Abstract
Attachment of particles and droplets to bubbles—the latter being of various fine sizes and created by different techniques (as described in detail)—forms the basis of flotation, a process which indeed was originated from mineral processing. Nevertheless, chemistry often plays a significant role in this area, in order for separation to be effective, as stressed. This (brief) review particularly discusses wastewater treatment applications and the effect of bubble size (from nano- to micro-) on the flotation process.
1. Flotation—Introduction
Flotation originated initially from the mineral-processing field, since for many years various particulate solids (besides minerals) have been extracted from water by using this effective separation method [1]. Flotation, depending on the method used to generate the bubbles, is classified as follows: dispersed-air flotation (DispAF), dissolved-air flotation (DAF), and electroflotation [2,3,4]. In DispAF, compressed air is forced through pores of sintered-glass discs in order to produce bubbles with diameters usually ranging from 75 to 655 μm. In DAF, air is first pressurized with water into a saturation vessel and then is released through needle valves into the flotation cell at atmospheric pressure. After the pressure is reduced, the air transfers out of solution and forms bubbles that rise to the surface of the liquid. The reported typical diameter range for bubbles generated using DAF is 10–120 μm, with a mean of 40 μm. In electroflotation, water is split into its molecular constituents by applying a current to the solution that is being treated. Bubbles of H2 are formed at the cathode and bubbles of O2 are formed at the anode. This method generates bubble diameters ranging from 22 to 50 μm, depending on the experimental conditions [2].
“Particle” attachment to air bubbles is the basis of the flotation process. These flotation applications mainly include the treatment of wastewater, but also bacteria, coal, clays, corn, resins, proteins, fats, rubber, dyes, glass, plastics, fruit juices, cane sugar, etc. Another possible scope constitutes the recovery of useful valuable minerals, contributing to recycling with environmental technology; for instance, arsenic-rich auriferous pyrite concentrates, often stockpiled in the mine area [5]. Additionally, solutions containing low concentrations of heavy metal could be produced from any industrial processes and it is important to remove these ions from aqueous solution due to environmental regulations [6,7]. For many of these applications, the process is best carried out by the dissolved-air generation method, rather than the dispersed-air one (typically in machines/cells or columns) used for minerals. Dissolved-air flotation (DAF) is usually operationally based on the theory of recycle chemical reactors, and certainly on the Henry’s law—governing the dissolution of gases (mostly air has been examined) in aqueous solutions as a function of pressure [8]. Currently, there are over 90 water treatment installations operating in the U.K. utilizing the DAF process and around the world it has been reported that there were 37 works in Finland, 26 in Australia, 26 in South Africa, 20 in the United States, etc. The development of flotation and filtration for treatment of potable water in five installations in the Northeastern USA was earlier described in another project. Although these numbers are still extremely small compared with the numerous applications in industrial and wastewater treatment, it clearly reflects the benefits of the DAF process and the established position it now holds in water treatment. In potable water treatment, the process chain of flocculation—flotation—filtration is a common concept. It is generally accepted that flocculation affects the flotation performance and in turn, the latter affects filter design and performance. Dissolved-air flotation has been considered not only an alternative to sedimentation plants, but as a clarification method to improve filtration. In DAF processes microbubbles of 10–80 µm in size were said to be generated. Among the other useful information given in a flotation forum, established for a recent flotation conference in Finland, a comparison of bubbles was found in different ranges that may give an illustrative idea of the significance of bubbles size applied and also the differences involved in the various flotation techniques (shown as Table 1).

Table 1.
The significance of bubbles size applied during flotation.
Alternatively, electroflotation or some other technique is feasible. Hence, a three-phase system is created, by various mechanisms, in which three interfacial tensions exert governing effects [9,10,11]. In the former, comparisons of the available flotation techniques were also attempted at the laboratory, applied to the same system under separation (magnesite and pyrite fines).
Perhaps due to its complexity, flotation has been characterized as being the encyclopedia of colloid science. The flotation of mixed sulfides is a common application in mineral processing used for selective separation, also noting that the specific gravity of the minerals is different. The impeller in a mechanical flotation tank plays a key role in keeping particles in suspension, dispersing and breaking-up air bubbles, and promoting particle–bubble collision; however, the turbulent regime generated by the impeller can also affect the pulp–froth interface [12]. Hydration phenomena, associations, and interactions between collectors, air bubbles, and water-soluble mineral particles were also presented [13]. Experimental research on the effect of (coal) particle size on the relative motion between mineral particles and bubbles was argued to be far more inadequate [14].
Fine particle recovery may be enhanced by using fine bubbles [15,16]. The problem of fine particle flotation is mainly due to their low collision and attachment efficiencies with bubbles. The application of microbubbles as flotation carriers, in fine mineral beneficiation processes, could be a promising approach for solving challenges of fine particle flotation [17]. The technique of electroflotation largely originated in the ex-USSR [18,19]. This technique is innovative, but the most common is electrocoagulation.
Foam (ion and precipitate) flotation was also suggested to be a promising green method of recovery of rare earth elements from leachates of the primary and secondary resources [20]. Since the beginning of the 20th century, xanthates (i.e., O-alkyl dithiocarbonates) have been widely used as reagents for the flotation concentration of a variety of sulfide and oxide minerals; the reduction of toxicity of the flotation process and improving its sustainability was investigated [21]. The effect of collectors and frothers was investigated on the electrical double layer and on the respective time for bubble coalescence [22].
Based on the above, it is clear how important the bubble size is in the flotation process. Therefore, this article will collect some important examples of the published works in flotation in which the commonly used microbubbles are taken part and compare them to the more recent trends of nanobubbles. The fundamentals of nanobubbles will also be given briefly to highlight the size effect impact on the process.
2. Fundamentals
Bubble–particle encounters include many kinds of interactions and forces—hydrodynamic, gravitational, surface, and capillary. The three identified subprocesses (collision, attachment, detachment) are not entirely discrete, but rather grade one into another; a theoretical model has been developed to describe such particle trajectories and velocities [23]. Certainly, bubbles (fine or larger) constitute the necessary transport medium of particulate matter for flotation. In this case (back in the 1970s), a photographic technique was used to measure the size of the bubbles during flotation; a special camera was constructed, based on earlier trials in solvent extraction—at the Chemical Engineering Department, University of Newcastle-upon-Tyne, UK—see also [24].
Using microbubbles has gained significant interest in many domestic and industrial applications due to bubble stability in solution and increased mass transfer area; the characterization of microbubble populations is therefore important and aids in the understanding of their behavior [25]. Examining gas bubbles, as found in different ranges, may give an illustrative idea of the significance of bubbles size applied and also the differences involved in the various flotation techniques [26]. So, a bubble size of 20 μm, means a number of created bubbles (×103) 1250, surface area (in cm2 cm−3) of 23, and a rising rate (in m h−1) of 1; compared with that of size 100 μm showing 14, 6.6, and 20, respectively. On the other hand, the size of the gas microbubbles produced by electroflotation and dissolved-air flotation is quite similar—on the order of 50 μm, always depending on the conditions.
Ion flotation, due to Sebba [27], is a technique that involves the removal of surface-inactive ions from dilute aqueous solutions by the addition of a surfactant, which is usually an ion of opposite charge, and the subsequent passage of gas through the solution [28]. This separation technique when first studied was not restricted to simple ions, but also included charged colloids, and there are many such pollutants in industrial wastewaters. Precipitate flotation of lead in the form of insoluble hydroxides, by simply regulating the solution pH and by the addition of a cationic surfactant, such as dodecylamine, proved to be an effective separation method—see Figure 1. The dotted line is a theoretical curve, showing the precipitation of zinc as Zn(OH)2, i.e. the free ion concentration remaining in the aqueous solution after precipitation. The impact of aqueous speciation during various flotation techniques was highlighted [29]. Thermodynamic equilibrium diagrams and software packages were employed to interpret the removal mechanism involved.
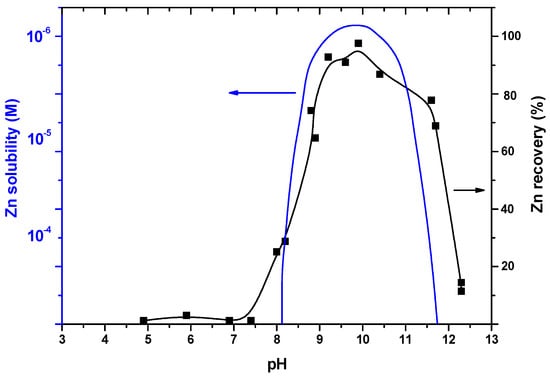
Figure 1.
Precipitate (dispersed-air) flotation for the removal of zinc. Effect of solution pH using the following initial concentrations: zinc 1 × 10−4 M and dodecylamine (laurylamine) 2 × 10−4 M. The dotted line is a theoretical curve, showing the precipitation of zinc. Redrawn from Nova Sci. (Zouboulis and Matis, 2005).
The laboratory dispersed-air flotation system (in our case) consisted of a nitrogen cylinder connected to the flotation cell through a gas humidifier, a filter, an open mercury U-tube manometer, a rotameter, and a low flow rate controller. The microcell had an approximate volume of 120 cm3 and was made from a Schott D4 fritted funnel, glass-blown to a piece of tubing, having the same diameter. Assuming a normal value of 60 dyn cm−1 for surface tension, an approximate bubble size of 0.79–0.83 mm was calculated for this diffuser (i.e. with pore diameter 10–16 μm), special arrangements provided for the collection of foam and also for sampling. Investigations have been performed in ion flotation (the term used here in the broad sense) using either low or high gas flow rates. The bubble size may be varied not only by using another diffuser or perhaps, another generation technique (i.e. electrolytically, etc.), but also by the application of a frother, hence changing the surface tension. A comparison between dispersed-air and dissolved-air flotation, applied to metal-loaded goethite, was presented; the dispersed-air technique looked preferable [30].
The effect of the type and concentration of selected frothers, the gas flow rate, and the pore size of the porous frit on the bubble sizes produced was published [31]. Similarly, measurements of bubble size distributions were examined (by changing surfactant dosage, solid percentage, air velocity, and particle size) as well as establishing the relationships between bubble size distribution properties and flotation performances in a bubble column [32]. Elsewhere, the Sauter mean diameter of bubbles was characterized for a gas–liquid system in a laboratory Jameson-type flotation cell, with focus on the size variation in the uprising path of the bubbles in the riser of the cell [33].
During galena flotation, the presence of slimes such as lead sulfate or pyrite, which adsorb or precipitate the collector, cause a depletion of the latter and hence, depression. Slime depression occurs when there is no energy barrier to heterocoagulation and is dependent on the zeta potentials and size of the mineral and slime. Another study of ZnS and NiS in Melbourne by detailed electrokinetic methods showed that by careful control of experimental parameters much information on sulfide–water interfaces could be obtained. Sphalerite activation with Cu2+, including the role of pH in this phenomenon, was examined, assisted by zeta-potential measurements; from the flotation point of view, the fast activation was emphasized [34]. The level of dissolved oxygen concentration is also a critical parameter (i.e. apart of pH, etc.) in differential flotation of sulfide minerals, which arises from the instability of the sulfur they contain [35]. The contribution of physical chemistry to flotation was stressed [36].
A possible utilization of an industrial by-product, such as iron sulfide, may be as an adsorbent/solid substrate material, which may be followed by their (dissolved-air) flotation as the scavenging mechanism; the technique was termed sorptive flotation [37]. Copper, among other heavy metals, is a common toxic ion encountered in many dilute leach solutions, spent process streams, and liquid effluents in industry. The respective electrokinetic measurements, illustrated in Figure 2, had applied increased quantities of copper ions.
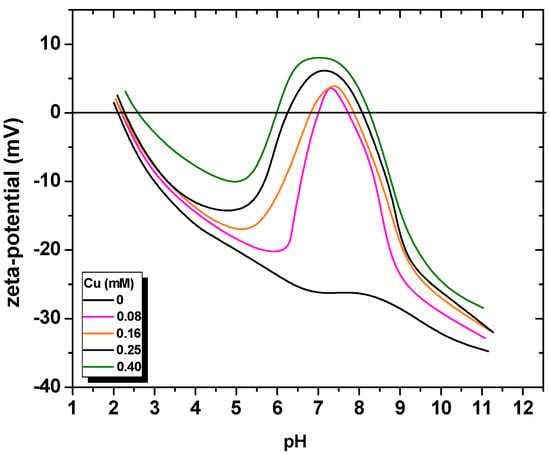
Figure 2.
Zeta-potential measurements of pyrite in the presence of copper ions. Redrawn from Taylor and Francis [38].
A reversal of zeta potential of pyrite was observed around the neutral and slightly alkaline pH region, which was due to the coverage of the mineral surface by hydrolyzed copper cationic species. A surface-induced hydrolysis reaction mechanism was suggested. An investigation into the adsorption performance of copper ions on arsenopyrite surfaces was also reported, as the removal of arsenic from base-metal ores is an important environmental objective, especially in relation to mineral processing [39].
In another study, chromium oxyanions were the target; the latter, particularly in its hexavalent form, is a known highly toxic priority pollutant, used in industry as strong oxidant. As shown in Figure 3, in the presence of chromium, an excessive positive surface charge of pyrite was observed, indicating a reduction process, since Cr(VI) exists in aqueous solutions only as an anionic species. For reasons of illustration, the behavior of chromium hydroxide precipitate (5 × 10−2 M) is also presented. The resulted hydroxo-trivalent chromium species were found to be precipitated and removed onto pyrite particles. Elsewhere, the separation of Cr(III)aq was achieved by ion and precipitate flotation using a multipolar collector [40].
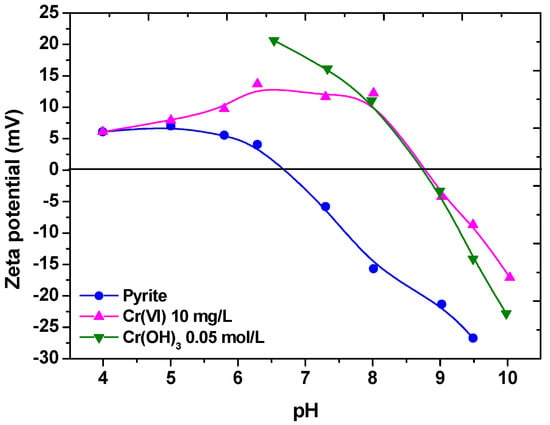
Figure 3.
Zeta-potential measurements of the pyrite system (with and without the presence of 10 mg L−1 Cr(VI) and also of Cr(OH)3). Reprinted with permission; copyright Elsevier [41].
Further, the performances of various flotation parameters, such as hydrodynamic variables (i.e. Reynolds number) and particle properties, and their impacts on recovery of coarse particles (>100 μm) in the presence of nanobubbles were looked at, in a mechanical cell and in presence of conventional flotation bubbles [42]. A foam separation method was said to effectively remove alkali metal ions from anionic surfactant solution by a combination of an air bubble generator and the flotation system [43].
3. Wastewater Treatment
One of the alternative but effective flotation techniques, mainly on a smaller scale, is electroflotation, or perhaps it should be correctly called electrolytic flotation, owing its name to the bubble generation method it uses (i.e., electrolysis of the aqueous medium); certain advantages of this technique have been stressed [44]. The used cell mainly consisted of a vertical Perspex tube (150 mm diameter and 1 m effective height); the electrodes used comprised two horizontal, circular stainless-steel discs equal to the column diameter: the lower, the cathode, was made of sheet steel and the anode, mounted above and parallel to it, was perforated to allow the upward passage of gas bubbles from the cathode. While examining the design aspects, a bipolar arrangement, and a cell having a cation exchange membrane separating the electrodes were constructed and tried, too. An investigation of hydrodynamics was also undertaken, as the efficiency is expected to be a function of the residence time distribution [11]. The stimulus-response technique was used—following Levenspiel [45]—in this experimentation, and the tracer input signal was an electrolyte and had the form of a step function. Assuming that the flow regime was composed of various flow types, a theoretical multiparameter model was applied (see Figure 4). It was proved, as shown, that the countercurrent operation mode in the column, between the effluent and the rising bubbles, was preferable.
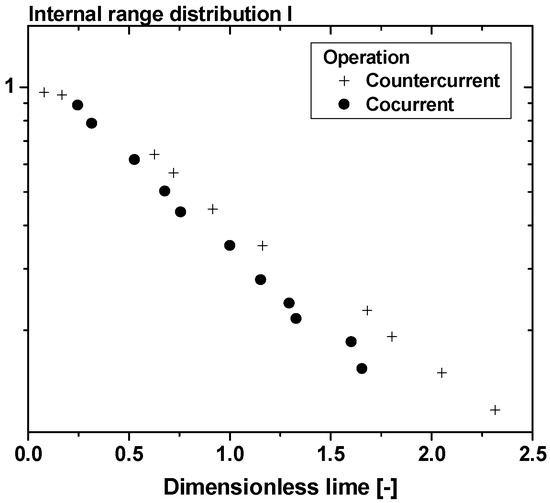
Figure 4.
A hydrodynamics study during electroflotation: internal age distribution function of the effluent. Reprinted with permission by Taylor and Francis [45].
Recently, a combined method for the treatment of complex wastewater based on sorption of the solution volume followed by extraction of the spent sorbent by the electroflotation method was suggested [46]. Another article has reviewed the experimental studies into mineral recovery by electroflotation and the potential application of electrolytic bubbles in the recovery of fine and ultrafine mineral particles [47]. The effect of hydrodynamic parameters on the nickel ion removal in an oscillating grid flotation cell was studied elsewhere [48]. Flotation, as a density/gravity separation process, has also been proposed and extensively tested in biotechnology—i.e., finding application in the solid–liquid separation of biomass; this can also be used for metal ion removal as a suitable biosorbent, even permitting its regeneration [49,50,51].
In the latter, a new hybrid cell of microfiltration combined with dispersed-air flotation has also been applied effectively; air bubbling is one of the techniques used to limit membrane fouling. So, a module with vertical membranes was used, with flat-sheet multi-channel geometry and hydrophilic surface properties and placed into the cell over the air diffuser. The finer the air bubbles used, the lower the pressure drop through the ceramic membranes. Backflushing contribution had no important influence and rather improved the operation. Attention was also paid to the economics of this integrated hybrid process, with application to heavy metals removal—usually following bonding onto a sorbent (zeolite) or precipitation. The aforementioned constituted an EU research project of the 5th Framework programme; a hybrid rig constructed by upt (from Saarbrücken, Germany) was transported at the Assarel-Medet open pit copper mine near Panagyurishte, Bulgaria [52].
Emerging contaminants (organics) in wastewater have recently attracted the attention of researchers as they pose significant risks to human health and wildlife [53]. Another study used coagulative colloidal gas aphrons to simultaneously remove microplastic particles and dissolved organic matter [54]. Fine bubbles were said to be cost effectively generated using a multi-orifice sparger with oscillatory air supply. On the other hand, it was published that the interaction between flocs and bubbles is crucial to achieve separation efficiency in the separation zone of the dissolved-air flotation process [55]. A CFD (computational fluid dynamics) model incorporating flotation kinetic expressions was developed to simulate the performance of dissolved-air flotation tanks utilized in water and wastewater treatment plants [56].
Bovine serum albumen (a protein) generated emulsion concentration had a significant impact on copper removal; separation of the copper-loaded microcells from the aqueous solution was also investigated by the so-called micro-flotation—i.e., dispersed-air [57]. Another process of adsorption onto silica nanoparticles coupled with flotation was validated for the recovery of trace Cu2+ from its aqueous solution [58]. A recent paper also reviewed the important studies on water treatment in this technological area and discussed the bubbles’ fundamental properties, such as stability, generation methods, and various chemical and physical features [34,59]; this investigation, however, suggested that despite the promising role of micro-nanobubbles in water-related applications, the current status of research has not reached its true potential.
4. Nanobubbles
The flotation performance (both recovery and kinetics) of ultrafine coal particles was said to be remarkably improved in the presence of nanobubbles; particle aggregates induced by nanobubble bridging has been recognized as one of the main contributors to improve the flotation performance of minerals [60]. It is generally believed that bubble miniaturization should be of significance to improve flotation efficiency [61]. Zhang et al. investigated the development of a new method that introduces interfacial nanobubbles (INBs) based on the temperature difference method for enhancing lignite flotation. The mechanism of lignite flotation enhanced by INBs is systematically explained through the induction time and bubble–particle collision-attachment behavior. Some interesting results revealed that the lignite flotation efficiency is evidently improved using the temperature difference method. In the presence of INBs, the induction time of lignite can be reduced from 400 ms to 27.21 ms, the number of bubble–particle collisions from 4 to 3, and the attachment time from 208 ms to 128 ms. When a macroscopic air bubble approaches a lignite surface with INBs, the air bubble first merges with the INBs, not only decreasing the induction time but also increasing the apparent contact angle and length of three-phase contact line [61].
Sobhy et al. [62] focused on reverse flotation experiments. This type of experiment, statistically designed using the Box-Behnken method, was carried out with a mechanical flotation cell integrated with nanobubbles. Response surface methodology was applied to evaluate the effects and interactions of three independent critical process parameters (dosages of hematite depressant starch, quartz activator lime, and collector TD-II used in the reverse hematite flotation in the presence of nanobubbles). By changing the starch dosages, nanobubbles improved the iron grade by up to 15% and iron recovery by 10–30%. In addition, TD-II collector and starch had the most significant impact on concentrate iron grade and recovery whereas lime had no direct independent effect over the examined dosage range. The maximum Fe recovery was determined to be 84.73% at a grade of 67.92% at the optimum conditions of 3 kg tn−1 starch, 1.5 kg tn−1 lime, and 0.78 kg tn−1 TD-II collector. The verification tests at optimum conditions established from the contour plots produced approximately the same grade and recovery as statistically predicted.
The existence of nanobubbles on the target mineral’s surface has also been proven, as well as their selective effectiveness through flotation separation [63]. The bubble size analysis results showed that the mean diameter of bubbles was between 60 and 70 nm; thus, they could be correctly called “nanobubbles”. Flotation test results showed a significant increase in the flotation recovery (by 37%) and grade (more than 1%) of fine phosphate ore sample (d80: 37 µm) using NBs that generated in the presence of a collector. Interestingly, surface analyses of flotation products showed that the amount of flotation collector adsorbed onto the surface of floated particles was decreased in the presence of NBs compared with their absence. The change in the particle surface (zeta) potential in the presence of NBs also provides additional evidence of NBs “adsorbed” (i.e., the surface NBs) onto the particle surface. These results indicated that NBs produced by hydrodynamic cavitation could adsorb onto the target mineral particles. This adsorption could change their surface properties, improve their hydrophobicity and surface potentials, and enhanced the bubble–particle attachment in flotation [63].
Another interesting study posed a question if nanobubbles can be produced by ultrasonication and what the contribution of ultrasonication is on flotation performance for the generation of nanobubbles. Li et al. [64] studied this point of ultrasonication by combining a series of techniques including flotation testing, AFM, and settling testing. AFM imaging showed that no surface nanobubbles were found at the HOPG–water interface before and after subjection to ultrasonication. Further, surface nanobubbles were generated with solution exchange before ultrasonciation and then they were subjected to ultrasonication. It was found that ultrasonication did not destroy the pre-existing surface nanobubbles at the HOPG (highly oriented pyrolytic graphite)–water interface. Settling tests and flotation tests indicate that ultrasonication has a negligible influence on the interaction between graphite particles and thus flotation performance. Nanobubbles were not one of the outcomes for ultrasonication.
The physico-chemical approach is also interesting in the study of nanobubbles regarding flotation. So, the effect of external electric field on nanobubbles adsorbed on the surface of hydrophobic particles during air flotation was studied by Wu et al. [65] by molecular dynamics simulations. The gas density distribution, diffusion coefficient, viscosity, and the change of the angle and number distribution of hydrogen bonds in the system with different amounts of gas molecules were calculated and compared with the results without an external electric field. The results show that the external electric field can make the size of the bubbles smaller. The diffusion coefficient of the gas increases and the viscosity of the system decreases when the external electric field is applied, which contributes to the reduction of the size of the nanobubbles. At the same time, compared with the results under no external electric field, the angle of hydrogen bonding under the external electric field will increase, and the proportion of water molecules containing more hydrogen bonds will reduce, which further explains the reason why the external electric field reduces the viscosity.
In a more applied work, raw water clarification by flotation was studied by injecting air into a centrifugal multiphase pump to generate microbubbles (MBs) and nanobubbles (NBs) [66]. Measurements of gas dispersion parameters were performed and optimal conditions were obtained using a pump pressure of 4 bar. Values showed a bubble Sauter diameter of 75 μm, an air holdup of 1.2%, a bubble surface area flux of 34 s−1, and an NB concentration of 1 × 108 NBs mL−1 (measuring 220 nm). Then, a study compared flotation with bubbles formed with the multiphase pump (F-MP) to lamellar settling at the clarification stage of a water treatment plant (WTP), in Brazil. The F-MP showed a higher separation efficiency at high hydraulic loads (9–15 m h−1), even without the use of a polymer, reaching 2 NTU (10–25 NTU raw water feed), which was much lower than the technical goal of the WTP (5 NTU).
Additionally, in a comparative study by Etchepare et al. [67], results were presented on the removal of Fe3+ ions as precipitates and nanoparticles of Fe(OH)3, at pH 7, by nanobubbles (NBs) and by dissolved-air flotation (DAF), a process which combines microbubbles (MBs) and NBs. DAF is a very well-known technology in the treatment of water and wastewater. Studies were conducted at low and high levels of saturation pressures (Psat of 2 and 4 bar) and with “isolated” (single) NBs. The latter were generated at 2.5 bar, after their separation from the MBs. Best results were obtained with flotation at 2 bar, when the concentration of MBs was low and NBs very high. The removal efficiency was 99% of the feed iron content (30 mg L−1), and the treated water had a residual total iron concentration of 0.3 mg/L and a turbidity of 0.6 NTU (95% reduction). The flotation at 4 bar (mostly by MBs) was faster (0.07 cm s−1) due to the high lifting power of the MBs but left a higher residual total iron content of 1.7 of mg/L. The flotation with isolated NBs (>108 bubbles mL−1) attained 91% iron removal due to “flotation” following NBs entrapment and the decrease of the relative density of the aggregates. Flotation of the nanoparticles with NBs was also studied and showed a removal efficiency of about 68%, considered high, for this difficult-to-separate system. It is believed that the combination of NBs and MBs has an excellent potential for future separation of particles, including nanoparticles, and will help to broaden applications in removing specific target pollutants from waters and wastewaters [67].
From a more fundamental point of view, the existence of nanobubbles (especially bulk nanobubbles) remains quite a “mystery”, mainly due to their stability and longevity; at the same time, high efforts are given to develop new generation methods and so far, their potential uses spread to high-value applications [68]. Bulk gaseous nanobubbles were produced mechanically through hydrodynamic cavitation, under working pressure of ~3 bar, and for the determination of their hydrodynamic diameter respective measurements were performed, as presented in Figure 5. The mean size was about 200 nm with a number of bubbles with size between 50 and 500 nm.
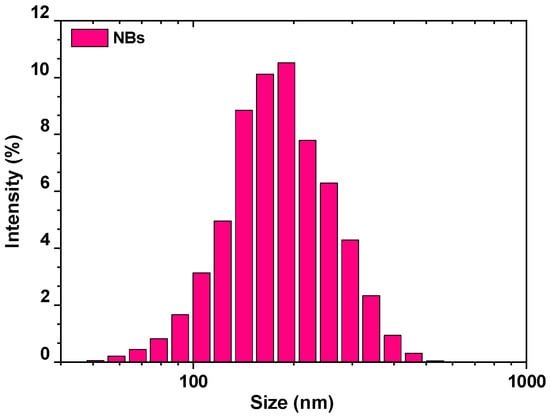
Figure 5.
Pore size distribution of air bulk nanobubbles observed from dynamic light scattering measurements, at 25 °C. Reprinted with permission; copyright Elsevier [69].
The inherent property of nanobubbles to accept charged particles onto their interface has been considered to assist diffusion and penetration phenomena of lead ions in activated carbon pores [70]. Models of adsorption kinetics were studied, and the comparison between fitted curves and experimental data is presented in Figure 6. A novel nanobubble generator with low energy demand, operating continuously, has also been recently presented [71]. One of the main factors supporting the stability of bulk nanobubbles in solution is known to be the negative zeta potential, which generates repulsive forces between neighboring bubbles. The zeta-potential value depends on the production time—as shown in Figure 7—and a decrease (as an absolute value) with the processing time was found to increase; in this work bulk nanobubbles with oxygen and air were generated in water by counterflow hydrodynamic cavitation.
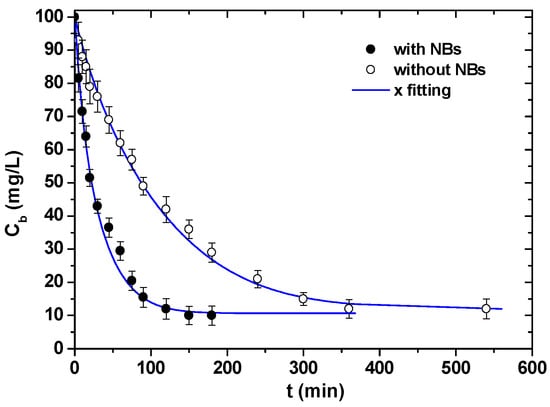
Figure 6.
Kinetic experiments for the adsorption of Pb(II) onto activated carbon with and without nanobubbles in water. Reprinted with permission; copyright Elsevier [70].
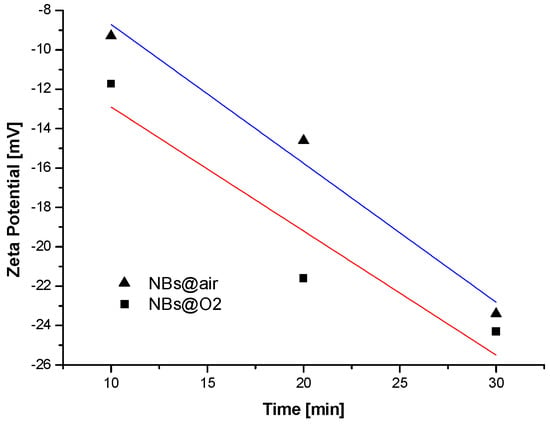
Figure 7.
Zeta-potential-processing time for air and oxygen bulk nanobubbles. Reprinted with permission; copyright Elsevier [71].
The effects of air bubbles and nanobubbles on flotation performance and kinetics of oxidized coal were studied [72]. It was found that surface nanobubbles were present on highly oriented pyrolytic graphite surface; coal agglomeration occurred in the presence of nanobubbles, indicated by the shear thinning of coal slurry [64,73]. Elsewhere, a new method was developed that introduces interfacial nanobubbles (based on the temperature difference method) for enhancing lignite flotation, which is more hydrophilic than other types of coal; hence, the separation and upgrading of its fine particles by froth flotation being quite ineffective [74].
Concluding, with the apparent advancements of technology and the respective progress in nanotechnology in different areas (including flotation), the grown interest in nanobubbles is apparently evident. The varying role and their interaction of micro-/nanobubbles were above highlighted in detail, starting from mineral processing and moving to water and wastewater treatment. Water, being a manufacturing tool that industry has taken for granted (as inexpensive and plentiful), is leading us to a new water constrained era due to population growth, globalization, and climate change.
Author Contributions
Writing—original draft preparation, G.Z.K., A.C.M. and K.A.M.; writing—review and editing, G.Z.K., A.C.M. and K.A.M.; supervision, G.Z.K. and K.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matis, K.A.; Zouboulis, A.I. Flotation Science and Engineering. In Flotation Science and Engineering; Matis, K.A., Ed.; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Burns, S.E.; Yiacoumi, S.; Tsouris, C. Microbubble generation for environmental and industrial separations. Sep. Purif. Technol. 1997, 11, 221–232. [Google Scholar] [CrossRef]
- Rodrigues, R.T.; Rubio, J. DAF-dissolved air flotation: Potential applications in the mining and mineral processing industry. Int. J. Miner. Process. 2007, 82, 1–13. [Google Scholar] [CrossRef]
- Szpyrkowicz, L. Hydrodynamic effects on the performance of electro-coagulation/electro- flotation for the removal of dyes from textile wastewater. Ind. Eng. Chem. Res. 2005, 44, 7844–7853. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Methods of arsenic wastes recycling: Focus on flotation. J. Mol. Liq. 2016, 214, 37–45. [Google Scholar] [CrossRef]
- Peleka, E.N.; Matis, K.A. Water separation processes and sustainability. Ind. Eng. Chem. Res. 2011, 50, 421–430. [Google Scholar] [CrossRef]
- Yenial, Ü.; Bulut, G. Examination of flotation behavior of metal ions for process water remediation. J. Mol. Liq. 2017, 241, 130–135. [Google Scholar] [CrossRef]
- Edzwald, J.K. Dissolved air flotation and me. Water Res. 2010, 44, 2077–2106. [Google Scholar] [CrossRef] [PubMed]
- Matis, K.A.; Mavros, P. Recovery of metals by ion flotation from dilute aqueous solutions. Sep. Purif. Methods 1991, 20, 1–48. [Google Scholar] [CrossRef]
- Simões, C.R.; Hacha, R.R.; Merma, A.G.; Torem, M.L. On the recovery of hematite from an iron ore fine fraction by electroflotation using a biosurfactant. Minerals 2020, 10, 1057. [Google Scholar] [CrossRef]
- Peleka, E.N.; Matis, K.A. Hydrodynamic aspects of flotation separation. Open Chem. 2016, 14, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Mesa, D.; Morrison, A.J.; Brito-Parada, P.R. The effect of impeller-stator design on bubble size: Implications for froth stability and flotation performance. Miner. Eng. 2020, 157, 106533. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Liu, H.; Zhang, H.; Miller, J.D. Some physicochemical aspects of water-soluble mineral flotation. Adv. Colloid Interface Sci. 2016, 235, 190–200. [Google Scholar] [CrossRef]
- Zhuo, Q.; Liu, W.; Zhang, H.; Zhang, W.; Cui, R. Effect of particle size on the relative motion between particles and bubbles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124956. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P. Dissolved-air and electrolytic flotation. In Mineral Processing at a Crossroads; Wills, B.A., Barley, R.W., Eds.; Martinus Nijhoff: Dordrecht, Germany, 1986; pp. 37–70. [Google Scholar]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of Fine Particles: A Review. Miner. Process. Extr. Metall. Rev. 2020, 1–11. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Sadovskiy, D.Y.; Rulyova, N.A.; Filippov, L.O. Column flotation of fine glass beads enhanced by their prior heteroaggregation with microbubbles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126398. [Google Scholar] [CrossRef]
- Da Mota, I.D.O.; de Castro, J.A.; de Góes Casqueira, R.; de Oliveira Junior, A.G. Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. J. Mater. Res. Technol. 2015, 4, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Kyzas, G.Z.; Matis, K.A. Electroflotation process: A review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Shetty, S.; Chernyshova, I.V.; Ponnurangam, S. Foam flotation of rare earth elements by conventional and green surfactants. Miner. Eng. 2020, 158, 106585. [Google Scholar] [CrossRef]
- Milosavljević, M.M.; Marinković, A.D.; Rančić, M.; Milentijević, G.; Bogdanović, A.; Cvijetić, I.N.; Gurešić, D. New Eco-Friendly Xanthate-Based Flotation Agents. Minerals 2020, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Roberto, P.-G.; Arturo, B.-T. Coalescence of air bubbles: Effect of the electrical double layer. Miner. Eng. 2020, 150, 106301. [Google Scholar] [CrossRef]
- Brabcová, Z.; Karapantsios, T.; Kostoglou, M.; Basařová, P.; Matis, K. Bubble–particle collision interaction in flotation systems. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 95–103. [Google Scholar] [CrossRef]
- Matis, K.A. Electrolytic flotation. In Innovations in Flotation Technology; Mavros, P., Matis, K.A., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1992; pp. 301–304. [Google Scholar]
- Swart, B.; Zhao, Y.; Khaku, M.; Che, E.; Maltby, R.; Chew, Y.J.; Wenk, J. In situ characterisation of size distribution and rise velocity of microbubbles by high-speed photography. Chem. Eng. Sci. 2020, 225, 115836. [Google Scholar] [CrossRef]
- Peleka, E.N.; Gallios, G.P.; Matis, K.A. A perspective on flotation: A review. J. Chem. Technol. Biotechnol. 2018, 93, 615–623. [Google Scholar] [CrossRef]
- Sebba, F. Ion Flotation. Available online: http://books.google.com/books?id=MYFKAAAAMAAJ (accessed on 10 May 2021).
- Zouboulis, A.I.; Matis, K.A. Ion flotation in environmental technology. Chemosphere 1987, 16, 623–631. [Google Scholar] [CrossRef]
- Matis, K.A.; Zouboulis, A.I. Flotation techniques in water technology for metals recovery: The impact of speciation. Sep. Sci. Technol. 2001, 36, 3777–3800. [Google Scholar] [CrossRef]
- Matis, K.A.; Lazaridis, N.K. Flotation techniques in water technology for metals recovery: Dispersed-air vs. dissolved-air flotation. J. Min. Metall. A Min. 2002, 38, 1–27. [Google Scholar]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals 2021, 11, 304. [Google Scholar] [CrossRef]
- Panjipour, R.; Karamoozian, M.; Albijanic, B. Bubble size distributions in gas–liquid–solid systems and their influence on flotation separation in a bubble column. Chem. Eng. Res. Des. 2021, 167, 96–106. [Google Scholar] [CrossRef]
- Zhu, H.; Valdivieso, A.L.; Zhu, J.; Song, S.; Min, F.; Corona Arroyo, M.A. A study of bubble size evolution in Jameson flotation cell. Chem. Eng. Res. Des. 2018, 137, 461–466. [Google Scholar] [CrossRef]
- Jain, S. Activation in the flotation of sphalerite. Flotat. Sulphide Miner. 1985, 6, 159–174. [Google Scholar]
- Gallios, G.P.; Kydros, K.A.; Matis, K.A. Electrokinetic behaviour of sulphide minerals: A contribution to the chemistry of flotation. In Mineral Processing and the Environment; Gallios, G.P., Matis, K.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 25–42. [Google Scholar]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Research activities related to flotation process. Trends Green Chem. 2015, 1, 1–5. [Google Scholar]
- Zamboulis, D.; Peleka, E.N.; Lazaridis, N.K.; Matis, K.A. Metal ion separation and recovery from environmental sources using various flotation and sorption techniques. J. Chem. Technol. Biotechnol. 2011, 86, 335–344. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Kydros, K.A.; Matis, K.A. Adsorbing Flotation of Copper Hydroxo Precipitates by Pyrite Fines. Sep. Sci. Technol. 1992, 27, 2143–2155. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.; Li, S.; Deng, J.; Luo, B.; Lai, H. Adsorption performance of copper ions on arsenopyrite surfaces and implications for flotation. Appl. Surf. Sci. 2019, 488, 185–193. [Google Scholar] [CrossRef]
- Craioveanu, M.G.; Stoica, L.; Constantin, C.; Oprea, O. Cr(III)aq separation by flotation with multipolar collector. Sep. Sci. Technol. 2020, 55, 346–357. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Kydros, K.A.; Matis, K.A. Removal of hexavalent chromium anions from solutions by pyrite fines. Water Res. 1995, 29, 1755–1760. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Shahbazi, B.; Chelgani, S.C. Study relationships between flotation variables and recovery of coarse particles in the absence and presence of nanobubble. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 284–288. [Google Scholar] [CrossRef]
- Matsuoka, K.; Hasegawa, S.; Yuma, T.; Goto, Y. Application of foam separation method for removal of alkaline earth metal ions from solution. J. Mol. Liq. 2019, 294, 111663. [Google Scholar] [CrossRef]
- Matis, K.A.; Peleka, E.N. Alternative flotation techniques for wastewater treatment: Focus on electroflotation. Sep. Sci. Technol. 2010, 45, 2465–2474. [Google Scholar] [CrossRef]
- Levenspiel, O. Mixed models to represent flow of fluids through vessels. Can. J. Chem. Eng. 1962, 40, 135–138. [Google Scholar] [CrossRef]
- Gaydukova, A.; Kolesnikov, V.; Aung, H.T. Electroflotosorption method for removing organic and inorganic impurities from wastewater. Sep. Purif. Technol. 2021, 267, 118682. [Google Scholar] [CrossRef]
- Tadesse, B.; Albijanic, B.; Makuei, F.; Browner, R. Recovery of Fine and Ultrafine Mineral Particles by Electroflotation—A Review. Miner. Process. Extr. Metall. Rev. 2019, 40, 108–122. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Rezai, B.; Safari, M.; Deglon, D.; Kowsari, E. Effect of hydrodynamic parameters on nickel removal rate from wastewater by ion flotation. J. Environ. Manag. 2019, 244, 408–414. [Google Scholar] [CrossRef]
- Gulden, S.J.; Riedele, C.; Kopf, M.H.; Nirschl, H. Potential of flotation as alternative separation process in biotechnology with focus on cost and energy efficiency. Chem. Eng. Sci. 2020, 218, 115117. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Flotation of biological materials. Processes 2014, 2, 293–310. [Google Scholar] [CrossRef]
- Matis, K.A.; Mavros, P. Foam/Froth Flotation. Sep. Purif. Methods 1991, 20, 163–198. [Google Scholar] [CrossRef]
- Nenov, V.; Lazaridis, N.K.; Blöcher, C.; Bonev, B.; Matis, K.A. Metal recovery from a copper mine effluent by a hybrid process. Chem. Eng. Process. Process Intensif. 2008, 47, 596–602. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Kang, Z.; Wu, X.; Tang, L.; Qiang, Z.; Zhang, D.; Pan, X. Removal of micron-scale microplastic particles from different waters with efficient tool of surface-functionalized microbubbles. J. Hazard. Mater. 2021, 404, 124095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, X.; Yang, S.; Wang, G.; Xu, L.; Jin, P.; Shi, X.; Shi, Y. Interactions between flocs and bubbles in the separation zone of dissolved air flotation system. Sci. Total Environ. 2021, 761, 143222. [Google Scholar] [CrossRef]
- Kostoglou, M.; Karapantsios, T.D.; Matis, K.A. CFD model for the design of large scale flotation tanks for water and wastewater treatment. Ind. Eng. Chem. Res. 2007, 46, 6590–6599. [Google Scholar] [CrossRef]
- Nazari, A.M.; Cox, P.W.; Waters, K.E. Biosorptive flotation of copper ions from dilute solution using BSA-coated bubbles. Miner. Eng. 2015, 75, 140–145. [Google Scholar] [CrossRef]
- Hu, N.; Liu, W.; Jin, L.; Li, Y.; Li, Z.; Liu, G.; Huang, D.; Wu, Z.; Yin, H. Recovery of trace Cu2+ using a process of nano-adsorption coupled with flotation: SNP as an adsorbing carrier. Sep. Purif. Technol. 2017, 184, 257–263. [Google Scholar] [CrossRef]
- Khan, P.; Zhu, W.; Huang, F.; Gao, W.; Khan, N.A. Micro–nanobubble technology and water-related application. Water Supply 2020, 20, 2021–2035. [Google Scholar] [CrossRef]
- Liu, L.; Hu, S.; Wu, C.; Liu, K.; Weng, L.; Zhou, W. Aggregates characterizations of the ultra-fine coal particles induced by nanobubbles. Fuel 2021, 297, 120765. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, L.; Yang, H.; Gui, X.; Schönherr, H.; Kappl, M.; Cao, Y.; Xing, Y. Recent advances for understanding the role of nanobubbles in particles flotation. Adv. Colloid Interface Sci. 2021, 291, 102403. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, A.; Wu, Z.; Tao, D. Statistical analysis and optimization of reverse anionic hematite flotation integrated with nanobubbles. Miner. Eng. 2021, 163, 06799. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M.; Nguyen, A.V.; Chelgani, S.C. Proving the existence of nanobubbles produced by hydrodynamic cavitation and their significant effects in powder flotation. Adv. Powder Technol. 2021, 32, 1810–1818. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Xu, M.; Zhang, H. Effect of ultrasonication on the flotation of fine graphite particles: Nanobubbles or not? Ultrason. Sonochemistry 2020, 69, 105243. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zhang, Q.; Zhao, S. Effect of external electric field on nanobubbles at the surface of hydrophobic particles during air flotation. RSC Adv. 2019, 9, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, A.; Etchepare, R.; Rubio, J. Raw water clarification by flotation with microbubbles and nanobubbles generated with a multiphase pump. Water Sci. Technol. 2017, 75, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Etchepare, R.; Azevedo, A.; Calgaroto, S.; Rubio, J. Removal of ferric hydroxide by flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 184, 347–353. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Effect of agitation on batch adsorption process facilitated by using nanobubbles. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125440. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Kyzas, G.Z.; Mitrikas, G.; Mitropoulos, A.C.; Efthimiadou, E.K.; Favvas, E.P. Bulk nanobubbles: Production and investigation of their formation/stability mechanism. J. Colloid Interface Sci. 2020, 564, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Xing, Y.; Zhang, F.; Yang, Z.; Liu, X.; Gui, X. Effect of nanobubbles on the flotation performance of oxidized coal. ACS Omega 2020, 5, 20283–20290. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, M.; Lei, W.; Yao, W.; Fan, R. Effect of Nanobubbles on the Slime Coating of Kaolinite in Coal Flotation. ACS Omega 2020, 5, 24773–24779. [Google Scholar] [CrossRef]
- Zhang, F.; Xing, Y.; Chang, G.; Yang, Z.; Cao, Y.; Gui, X. Enhanced lignite flotation using interfacial nanobubbles based on temperature difference method. Fuel 2021, 293, 120313. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).