Titanium Dioxide/Activated Carbon Electrode with Polyurethane Binder for the Removal of Indium Ions via Capacitive Deionization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Preparation of Polyurethane Elastomer
2.3. Microwave-Assisted Ionothermal Synthesis of TiO2/AC Composite
2.4. Fabrication of the Electrodes
2.5. Electrochemical Characterization
2.6. CDI Performance
3. Results
3.1. Characterization of the Electrode Materials
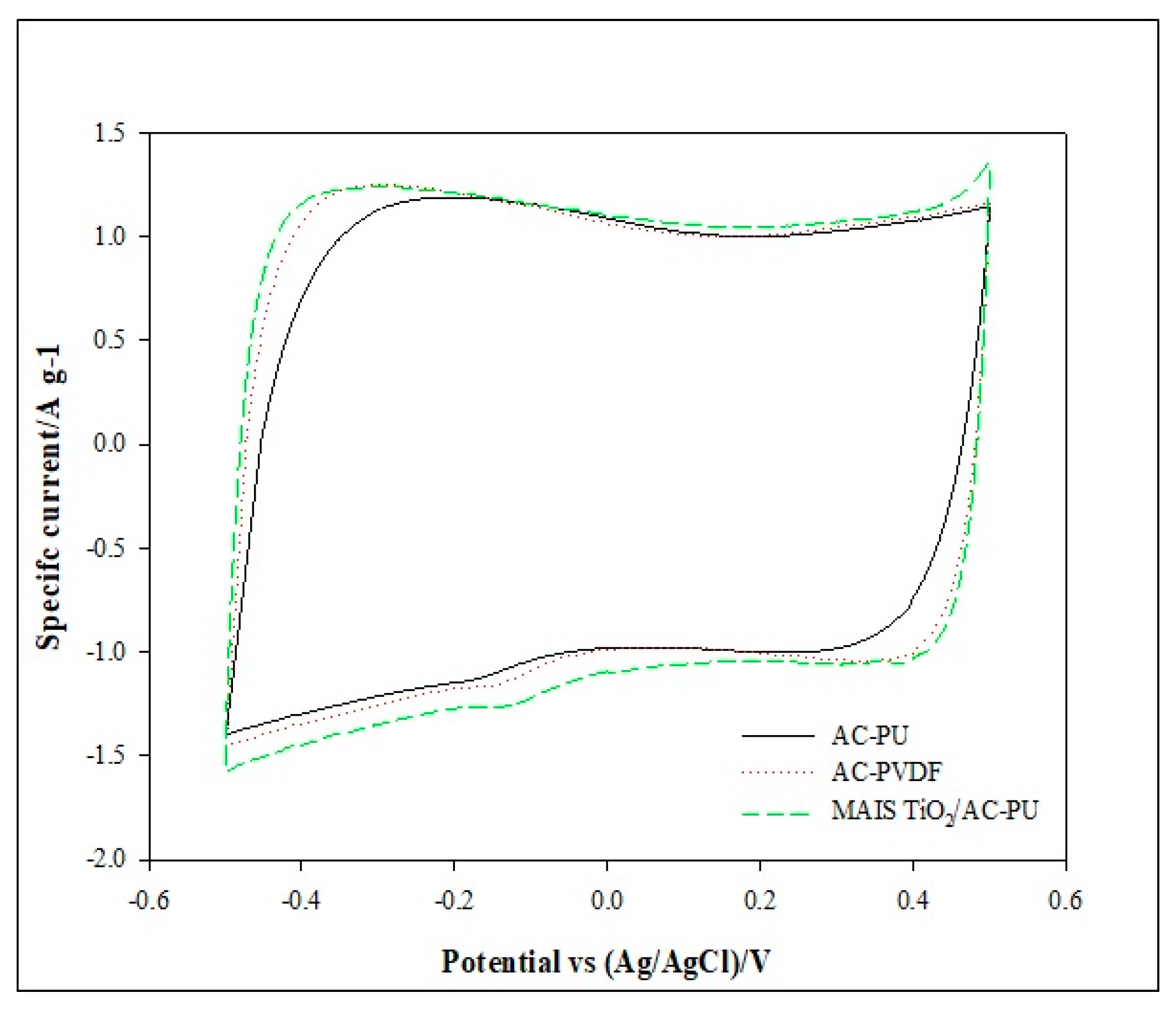
3.2. Cyclic Voltammetry Analysis of the Electrodes
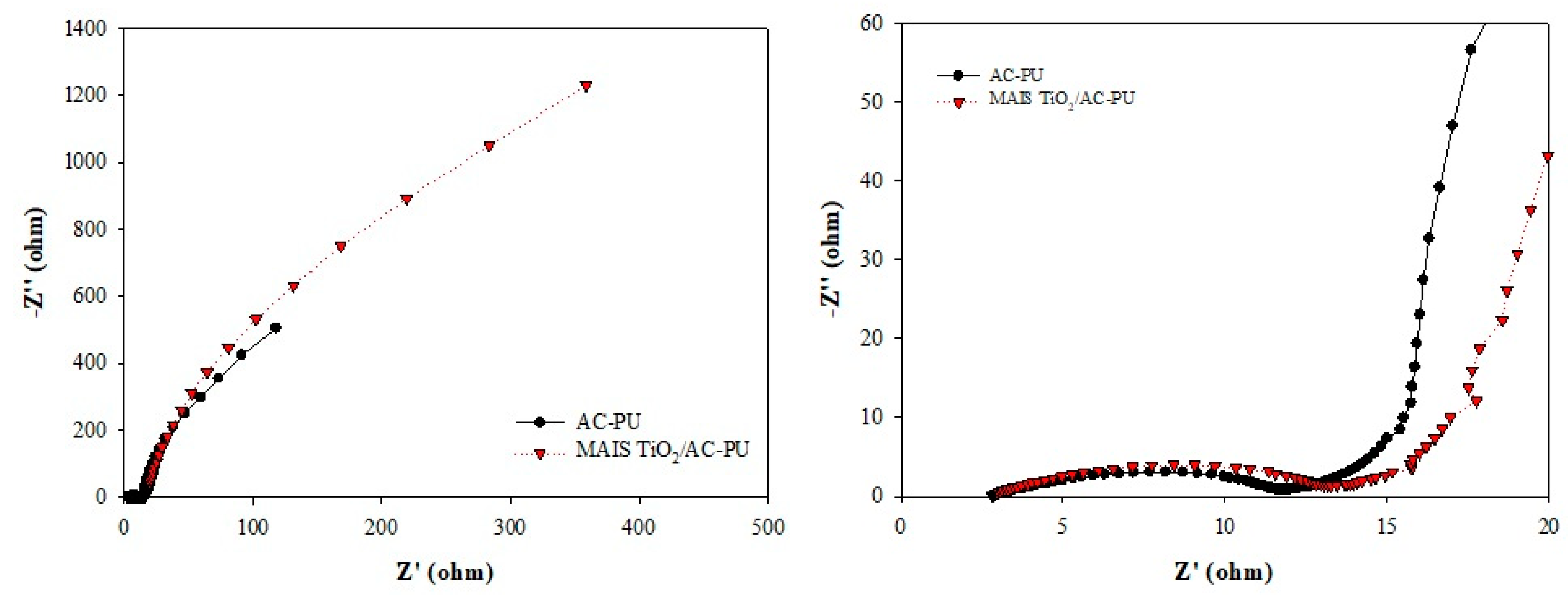
3.3. Electrochemical Impedance Spectroscopy Analysis of the Electrodes
3.4. CDI Performance of the MAIS TiO2/AC-PU Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of capacitive deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, K.; Zhang, X.; Zhang, C.; Liang, P. Selective ion separation by capacitive deionization (CDI) based technologies: A state-of-the-art review. Environ. Sci. Water Res. Technol. 2020, 6, 243–257. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Nesaraj, A.S.; Noel, M. Review on carbon-based electrode materials for application in capacitive deionization process. Int. J. Environ. Sci. Technol. 2016, 13, 2961–2976. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Lu, L.; Cai, Z.; Ren, Z.J. Individual and competitive removal of heavy metals using capacitive deionization. J. Hazard. Mater. 2016, 302, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Fan, C.S.; Hou, C.H. Electro-enhanced removal of copper ions from aqueous solutions by capacitive deionization. J. Hazard. Mater. 2014, 278, 8–15. [Google Scholar] [CrossRef]
- Liu, L.; Guo, X.; Tallon, R.; Huang, X.; Chen, J. Highly porous N-doped graphene nanosheets for rapid removal of heavy metals from water by capacitive deionization. Chem. Commun. 2017, 53, 881–884. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.Y.; Wu, S.W.; Hou, C.H. Exploring the electrosorption selectivity and recovery of indium ions with capacitive deionization in acidic solution. J. Colloid Interface Sci. 2021, 586, 819–829. [Google Scholar] [CrossRef]
- Liu, H.M.; Wu, C.C.; Lin, Y.H.; Chiang, C.K. Recovery of indium from etching wastewater using supercritical carbon dioxide extraction. J. Hazard. Mater. 2009, 172, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Mishra, C.; Hong, H.S.; Cho, S.S. Treatment of indium-tin-oxide etching wastewater and recovery of In, Mo, Sn and Cu by liquid–liquid extraction and wet chemical reduction: A laboratory scale sustainable commercial green process. Green Chem. 2015, 17, 4418–4431. [Google Scholar] [CrossRef]
- Hasegawa, H.; Rahman, I.M.M.; Umehara, Y.; Sawai, H.; Maki, T.; Furusho, Y.; Mizutani, S. Selective recovery of indium from the etching waste solution of the flat-panel display fabrication process. Microchem. J. 2013, 110, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Chou, W.S.; Shen, Y.H.; Yang, S.J.; Hsiao, T.C.; Huang, L.F. Recovery of indium from the etching solution of indium tin oxide by solvent extraction. Environ. Prog. Sustain. Energy 2016, 35, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Hsu, K.U.; Hu, Y.T.; Chu, C.P.; Chung, Y.R. Current investigation and control of new pollutants in wastewater and recycled water (translated from Chinese). Sinotech Eng. 2014, 123, 3–15. [Google Scholar]
- Atanacio, A.J.; Bak, T.; Nowotny, J. Effect of indium segregation on the surface versus bulk chemistry for indium-doped TiO2. ACS Appl. Mater. Interfaces 2012, 4, 6626–6634. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Guo, X.; Yuan, Z.; Zhao, Z. Separation and preconcentration of trace indium(III) from environmental samples with nanometer-size titanium dioxide. Hydrometallurgy 2009, 95, 92–95. [Google Scholar] [CrossRef]
- Hang, Y.; Qin, Y.; Jiang, Z.; Hu, B. Study on the adsorption behavior of Ga, In and Tl on nanometer-size titanium dioxide by ICP-AES. Spectrosc. Spect. Anal. 2005, 25, 1131–1134. [Google Scholar]
- Choi, J.Y.; Choi, J.H. A carbon electrode fabricated using a poly(vinylidene fluoride) binder controlled the Faradaic reaction of carbon powder. J. Ind. Eng. Chem. 2010, 16, 401–405. [Google Scholar] [CrossRef]
- Fang, C.H.; Liu, P.I.; Chung, L.C.; Shao, H.; Ho, C.H.; Chen, R.S.; Fan, H.T.; Liang, T.M.; Chang, M.C.; Horng, R.Y. A flexible and hydrophobic polyurethane elastomer used as binder for the activated carbon electrode in capacitive deionization. Desalination 2016, 399, 34–39. [Google Scholar] [CrossRef]
- Liu, P.I.; Chung, L.C.; Shao, H.; Liang, T.M.; Horng, R.Y.; Ma, C.C.M.; Chang, M.C. Microwave-assisted ionothermal synthesis of nanostructured anatase titanium dioxide/activated carbon composite as electrode material for capacitive deionization. Electrochim. Acta 2013, 96, 173–179. [Google Scholar] [CrossRef]
- Liu, P.I.; Chung, L.C.; Ho, C.H.; Shao, H.; Liang, T.M.; Horng, R.Y.; Chang, M.C.; Ma, C.C.M. Effects of activated carbon characteristics on the electrosorption capacity of titanium dioxide/activated carbon composite electrode materials prepared by a microwave-assisted ionothermal synthesis method. J. Colloid Interface Sci. 2015, 446, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.S.; Keesman, K.J.; Kaskel, S.; Biesheuvel, P.M.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yan, T.; Wang, Z.; Zhang, J.; Shi, L.; Zhang, D. Removal of NaCl from saltwater solutions using micro/mesoporous carbon sheets derived from watermelon peel via deionization capacitors. RSC Adv. 2017, 7, 4297–4305. [Google Scholar] [CrossRef] [Green Version]
- Engineering ToolBox. Relative Permittivity—the Dielectric Constant. 2010. Available online: https://www.engineeringtoolbox.com/relative-permittivity-d_1660.html (accessed on 6 August 2021).
- Khan, T.A.; Nazir, M.; Khan, E.A.; Riaz, U. Multiwalled carbon nanotube-polyurethane (MWCNT/PU) composite adsorbent for safranin T and Pb(II) removal from aqueous solution: Batch and fixed-bed studies. J. Mol. Liq. 2015, 212, 467–479. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; pp. 436–442. [Google Scholar]
- Nowotny, J.; Alim, M.A.; Bak, T.; Atanacio, A.J.; Malik, A. Electrical properties and defect chemistry of indium-doped TiO2. Thermoelectric power. Ionics 2015, 21, 2019–2029. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, J.; Bak, T.; Ionescu, M.; Alim, M.A. Electrical Properties and Defect Chemistry of In-Doped TiO2 in Terms of the Jonker Formalism. J. Phys. Chem. A 2015, 119, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Atanacio, A.J.; Alim, M.A.; Bak, T.; Ionescu, M.; Nowotny, J. Segregation in Titanium Dioxide Co-Doped with Indium and Niobium. J. Am. Ceram. Soc. 2017, 100, 419–428. [Google Scholar] [CrossRef] [Green Version]
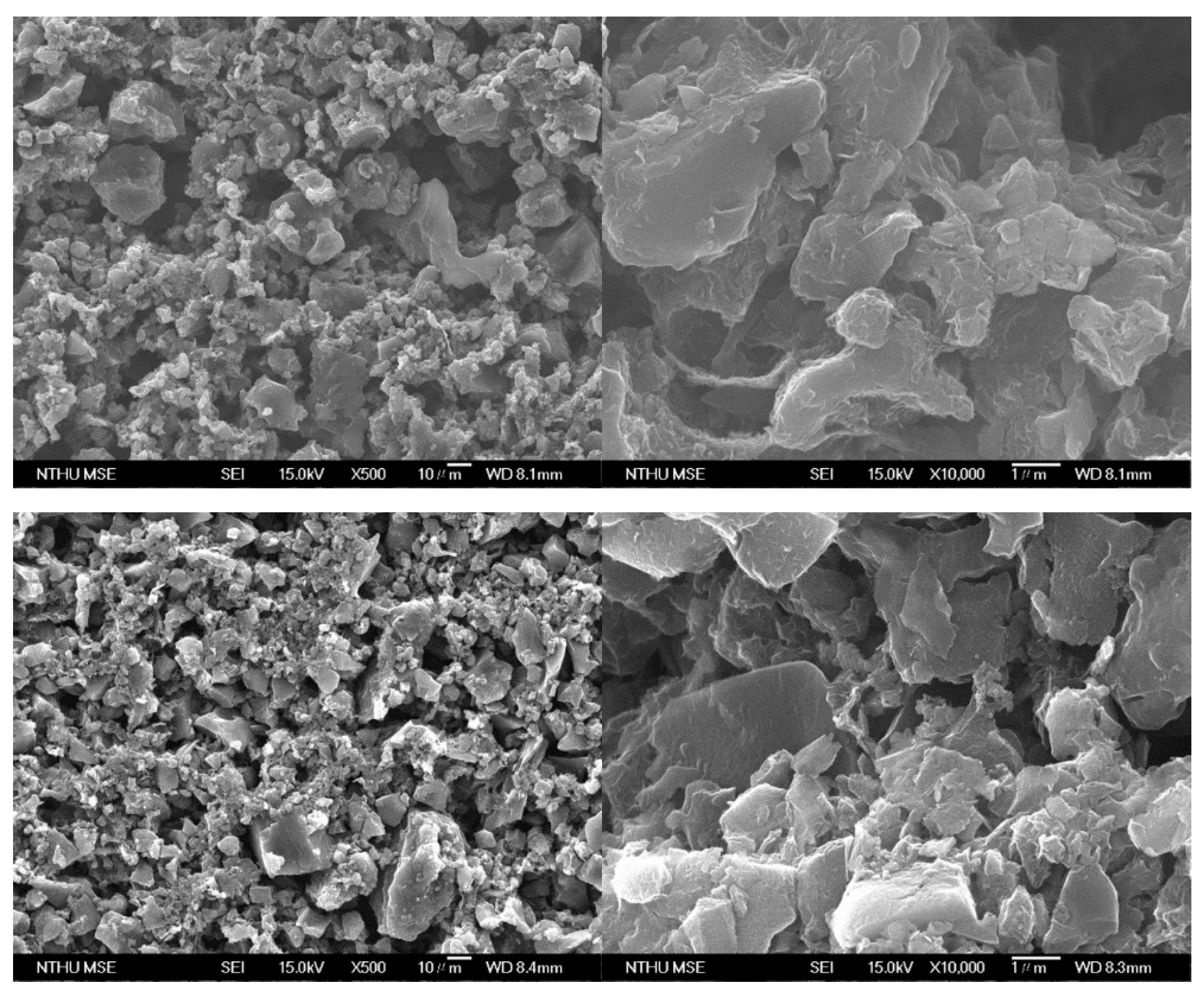

| Specific Surface Area | Total Pore Volume | Micropore Volume (<2 nm) | Mesopore Volume (2–50 nm) | |
|---|---|---|---|---|
| AC | 2715 m2/g | 1.55 cm3/g | 0.06 cm3/g | 1.10 cm3/g |
| CDI Electrode | Average Capacitance (F/g) |
|---|---|
| AC-PU | 122.4 ± 4.3 |
| AC-PVDF | 129.6 ± 2.3 |
| MAIS TiO2/AC-PU | 135.2 ± 4.0 |
| Electrode Type | Initial Concentration (ppm) | Final Concentration (ppm) | Final pH 1 | Electrosorption Capacity (mg/g) | Removal Efficiency (%) |
|---|---|---|---|---|---|
| MAIS TiO2/AC-PU | 0 | 0 | 4.6 | n/a | n/a |
| MAIS TiO2/AC-PU | 1.2 | 0.2 | 4.7 | 0.4 | 84.8 |
| MAIS TiO2/AC-PU | 5.3 | 0.8 | 5.6 | 2.5 | 84.3 |
| MAIS TiO2/AC-PU | 10.3 | 2.1 | 4.5 | 4.6 | 79.6 |
| MAIS TiO2/AC-PU | 23.5 | 13.6 | 3.9 | 5.4 | 42.2 |
| MAIS TiO2/AC-PU | 48.8 | 30.3 | 3.5 | 7.5 | 37.9 |
| AC-PU | 10.2 | precipitated | 7.8 | n/a | n/a |
| AC-PVDF | 12.3 | precipitated | 7.5 | n/a | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.H.; Chuang, S.-H.; Huang, R.-Y.; Liu, P.-I.; Chang, M.-C.; Horng, R.-Y. Titanium Dioxide/Activated Carbon Electrode with Polyurethane Binder for the Removal of Indium Ions via Capacitive Deionization. Processes 2021, 9, 1427. https://doi.org/10.3390/pr9081427
Yen CH, Chuang S-H, Huang R-Y, Liu P-I, Chang M-C, Horng R-Y. Titanium Dioxide/Activated Carbon Electrode with Polyurethane Binder for the Removal of Indium Ions via Capacitive Deionization. Processes. 2021; 9(8):1427. https://doi.org/10.3390/pr9081427
Chicago/Turabian StyleYen, Clive H., Shun-Hsing Chuang, Ren-Yi Huang, Po-I Liu, Min-Chao Chang, and Ren-Yang Horng. 2021. "Titanium Dioxide/Activated Carbon Electrode with Polyurethane Binder for the Removal of Indium Ions via Capacitive Deionization" Processes 9, no. 8: 1427. https://doi.org/10.3390/pr9081427
APA StyleYen, C. H., Chuang, S.-H., Huang, R.-Y., Liu, P.-I., Chang, M.-C., & Horng, R.-Y. (2021). Titanium Dioxide/Activated Carbon Electrode with Polyurethane Binder for the Removal of Indium Ions via Capacitive Deionization. Processes, 9(8), 1427. https://doi.org/10.3390/pr9081427






