–125. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Zhang, J.; Goodyer, C.G.; Hayward, S.; Cooke, G.M.; Curran, I.H. Bisphenol A in human placental and fetal liver tissues collected from Greater Montreal area (Quebec) during 1998–2008. Chemosphere 2012, 89, 505–511. [Google Scholar] [CrossRef]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef]
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; van Vugt-Lussenburg, B.M.A.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 139, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.B.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecker, M.; Giesy, J.P. Novel trends in endocrine disruptor testing: The H295R Steroidogenesis Assay for identification of inducers and inhibitors of hormone production. Anal. Bioanal. Chem. 2008, 390, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Knazicka, Z.; Forgacs, Z.; Lukacova, J.; Roychoudhury, S.; Massanyi, P.; Lukac, N. Endocrine disruptive effects of cadmium on steroidogenesis: Human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2015, 50, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Oie, H.K.; Shackleton, C.H.; Chen, T.R.; Triche, T.J.; Myers, C.E.; Chrousos, G.P.; Brennan, M.F.; Stein, C.A.; La Rocca, R.V. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990, 50, 5488–5496. [Google Scholar] [PubMed]
- Rainey, W.E.; Saner, K.; Schimmer, B.P. Adrenocortical cell lines. Mol. Cell. Endocrinol. 2004, 228, 23–38. [Google Scholar] [CrossRef]
- Strajhar, P.; Tonoli, D.; Jeanneret, F.; Imhof, R.M.; Malagnino, V.; Patt, M.; Kratschmar, D.V.; Boccard, J.; Rudaz, S.; Odermatt, A. Steroid profiling in H295R cells to identify chemicals potentially disrupting the production of adrenal steroids. Toxicology 2017, 381, 51–63. [Google Scholar] [CrossRef]
- OECD. Test No. 456: H295R Steroidogenesis Assay; OECD: Paris, France, 2011. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Schirmer, K.; Chan, A.G.; Greenberg, B.M.; Dixon, D.G.; Bols, N.C. Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 1997, 11, 107–119. [Google Scholar] [CrossRef]
- Yu, P.L.; Lin, H.W.; Wang, S.W.; Wang, P.S. Effects of nonylphenol on the production of progesterone on the rats granulosa cells. J. Cell. Biochem. 2011, 112, 2627–2636. [Google Scholar] [CrossRef]
- Desdoits-Lethimonier, C.; Albert, O.; Le Bizec, B.; Perdu, E.; Zalko, D.; Courant, F.; Lesné, L.; Guillé, F.; Dejucq-Rainsford, N.; Jégou, B. Human testis steroidogenesis is inhibited by phthalates. Hum. Reprod. 2012, 27, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Goldinger, D.M.; Demierre, A.L.; Zoller, O.; Rupp, H.; Reinhard, H.; Magnin, R.; Becker, T.W.; Bourqui-Pittet, M. Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul. Toxicol. Pharmacol. 2015, 71, 453–462. [Google Scholar] [CrossRef]
- Lan, H.C.; Wu, K.Y.; Lin, I.W.; Yang, Z.J.; Chang, A.A.; Hu, M.C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 2017, 185, 237–246. [Google Scholar] [CrossRef]
- Huang, M.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere 2020, 253, 126707. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, L.; Yang, J.; Gao, X.; Zhang, W. Bisphenol A decreases progesterone synthesis in human ovarian granulosa cells. Birth Defects Res. 2020, 112, 1843–1849. [Google Scholar] [CrossRef]
- Roelofs, M.J.; van den Berg, M.; Bovee, T.F.; Piersma, A.H.; van Duursen, M.B. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology 2015, 329, 10–20. [Google Scholar] [CrossRef]
- Pogrmic-Majkic, K.; Samardzija Nenadov, D.; Fa, S.; Stanic, B.; Trninic Pjevic, A.; Andric, N. BPA activates EGFR and ERK1/2 through PPARγ to increase expression of steroidogenic acute regulatory protein in human cumulus granulosa cells. Chemosphere 2019, 229, 60–67. [Google Scholar] [CrossRef]
- Amar, S.; Binet, A.; Téteau, O.; Desmarchais, A.; Papillier, P.; Lacroix, M.Z.; Maillard, V.; Guérif, F.; Elis, S. Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro. Int. J. Mol. Sci. 2020, 21, 1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocco, D.M.; Wang, X.; Jo, Y.; Manna, P.R. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, P.W.; Yang, Z.J.; Huang, H.H.; Chang, A.A.; Cheng, Y.C.; Wu, G.J.; Lan, H.C. Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol. Reprod. 2018, 98, 250–258. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knížatová, N.; Greifová, H.; Tokárová, K.; Jambor, T.; Binkowski, Ł.J.; Lukáč, N. Assessment of the Effective Impact of Bisphenols on Mitochondrial Activity, Viability and Steroidogenesis in a Dose-Dependency in Human Adrenocortical Carcinoma Cells. Processes 2021, 9, 1471. https://doi.org/10.3390/pr9081471
Knížatová N, Greifová H, Tokárová K, Jambor T, Binkowski ŁJ, Lukáč N. Assessment of the Effective Impact of Bisphenols on Mitochondrial Activity, Viability and Steroidogenesis in a Dose-Dependency in Human Adrenocortical Carcinoma Cells. Processes. 2021; 9(8):1471. https://doi.org/10.3390/pr9081471
Chicago/Turabian StyleKnížatová, Nikola, Hana Greifová, Katarína Tokárová, Tomáš Jambor, Łukasz J. Binkowski, and Norbert Lukáč. 2021. "Assessment of the Effective Impact of Bisphenols on Mitochondrial Activity, Viability and Steroidogenesis in a Dose-Dependency in Human Adrenocortical Carcinoma Cells" Processes 9, no. 8: 1471. https://doi.org/10.3390/pr9081471
APA StyleKnížatová, N., Greifová, H., Tokárová, K., Jambor, T., Binkowski, Ł. J., & Lukáč, N. (2021). Assessment of the Effective Impact of Bisphenols on Mitochondrial Activity, Viability and Steroidogenesis in a Dose-Dependency in Human Adrenocortical Carcinoma Cells. Processes, 9(8), 1471. https://doi.org/10.3390/pr9081471






