Abstract
This paper is a review of 20 years of full-scale experience with the granular sludge-based ANAMMOX process. The ANAMMOX process is a biological deammonification process for energy-efficient removal of ammoniacal nitrogen, which has been successfully applied on dewatering reject liquors from biosolids sludge digesters (e.g., mesophilic anaerobic digestions, codigestion, thermal sludge hydrolysis process (THP)) and nutrient-rich anaerobically treated industrial effluents (e.g., fermentation industry, food industry). The ANAMMOX process is a continuously operated biological process using granular biomass. The highly active concentrated granular biomass allows for compact reactor systems and a fast start-up. Long term operations of various case studies show stable process performance of full-scale reactors treating municipal and industrial effluents, achieving ammoniacal nitrogen (NH4-N) removal in excess of 90% at low and high loading rates up to 2.5 kgNH4-N/(m3·d). Some special aspects (e.g., micro-nutrients, inhibition, alkalinity consumption) of treating various wastewaters are discussed in detail. The ANAMMOX process is demonstrated to be resilient in handling process upsets and off-spec wastewater composition.
1. Introduction
Anaerobic treatment has been applied increasingly in treating sewage sludge (biosolids), organic residues, and industrial effluents to generate renewable energy (methane rich biogas). Although anaerobic digestion (AD) is an effective method to remove organic compounds (measured as chemical oxygen demand: COD), the removal of nutrients (N, P) is fairly limited [1]. As a result, liquors derived from anaerobic processes have a reduced COD (carbon source) content but might still contain relatively high concentrations of ammoniacal nitrogen (NH4-N) and sometimes also ortho-phosphate (PO4). These low carbon to nitrogen (C/N) effluents require further dedicated treatment for nitrogen removal.
As opposed to conventional biological nitrogen removal by involving full nitrification (NH4 + O2 → NO2 + O2 → NO3) and denitrification (NO3 + COD → N2), combined partial nitritation (Equation (1)) and anammox (anaerobic ammonium oxidation—Equation (2) allows for the biological removal of ammoniacal nitrogen without the requirement for an organic carbon source. As a result, the addition of an external carbon source (e.g., methanol or glycerol) or an internal carbon source (e.g., COD in the influent bypassing the anaerobic treatment) is not required. Hence, the combination of anaerobic treatment and anammox does allow for maximizing the production of renewable energy by converting all carbon sources. Combined partial nitritation (PN) by ammonia oxidizing bacteria (AOB) and anammox by anammox micro-organisms (AMX) is often referred to as PN/A. Equations for the various reactions are as follows [2,3]:
Nitritation: 1.32 NH4+ + 1.98 O2 → 1.32 NO2− + 1.4 H2O + 2.64 H+
Anammox: 1 NH4+ + 1.32 NO2− + 0.13 H+ → 1.02 N2 + 0.26 NO3− + 2.03 H2O
Overall (PN/A):1 NH4+ + 0.85 O2 → 0.45 N2 + 0.11 NO3− + 1.08 H+ + 1.44 H2O
As in the PN/A process, only a part of the ammoniacal nitrogen is oxidized to nitrite (NO2) instead of nitrate (NO3), up to 60% savings on aeration energy can be achieved. Over the last two decades, ammonium removal via the PN/A route has become widely accepted with applications in industry and municipal sidestream treatment, in different process configurations [2]. ANAMMOX (acronym for ANaerobic AMMonium OXidation) is a trade name for the proprietary Paques deammonification process filed by Paques Technology and registered in several countries.
1.1. Historical Background and Applications
Paques installed the world’s first full-scale anammox reactor in 2002 at the Sluisjesdijk sludge treatment plant in Rotterdam, the Netherlands. This ANAMMOX reactor was implemented as a two-step anammox process treating the effluent of an existing nitritation reactor. The Rotterdam ANAMMOX reactor is still in operation, having demonstrated an average N removal of 92% (4-year period) at volumetric loading rates over 10 kgN/(m3·d) [4]. While the first ANAMMOX reactor was developed to treat effluent after a separate partial nitritation reactor to convert nitrite and ammonia into nitrogen gas, Paques soon developed one-step (PN/A) ANAMMOX® reactors integrating partial nitritation and anammox in a single reactor. In 2006, the first one-step PN/A ANAMMOX reactor was built and started by Waterstromen at the sewage treatment works (STW) of Olburgen in the Netherlands [5]. Whereas the two-step ANAMMOX reactors were mostly built as tall towered steel tank (height 8–16 m) reactors, the current one-step PN/A ANAMMOX reactors are built as lower cylindrical (steel or concrete) or rectangular tanks (height 5–10 m). Some examples are depicted in Figure 1. When appropriate, available tanks might be retrofitted using existing assets [6].

Figure 1.
Photographs of various ANAMMOX reactor installations constructed in time (left to right): Rotterdam (2002): biosolids reject; Olburgen (2006): biosolids reject + food industry (PN/A reactor + Phospaq struvite reactor); Son (2014): Industrial effluent and Tilburg (2017): THP-biosolids reject (PN/A + Phospaq struvite reactor).
To date (June 2021), 66 ANAMMOX plants have been installed worldwide with a total installed treatment capacity of close to 150,000 kgN/d (Figure 2). The treatment capacities of the reactors vary between 25 and 11,000 kgN/d. The ANAMMOX process has been applied on a wide variety of municipal and industrial effluents as presented in Table 1.
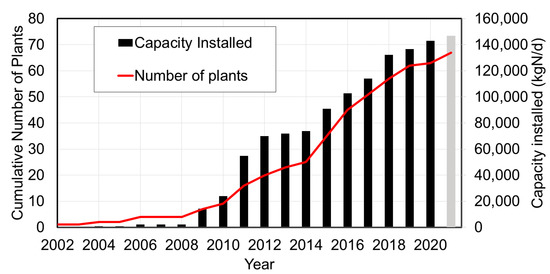
Figure 2.
Historical development of the number and installed capacity of ANAMMOX plants (status June 2021) since the first full scale reactor at Sluisjesdijk in Rotterdam in 2002.

Table 1.
Market sectors in which ANAMMOX reactors have been applied.
In municipal projects, the anammox process is mostly applied in the sidestream of the main STW treating the dewatering reject liquors from anaerobic sludge digestion. Although the sidestream dewatering reject liquors represent less than 1% of the overall flow of the mainstream STW, these reject liquors may represent typically 10–15% of the overall nitrogen load to the main STW. The reject liquors’ ammoniacal nitrogen load might increase up to 30% in case of co-digestion or even up to 50% if thermal sludge hydrolysis (THP) is applied and the STW acts as a sludge center that handles external sludge imports from other STWs [7]. THP has gained increasing interest for the pre-treatment of biosolids prior to anaerobic digestion. Treatment of THP-AD reject liquors by ANAMMOX has been demonstrated successfully at full scale [7,8].
In case biological phosphorus removal is applied in the main STW, phosphate is released during anaerobic sludge digestion. This potentially increases the phosphate concentration in the reject liquor and the P- loadings to the main STW. In such cases, phosphorus removal via controlled struvite precipitation might become interesting. Anammox is also applied in agro-food industries (e.g., fermentation industry, food industry, animal byproducts), often treating anaerobically pre-treated effluent having a low C/N ratio. As anammox is an autotrophic biological process requiring no carbon source, all effluent can be treated anaerobically, requiring no bypass of COD for downstream denitrification. Combined treatment with granular sludge-based anaerobic reactors and ANAMMOX has been demonstrated on fermentation industry, yeast industry, and rendering effluent with organic residue co-digestion [9].
1.2. ANAMMOX Reactor Technology
The PN/A ANAMMOX® reactor is a reactor system in which partial nitritation and anammox conversion occur simultaneously in one single process unit. This one-step PN/A ANAMMOX reactor is continuously aerated, uses granular biomass, and has an internal biomass separator. Figure 3 depicts the main features of the ANAMMOX reactor technology.
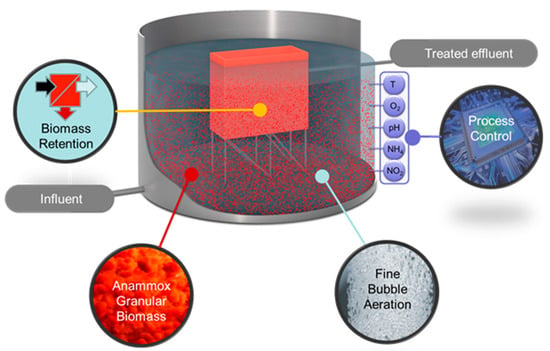
Figure 3.
Typical features of the PN/A ANAMMOX reactor with an internal biomass retention system, granular biomass, fine bubble aeration system, and multiple parameter control unit.
As the anammox bacteria have a relatively low growth rate, effective retention of biomass is of essence for a sustainable process. The granular biomass has superior settling properties (sludge volume index (SVI) typically 20–25 mL/g) as compared to flocculent biomass. Due to the special design of a proprietary granular biomass separator, fine light weighted solids in the influent and flocculent biomass present in the reactor are selectively washed out from the reactor, while the dense well settleable granular biomass is effectively retained [5]. The concentrated highly active biomass allows for high volumetric loading rates and the construction of compact reactor systems. Due to the concentrated nature and the large surface area of the granular sludge, high volumetric loading rates up to 2.5 kg N/(m3·day) are achieved. Any excess granular biomass is easily harvested from the reactor and can be used for inoculation of new reactors or stored for back-up in case of any calamities at other sites. The ANAMMOX reactor is typically equipped with a fine bubble aeration system for the energy-efficient transfer of oxygen (typically 1 kWh/kgNH4-N removed). The aeration system operates on a continuous basis, providing good contact between the biomass and the wastewater. The ANAMMOX reactor system is typically equipped with a PLC/SCADA control unit using a versatile multiple parameters-based algorithm, ensuring an optimal operation of the process under various conditions.
1.3. Anammox Granular Sludge
The advantages of using particulate biofilm systems, such as granular sludge in biological reactor systems (anaerobic or aerobic), have been discussed by Lettinga et al. and Nicolella et al. extensively [10,11]. The anammox granules in a one-step PN/A ANAMMOX reactor consist of an outer aerobic layer containing AOB and an anoxic core of AMX bacteria (Figure 4). In practice, the granule may also contain other micro-organisms, such as heterotrophic bacteria, converting COD present in the effluent.
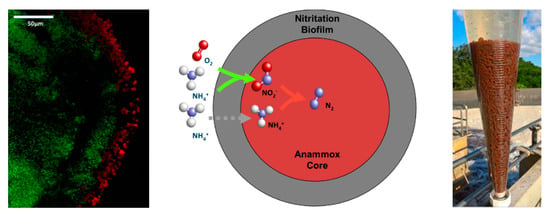
Figure 4.
A FISH image of a cross-section of an ANAMMOX granule (left) indicating the anammox bacteria (green) and the outer-layer biofilm indicating the aerobic ammonia-oxidizing bacteria (red) and the simplified layered granular sludge model with the associated biological reactions (middle) and an Imhoff cone with PN/A ANAMMOX granules (right).
The granular matrix protects the anammox bacteria in the inner core from any extreme conditions in the bulk solution (e.g., NO2, pH, DO, etc.). As a result, the Anammox granules proved to be more resilient in coping with potential inhibiting components, when compared to flocculent biomass. Granular biomass in general was demonstrated to be less susceptible for incidents with high nitrite levels, solids, or COD than reactor systems incorporating flocculent sludge [5]. The granular sludge consists of spherical particles with a typical diameter of 2–4 mm (range 1–10 mm). Some typical ANAMMOX granules are depicted in Figure 5.

Figure 5.
Examples of ANAMMOX granules: AMX only (left) and PN/AMX (4× right) with typical diameters of 1, 1–2, 2–5, 3–5, and 4–6 mm, respectively.
The granular matrix also plays an important role in process control, by facilitating a natural mechanism to outcompete NOB (nitrite oxidizing bacteria). The design and operation of the ANAMMOX process are such that only granular biomass is retained in the reactor, while NOB present in flocs will be washed out continuously. For NOB to grow inside the reactor, they must do so in/on the granular biomass. Additionally, for NOB to have any access to substrate (nitrite and dissolved oxygen), they will grow in the outer aerobic layer of the granules [12]. The control of the ANAMMOX process is such that AOB activity naturally creates a steep oxygen gradient inside the aerobic layer, thus reducing the oxygen availability for NOB in the granule to a minimum. As a result, the nitrate production in the ANAMMOX process is limited to what is stoichiometrically produced by anammox bacteria, which is reported to be 11% of ammonium conversion [3], i.e., a ratio of 0.11 mol NO3 produced per mol NH4 removed by PN/A (Equation (3) [3]).
2. Operational Experience
2.1. Start-Up
The high settling velocity of the anammox granules allows for easy harvesting by gravity or screening. Hence, using surplus anammox biomass for inoculation of other reactors does not require external carrier material or special devices for further thickening. The granules are mostly transported by truck or ship. When required, the amount of granules in the reactor can be adjusted to meet a certain food to microorganisms (F/M) loading. The biomass inventory of the ANAMMOX reactor is easily checked by gravity settling in an Imhoff cone (Figure 4). Any amount of excess granules produced is simply stored in the reactor or if required used for seeding other reactors.
At the time of starting-up the world’s first anammox reactor at Rotterdam, there was no seed sludge available. Because of this, and because of other operational disturbances, the start-up period of this reactor took almost 3 years [13,14]. The experience gained from this unique project in combination with the increasing availability of granular seed sludge has shortened the start-up time significantly. Ultimately, the start-up time depends on the amount of seed sludge used for inoculation as compared to the amount required for treating the full ammonium load. Figure 6 presents the start-up time of several ANAMMOX reactors required for treating all effluents available. The first case presents the start-up of the first 600 m3 PN/A ANAMMOX plant in 2006 treating combined effluents from biosolids reject liquor and food industry. With only little limited granular sludge available from the Rotterdam ANAMMOX reactor, the start-up time took almost 6 months. The second case presents the start-up of a 1240 m3 PN/A ANAMMOX plant in 2012, treating fermentation industry effluent. The start-up was done by inoculation with granular biomass and took about 50 days. The third case presents the start-up of a 425 m3 PN/A ANAMMOX reactor in 2019, treating reject liquor from co-digestion of biosolids and industrial residues. As this reactor was seeded with an amount of granular biomass that was sufficient for the design load, this start-up took less than 1 week. The availability of granular anammox biomass shortens the start-up time significantly. Whereas the start-up of the first one-step PN/A ANAMMOX reactor in 2006 lasted 6 months, nowadays, ANAMMOX reactors have been started up within week(s).
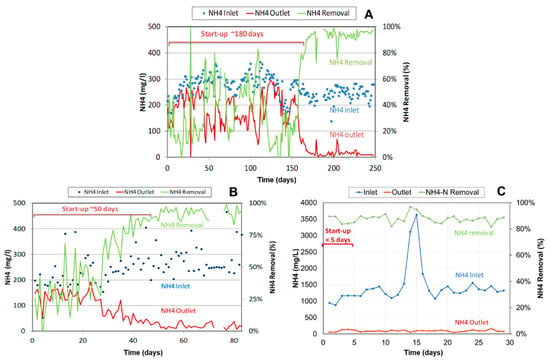
Figure 6.
Start-up time for 3 PN/A ANAMMOX reactors: Case (A): (600 m3) in 2006 taking 180 days; Case (B): (1230 m3) in 2012 taking 50 days; and Case (C): (425 m3) in 2019 taking less than 5 days.
2.2. Long-Term Operational Performance at Elevated Loading Rates
Long-term (years) operational results for a number of case studies (see Table 2 and Figure 7) are presented. The first case study with a relatively low total Kjeldahl nitrogen (TKN) concentration of 300–600 mg/L shows a 7-year average TKN removal of 92% at loadings up to 2.5 kgTKN/(m3·d). The second case study demonstrates a 7-year average NH4-N removal of 95% at loadings up to 2.7 kg NH4-N/(m3·d). The third case study shows a 1.4-year average of almost 90% at loadings rates up to 2 kgNH4-N/(m3·d).

Table 2.
Characteristics of the long-term case studies discussed.
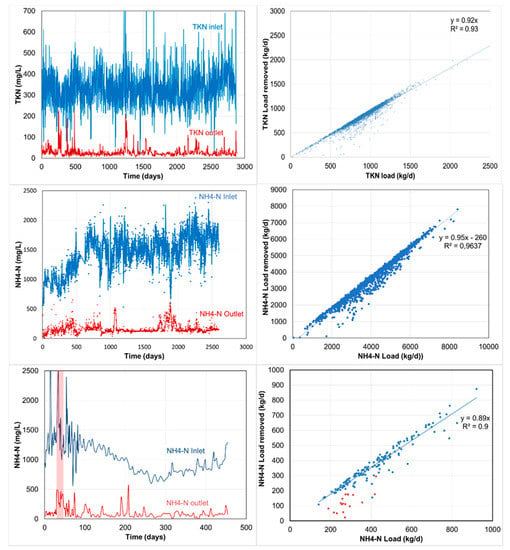
Figure 7.
Long-term operation performance of ANAMMOX reactors of case studies described in Table 2: Case I (top); Case II (middle) and Case III (bottom). The graph on the left depicts the nitrogen concentrations (mg/L) while the graph on the right depicts the nitrogen removal as a function of the nitrogen loadings (kg/d).
Figure 7 (graphs on the right) shows that in all case studies, the nitrogen removal efficiency is not compromised at high(er) loadings even above design loadings.
It should be mentioned that the aforementioned volumetric loading rates (>2 kg/(m3·d)) are well achievable at reactor temperatures between 30 and 39 °C. At lower temperatures, the activity of the biomass (anammox) will be reduced [15,16]. In such cases, the amount of biomass is increased by either increasing the biomass concentration in the reactor or by increasing the reactor volume.
2.3. Long Term NOB Suppression
A key factor in achieving long-term stability is to ensure that the conversion is selective, meaning that conversion takes place via the PN/Anammox route, without significant nitrate (NO3) formation by NOB. As explained in the section on Anammox Granular Biomass, simulation studies have shown that the use of granular PN/A biomass offers a natural mechanism to outcompete NOB [12,17]. The following two cases show operational data for two full-scale installations (details in Table 3), where the effluent nitrate concentration was routinely measured, so that the NO3/NH4 ratio could be calculated. This ratio can then be compared to the stoichiometry of the Anammox reaction and gives insight into whether NOB are active, as they would increase the nitrate production to above the stoichiometric ratio.

Table 3.
Characteristics of the long-term NOB suppression case studies discussed.
The long-term operational data for case A is shown in Figure 8, which shows a stable effluent nitrate concentration over a period of more than 3 years. At the same time, the influent ammonium concentration showed some fluctuations, between 240 and 450 mg NH4-N/L. Based on the results shown in Figure 9, the long-term average ratio was 0.080 mol NO3 produced per mol NH4 removed. This is slightly below the value of the nitrate production (0.11 NO3/NH4) according to the anammox stoichiometry (Equation (3)), meaning that no nitrate production by NOB took place. Case B is presented in Figure 9, which shows that effluent nitrate concentration is stable throughout the presented period, except for a short period around day 420. In this short period, the effluent nitrate concentration was lower, which is explained by the lower influent ammonium concentration at that time, but a stable fraction of nitrate produced per ammonium removed. Over the total period presented in the graph, the average ratio of produced nitrate per ammonium removed was 0.098 mol NO3/mol NH4. Additionally, for this case, the ratio is slightly below the value of the stoichiometric nitrate production (0.11 NO3/NH4) of the PN/A conversion (Equation (3)), thus demonstrating that no nitrate production by NOB took place.
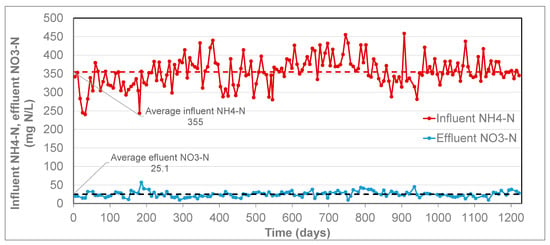
Figure 8.
Influent ammonium and effluent nitrate from the Anammox reactor. Each data point represents a single measurement (NO3-N) or week average value (NH4-N). The horizontal dashed lines show the average values of influent NH4-N and effluent NO3 in the presented period. The average effluent ammonium in this period was 61 mg NH4-N/L (not shown in the graph).
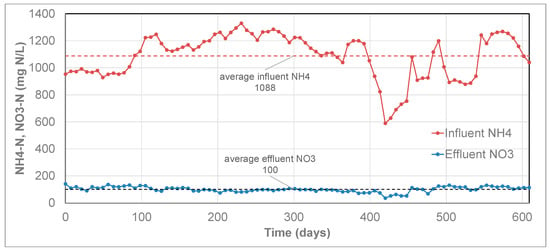
Figure 9.
Influent ammonium and effluent nitrate from the Anammox reactor. Each data point represents the week average values for influent NH4-N or effluent NO3-N. The horizontal dashed lines show the average values of influent NH4-N and effluent NO3-N in the presented period. The average effluent ammonium in this period was 79 mg NH4-N/L (not shown in the graph).
These two cases demonstrate the long-term stability of the ANAMMOX process in terms of selectivity via the Anammox route, without nitrate formation by NOB. This confirms that, in full-scale installations, NOB can effectively be outcompeted by the natural substrate gradients in the granular PN/A biomass, as previously proven in simulation studies [18]. Both cases showed a ratio that was slightly below the value of the anammox stoichiometry. This could be caused by partial removal of nitrate by heterotrophic denitrification. Another possibility is the effect of sludge age on this ratio, since the formation of nitrate by Anammox is linked to growth [3]. This was not further looked into. Previously published operational data on treated reject liquor from thermally hydrolyzed and mesophilic anaerobic digested biosolids (THP-AD) also showed a low nitrate production of less than 5% of the converted ammonium, indicating effective suppression of the NOB activity [8].
2.4. Micronutrients
Although most effluents contain sufficient quantities of essential trace elements, there are certain cases where dosing of micro-nutrients is to be considered to ensure the bioavailability of these elements. It is clear that as condensates lack any minerals, dosing of micro-nutrients is necessary. Other cases for possible dosing of micro-nutrients are effluents containing high-molecular-weight organic components (alike fulvic acid), such as, for example, reject liquors from certain organic waste digestion or dewatering reject liquors from anaerobic sludge digestion, including thermal sludge hydrolysis (THP-AD). Because of the chelating properties of these organic compounds, the bioavailability of the trace metals might be limited [7,8]. Recent research at the Delft University (Netherlands) indicated that essential metals, such as iron (Fe) and copper (Cu), are reduced in concentration when brought in contact with melanoidins and subsequently filtered by a membrane, retaining the high-molecular-weight melanoidins and bound metals [19].
Figure 10 presents the operation of an ANAMMOX reactor treating dewatering reject liquors from anaerobic digestion of sewage sludge in combination with co-digestion of industrial and agricultural waste residues. As the composition of the feedstock to the digesters changes over time, periods with insufficient bioavailability of micronutrients might occur at times (due to lack of nutrients or by precipitation with phosphates). The NH4-N removal of the ANAMMOX plant (typically over 90%) deteriorated after some time (day 50–120), showing increased NH4-N loadings in the outlet. Looking for the root cause, it was found that additional dosing of micro-nutrients did restore the performance again.
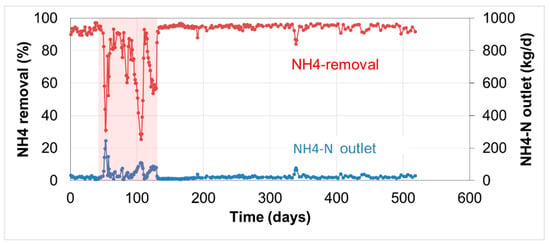
Figure 10.
NH4-N removal and NH4-N outlet load before and after dosing of micro-nutrients at an ANAMMOX reactor treating MAD reject liquor in combination with co-digestion industrial residues.
2.5. Examples of Inhibition
2.5.1. Nitrite
Nitrite is an essential substrate for Anammox bacteria, but at the same time, a too high nitrite concentration can be inhibiting for bacteria. As an intermediate product of the PN/A process, an increase in nitrite concentration can be a possible sign of a disturbed process. Prolonged exposure of granular anammox biomass to nitrite levels up to 50 mg/L did not result in inhibition of the bacterial activity [5]. Although the nitrite concentration is generally low in full-scale installations, high peaks in the nitrite concentration may occasionally occur due to, e.g., interruptions in the power supply, or extreme fluctuations in the ammonium load to the reactor. Figure 11 shows the operational data of a full-scale ANAMMOX reactor. As shown, the nitrite concentration was typically below 10 mg NO2-N/L in the reactor, but at a certain moment it peaked at 60 mg NO2-N/L (pH 7.0). At the time of the event, the effluent ammonium concentration increased by nearly a factor 4, from ~25 to ~100 mg NH4-N/L. Once the nitrite concentration was reduced again, the effluent ammonium concentration returned within days to its previous level of 30–35 mg NH4-N/L. This demonstrates that also under full-scale conditions, the ANAMMOX granules can cope with high nitrite concentrations with a limited effect on the removal efficiency and that the process can recover quickly to its original capacity.
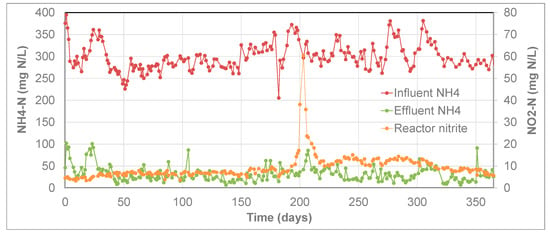
Figure 11.
Operational data from a PN/A ANAMMOX reactor.
It should be noted that no frequent biomass activity assays with granules from the full-scale reactor were performed, and thus it cannot be excluded that the biomass-specific activity did suffer to a larger extent from the nitrite peak. In the full-scale reactor, this could have been compensated by an overcapacity of available anammox activity. In addition to the protection by the aerobic layer, such an overcapacity is an extra protection of process efficiency against process upsets, such as high nitrite concentrations. This overcapacity is not only created by a large amount of granular biomass in the reactor, but also within each of the granules itself. Anammox bacteria near the core of the granule normally receive low substrate concentrations (due to gradients in the granule). After a process upset that affects the anammox bacteria, these bacteria near the core will receive higher substrate concentrations (and fluxes) and thus contribute more towards the overall conversion.
2.5.2. Hypochlorite Incident
At an ANAMMOX installation, an incident happened by which bleach (sodium-hypochlorite) entered the ANAMMOX reactor. At this site, dewatering reject from co-digestion of biosolids and industrial residue was treated by a PN/A ANAMMOX reactor. Since dewatering is only operated for 5 working days, a hydraulic buffer tank is used to also supply flow to the ANAMMOX reactor outside of the working days. During weekends, the reactor is operated at significant lower loadings as the stored reject in the buffer is pumped to the reactor. To maintain a certain hydraulic load, during the weekend, final effluent from the main STW is added to the reject liquor. By error, concentrated hypochlorite used for disinfection of the final effluent entered the PN/A ANAMMOX reactor during the weekend, resulting in a drop of the performance (since day 29 in Figure 12). In time, the reactor started to pick up activity after 15 days and regular loadings were achieved again after approximately 25 days. The granular biomass recovered without any addition of external seed sludge.
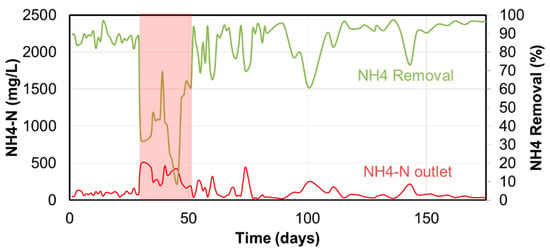
Figure 12.
NH4-N removal and NH4-N outlet concentration of a PN/A ANAMMOX reactor while hypochlorite was accidentally spilled at day 29 and activity was restoring around day 44 and regular loadings treated at day 55.
2.6. Alkalinity
Ammonium removal consumes alkalinity. Conversion of ammonium in a PN/A process consumes 1.1 mol alkalinity per mol NH4 removed (see Equation (3) and [3]). This is the sum of alkalinity consumption by nitritation (2 mol alkalinity per mol ammonium, Equation (1)) and of alkalinity recovery by the anammox conversion (0.13 mol alkalinity per mol ammonium, Equation (2)).
Typically, anaerobically pre-treated waters contain sufficient alkalinity to reach a high ammonium removal efficiency. However, in some cases, there is insufficient alkalinity, often due to upstream alkalinity-consuming reactions. For example, dosing ferric iron (FeCl3) to the anaerobic digester reduces the amount of bicarbonate [7]. The ferric reacts with the phosphate, producing vivianite (Fe3(PO4)2·8(H2O)) [20]. The use of magnesium chloride (MgCl2) for upstream struvite precipitation is another example. In such cases, there are basically three solutions:
- Accept lower NH4 removal efficiency: conversion stops once alkalinity is depleted.
- Increase alkalinity (e.g., indirect alkalinity increases by dosing caustic soda NaOH).
- Replace upstream dosing by an alkaline chemical (e.g., replace MgCl2 by magnesium hydroxide Mg(OH)2).
The following case demonstrates two of these solutions. The treatment sequence of this case is anaerobic sludge digestion followed by a struvite precipitation reactor and finally an ANAMMOX reactor. Initially, MgCl2 was used in the struvite reactor, but this was later replaced by Mg(OH)2.
The operational data presented in Figure 13 show an initial period of 32 days in which magnesium chloride was used in the struvite reactor. The measured alkalinity to ammonium ratio in the effluent from the struvite reactor was 0.74 mol alkalinity per mol ammonium (average of three measurements). Due to this low alkalinity and since no NaOH was dosed to supply additional alkalinity, the resulting ammonium removal efficiency was low, in this case around 40%. Additionally, from the graph, the pH was just below 7 (6.86 on average) in this period, confirming the limited availability of buffering capacity.
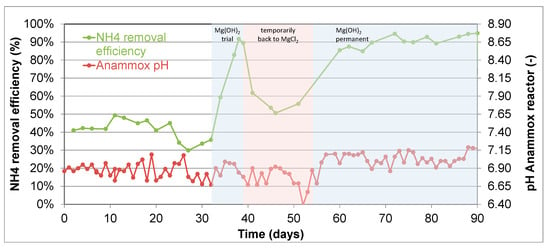
Figure 13.
Operational data from the ANAMMOX reactor downstream of a struvite precipitation reactor. The shaded areas refer to periods in which changes were made in the use of magnesium source in the struvite reactor. Average influent NH4 in the presented period was 621 ± 45 mg NH4-N/L.
When switching to the use of Mg(OH)2 in the struvite reactor, the ammonium removal efficiency quickly increased to >90%. This was seen in the one-week test period (day 32–39) and also after the permanent switch from day 54 onwards. Additionally, the pH was clearly higher, with an average of 7.02. The higher availability of alkalinity was confirmed by a measured alkalinity to ammonium ratio of 1.3 mol/mol (average of two measurements) in the test period. The drop in removal efficiency in the brief period between the test and permanent use of Mg(OH)2 further confirmed that the removal efficiency is directly linked to the type of magnesium chemical used in the upstream struvite reactor. Using Mg(OH)2 for controlled precipitation of phosphate in struvite reactors might reduce the alkalinity deficit in a downstream PN/A anammox reactor. The PHOSPAQTM is an example of struvite technology designed to work with Mg(OH)2 as a magnesium source forming struvite (MgNH4PO4.6H2O) [21].
The operational data presented for this case study clearly demonstrate the relevance of sufficient alkalinity to achieve high ammonium removal efficiencies. It also demonstrates that the ANAMMOX process is flexible in its process control. At low alkalinity availability, and without NaOH dosing, the control can be governed by the pH, thus preventing the pH from becoming too low. The resulting ammonium removal efficiency is then determined by the wastewater characteristics. However, at high alkalinity availability, or when NaOH addition is applied, good process control at a higher pH is feasible, by using ammonium as the lead control parameter. Such a multi parameter-based control will enable for a more flexible and secure process control. This in contrast to process control on the basis of a single parameter, for example pH, which will fail in case NaOH is dosed to the process
2.7. Green House Gas Emissions
The overall emission of nitrous oxide (N2O) in wastewater treatment plants in general is estimated to be 3% of the estimated world’s total anthropogenic N2O emission [22]. Emission data from activated sludge plants show a large variation varying from 0 to 14.6% [22]. N2O and NO emission is generally linked to the activity of AOB and heterotrophic bacteria and not to anammox bacteria [23]. Fluctuations in load, aeration control, and the presence of flocculent sludge are suggested to have an impact on biological N2O emission [22,24]. Simulation studies suggest the control of NH4 effluent concentrations to be a possible means to mitigate N2O emissions [24]. Based on this, the N2O emission from the PN/A ANAMMOX process (continuous aeration, no flocculent biomass, control on NH4) can be expected to be relatively low. Preliminary measurements indicate the relative N2O emission (as fraction of kg N removed) in reject liquor ANAMMOX reactors to be less than for the mainstream STW [23]. Representative and reliable data on N2O and NO emission are, however, scarce as it requires uniformity on analysis methods and long-term testing periods (including the regular loadings). Hence, critical evaluation of research results is important prior to drawing any general conclusions.
3. Conclusions
The Paques ANAMMOX process is a continuously operated biological process using granular PN/A biomass. Since 2002, there have been 66 projects with an overall installed capacity of almost 150,000 kgN/d. Based on the presented operational data from the past 20 years, the following can be concluded:
- A consistent high loading rate to up to 2.5 kg NH4-N/(m3·d) can be achieved;
- Stable long-term removal efficiencies can be maintained;
- The advanced process control is capable of dealing with specific situations, such as the lack of alkalinity or the addition of an excess of alkalinity;
- Knowledge of the (micro)nutrient requirements for specific waste waters contributes towards long-term stability and high removal efficiencies.
The granular PN/A ANAMMOX biomass has been demonstrated to be characterized by:
- A natural selection mechanism to outcompete undesired NOB activity;
- A high resistance against inhibiting compounds, such as bleach and high nitrite;
- A high settling velocity, making it easy to retain the granules in the reactor, but also to remove, concentrate, and store excess granular biomass;
- Excess granular biomass facilitates a quick start up of new ANAMMOX installations.
Author Contributions
Both authors contributed substantially to the realization of this paper, including data analysis, original draft preparation, and reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank all staff of the treatment plants for making the data available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications, 2nd ed.; McGraw-Hill: New York, NY, USA, 2020. [Google Scholar]
- Metcalf & Eddy Inc.; Tchobanoglous, G.; Burton, F.L.; Tsuchihashi, R.; Stensel, H.D. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2013. [Google Scholar]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Driessen, W.; Hendrickx, T.; de Reus, P. Long term experience with the Anammox® process—Treatment of industrial effluent, sludge dewatering liquors and the potential for application in mainstream sewage treatment. In Stickstoffrückbelastung—Stand der Technik 2018—Erweiterter Tagungsband zur 10. Aachener Tagung mit 7. Erfahrungsaustausch Deammonifikation; Grömping, M., Schäpers, D., Eds.; Verlagsgrupppe Mainz GmbH: Leverkusen, Germany, 2018; 13p, ISBN 978-3-95886-211-1. [Google Scholar]
- Abma, W.R.; Driessen, W.; Haarhuis, R.; van Loosdrecht, M.C.M. Upgrading of sewage treatment plant by sustainable & cost-effective separate treatment of industrial wastewater. Water Sci. Technol. 2010, 61, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Narroway, Y.; Barker, R.; Vale, P.; Chadha, M. Linking up nutrient removal, liquor treatment and nutrient recovery. In Proceedings of the 18th European Biosolids & Organic Resources Conference, Manchester, UK, 18–20 November 2013. [Google Scholar]
- Driessen, W.J.B.M.; Van Veldhoven, J.T.A.; Janssen, M.P.M.; Went, C.; Hobbs, E.; van Loosdrecht, M.C.M. Operational experience and lessons learned on treatment of dewatering of reject liquors from thermally hydrolysed and anaerobically digested (THP-MAD) biosolids—Two case studies. In Proceedings of the IWA 2020 Nutrient Recovery & Removal Conference, Virtual Conference, Helsinki, Finland, 1–3 September 2020. [Google Scholar]
- Driessen, W.; van Veldhoven, J.T.A.; Janssen, M.P.M.; van Loosdrecht, M.C.M. Treatment of sidestream dewatering liquors from thermally hydrolysed and anaerobically digested biosolids. Water Pract. Technol. 2020, 15, 142–150. [Google Scholar] [CrossRef]
- Driessen, W.; Sandstra, E.; Tielbaard, M.; Vlaardingerbroek, A. Sustainable effluent treatment combining anaerobic treatment and Anammox—First rendering plant to apply high-rate anaerobic treatment and Anammox to treat its effluent. IWC 20-17. In Proceedings of the ESWP 2020 International Water Conference, Virtual Conference, San Antonio, TX, USA, 9–11 November 2020. [Google Scholar]
- Lettinga, G.; van Velsen, A.F.M.; Hobma, S.; de Zeeuw, W.; Klapwijk, A. Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol. Bioeng. 1980, 22, 699–734. [Google Scholar] [CrossRef]
- Nicolella, C.; van Loosdrecht, M.C.M.; Heijnen, J.J. Wastewater treatment with particulate biofilm reactors. J. Biotechnol. 2000, 80, 1–33. [Google Scholar] [CrossRef]
- Picioreanu, C.; Pérez, J.; van Loosdrecht, M.C.M. Impact of cell cluster size on apparent half-saturation coefficients for oxygen in nitrifying sludge and biofilms. Water Res. 2016, 106, 371–382. [Google Scholar] [CrossRef] [PubMed]
- van der Star, W.R.L.; Abma, W.R.; Blommers, D.; Mulder, J.-W.; Tokutomi, T.; Strous, M.; Picioreanu, C.; van Loosdrecht, M.C.M. Start-up of reactors for anoxic ammonium oxidation: Experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007, 41, 4149–4163. [Google Scholar] [CrossRef] [PubMed]
- Abma, W.R.; Schultz, C.E.; Mulder, J.W.; van der Star, W.R.L.; Strous, M.; Tokutomi, T.; van Loosdrecht, M.C.M. Full-scale granular sludge Anammox process. Water Sci. Technol. 2007, 55, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, D.; Czerwionka, K.; Makinia, J. Influence of temperature on the activity of anammox granular biomass. Water Sci. Technol. 2016, 73, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; van Loosdrecht, M.C.M. Effect of Temperature Change on Anammox Activity. Biotechnol. Bioeng. 2015, 112, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Heijnen, J.J.; van Loosdrecht, M.C.M. Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnol. Bioeng. 2002, 77, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Lotti, T.; Kleerebezem, R.; Picioreanu, C.; van Loosdrecht, M.C.M. Outcompeting nitrite-oxidizing bacteria in single-stage nitrogen removal in sewage treatment plants: A model-based study. Water Res. 2014, 66, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Pavez Jara, J.A.; van Lier, J.B.; de Kreuk, M.K. Analysis and effect of thermal hydrolysis process (THP) on conversion rates of partial nitrification/anammox (PN/A) process. In Proceedings of the 6th IWA YWP Benelux Conference, Luxembourg, Germany, 12–14 February 2020. [Google Scholar]
- Wilbert, P.; Dugulan, A.I.; Goubitz, K.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C.M. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery. Water Res. 2018, 144, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Driessen, W.; Hendrickx, T.; Remy, M.; Haarhuis, R. The Phospaq Process. Chapter 18. In Phosphorus: Polluter and Resource of the Future—Removal and Recovery from Wastewater; Schaum, C., Ed.; IWA Publishing: London, UK, 2018; pp. 351–357. ISBN 9781780408354. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; van der Star, W.R.L.; Wielders, H.A.; Mulder, J.W.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Dynamics of nitric oxide and nitrous oxide emission during full scale reject water treatment. Water Res. 2008, 42, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Baeten, J.E.; Laureni, M.; Volcke, E.I.P. Ammonium-based aeration control improves nitrogen removal efficiency and reduces N2O emissions for partial nitritation-anammox reactors. Chemosphere 2021, 274, 129720. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).