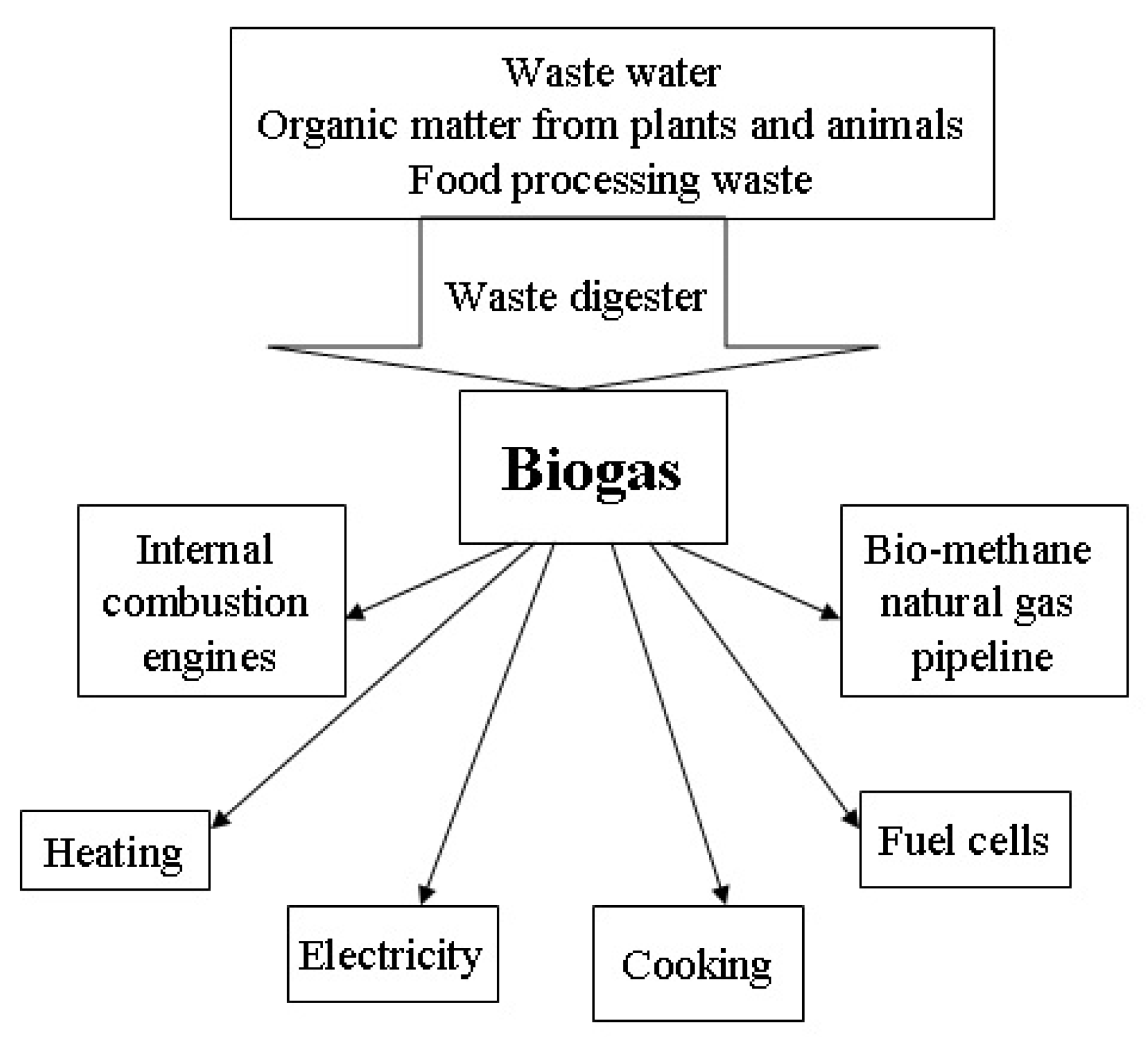
Laminar Burning Velocity of Biogas-Containing Mixtures. A Literature Review
Abstract
1. Introduction
2. General Characteristics of Flames in Gaseous Mixtures. The Laminar Burning Velocity
3. Experimental Methods for Determining the Laminar Burning Velocity
4. Computing Methods
5. Initial Conditions of Studied Biogas-Air Mixtures
6. Discussions
6.1. Laminar Burning Velocities of Biogas-Air Flames
6.2. Effect of Hydrogen Addition on the Laminar Burning Velocity of Biogas-Air Flames
6.3. Effect of Oxygen Addition on the Laminar Burning Velocity of Biogas-Air Flames
7. Conclusions
- -
- The increase in carbon dioxide content decreases laminar burning velocities no matter the initial biogas composition, pressure and temperature. This phenomenon significantly affects the stability of the flame, which can make it difficult to use biogas as a fuel in spark ignited engines and conventional burners.
- -
- Addition of hydrogen to a biogas-air mixture increases the laminar burning velocity due to the increase in thermal diffusivity and the reaction rate, as well as the flame temperature of the mixture. For practical applications, large quantities of H2 added to biogas could avoid the need to completely remove of the carbon dioxide from the biogas blends.
- -
- The addition of oxygen to biogas mixtures leads to increase of the laminar burning velocity due to the increase in the adiabatic flame temperature. Oxygen-enriched combustion of biogas can be efficient for power generation not only because of the lower NOx emissions but also because of the suitability for CO2 capture and storage.
- -
- Small quantities of hydrogen and oxygen added to biogas leads to greater flame stability and improve the combustion properties of these flames.
- -
- The increase of the initial temperature and the decrease of the initial pressure of fuel-oxidant mixture cause the increase of both, experimental and computed laminar burning velocities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Chin, M.J.; Poh, P.E.; Tey, B.T.; Chan, E.S.; Chin, K.L. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sust. Energ. Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Feasibility study of biogas production and utilization as a source of renewable energy in Malaysia. Renew. Sust. Energ. Rev. 2013, 19, 454–462. [Google Scholar] [CrossRef]
- Mengistu, M.G.; Simane, B.; Eshete, G.; Workneh, T.S. A review on biogas technology and its contributions to sustainable rural livelihood in Ethiopia. Renew. Sust. Energ. Rev. 2015, 48, 306–316. [Google Scholar] [CrossRef]
- Wang, Z.; Yelishala, S.C.; Yu, G.; Metghalchi, H.; Levendis, Y.A. Effects of carbon dioxide on laminar burning speed and flame instability of methane/air and propane/air mixtures: A literature review. Energy Fuels 2019, 33, 9403–9418. [Google Scholar] [CrossRef]
- Sulaiman, S.Z.; Khan, N.A.M.H.; Izhab, I.; Shaarani, S.M.; Mudalip, S.K.A.; Man, R.C.; Arshad, Z.I.M.; Kasmani, R.M.; Sulaiman, S. Explosion characteristics assessment of premixed biogas/air mixture in a 20-L spherical vessel. Chem. Eng. Commun. 2021, 208, 583–591. [Google Scholar] [CrossRef]
- Hafner, S.; Rennuit, C. Predicting Methane and Biogas Production with the Biogas Package. Biogas Software. 2015. Available online: https://CRAN.R-project.org/package=biogas (accessed on 3 February 2021).
- Coward, H.F.; Jones, G.W. Limits of flammability of gases and vapors. Bur. Mines Bull. 1952, 503. [Google Scholar] [CrossRef][Green Version]
- Schroeder, V.; Schalau, B.; Molnare, M. Explosion protection in biogas and hybrid power plants. Procedia Eng. 2014, 84, 259–272. [Google Scholar] [CrossRef]
- Casson Moreno, V.; Papasidero, S.; Scarponi, G.E.; Guglielmi, D.; Cozzani, V. Analysis of accidents in biogas production and upgrading. Renew. Energy 2016, 96, 1127–1134. [Google Scholar] [CrossRef]
- Hedlund, F.H. Biomass Accident Investigations-Missed Opportunities for Learning and Accident Prevention. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden, 12–15 June 2017; pp. 1804–1814. [Google Scholar] [CrossRef]
- Boscolo, M.; Bregant, L.; Miani, S.; Padoano, E.; Pille, M. An enquiry into the causes of an explosion accident occurred in a biogas plant. Process Saf. Prog. 2019, e12063. [Google Scholar] [CrossRef]
- Mandal, T.; Kiran, B.A.; Mandal, N.K. Determination of the quality of biogas by flame temperature measurement. Energy Convers. Manag. 1999, 40, 1225–1228. [Google Scholar] [CrossRef]
- Forsich, C.; Lackner, M.; Winter, F.; Kopecek, H.; Wintner, E. Characterization of laser-induced ignition of biogas–air mixtures. Biomass Bioenergy 2004, 27, 299–312. [Google Scholar] [CrossRef]
- Dupont, L.; Accorsi, A. Explosion characteristics of synthesized biogas at various temperatures. J. Hazard. Mater. 2006, 136, 520–525. [Google Scholar] [CrossRef]
- Molnarne, M.; Schröder, V. Using explosion diagrams for estimation of the explosion limits of biogas mixtures. In Proceedings of the 2nd International Conference on Safety & Environment in Process Industry (CISAP-2), Neaples, Italy, 21–24 May 2006. [Google Scholar]
- Porpatham, E.; Ramesh, A.; Nagalingam, B. Effect of hydrogen addition on the performance of a biogas fuelled spark ignition engine. Intern. J. Hydrog. Energy 2007, 32, 2057–2065. [Google Scholar] [CrossRef]
- Liu, C.; Yan, B.; Chen, G.; Bai, X.S. Structures and burning velocity of biomass derived gas flames. Intern. J. Hydrog. Energy 2010, 35, 542–555. [Google Scholar] [CrossRef]
- Ghenai, C.; Janajreh, I. Combustion of renewable biogas fuels. J. Energy Power Eng. 2015, 9, 831–843. [Google Scholar] [CrossRef]
- Dou, Z.; Zheng, L.; Zheng, K.; Pan, R.; Yang, W.; Fu, Y. Effect of film thickness and methane fraction on explosion characteristics of biogas/air mixture in a duct. Process Saf. Environ. Prot. 2020, 139, 26–35. [Google Scholar] [CrossRef]
- Anggono, W.; Wardana, I.N.G.; Lawes, M.; Hughes, K.J.; Wahyudi, S.; Hamidi, N.; Hayakawa, A. Biogas laminar burning velocity and flammability characteristics in spark ignited premix combustion. J. Phys. Conf. Ser. 2013, 423, 012015. [Google Scholar] [CrossRef]
- Anggono, W.; Wardana, I.N.G.; Lawes, M.; Hughes, K.J.; Wahyudi, S.; Hamidi, N. Laminar burning velocity and flammability characteristics of biogas in spark ignited premix combustion at reduced pressure. Appl. Mech. Mater. 2013, 376, 79–85. [Google Scholar] [CrossRef]
- Askari, M.H.; Ashjaee, M.; Karaminejad, S. Experimental and numerical investigation of laminar burning velocity and combustion characteristics of biogas at high pressures. Energy Fuels 2017, 31, 14169–14179. [Google Scholar] [CrossRef]
- Pizzuti, V.; Martins, C.A.; dos Santos, L.R. Experimental determination of laminar burning velocity of biogas at pressures up to 5 bar. Engenharia Térmica (Therm. Eng.) 2018, 17, 3–11. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ray, A.; Ravi, M.R. Experimental and computational investigation of the laminar burning velocity of hydrogen-enriched biogas. Fuel 2019, 235, 810–821. [Google Scholar] [CrossRef]
- Hinton, N.; Stone, R. Laminar burning velocity measurements of methane and carbon dioxide mixtures (biogas) over wide ranging temperatures and pressures. Fuel 2014, 116, 743–750. [Google Scholar] [CrossRef]
- Anggono, W.; Hayakawa, A.; Okafor, E.C.; Gotama, G.J. Experimental and numerical investigation of laminar burning velocities of artificial biogas under various pressure and CO2 concentration. In Proceedings of the 1st International Conference on Automotive, Manufacturing, and Mechanical Engineering (IC-AMME 2018), Kuta, Indonesia, 26–28 September 2018. [Google Scholar]
- Mitu, M.; Prodan, M.; Giurcan, V.; Razus, D.; Oancea, D. Influence of inert gas addition on propagation indices of methane–air deflagrations. Process Saf. Environ. Prot. 2016, 102, 513–522. [Google Scholar] [CrossRef]
- Prodan, M.; Mitu, M.; Razus, D.; Oancea, D. Spark ignition and propagation properties of methane-air mixtures from early stages of pressure history. Rev. Roumaine Chim. 2016, 61, 299–307. [Google Scholar]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Prodan, M.; Oancea, D. Propagation indices of methane-air explosions in closed vessels. J. Loss Prev. Process Ind. 2017, 47, 110–119. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on propagation velocity of methane–air laminar flames. Rev. Chim. 2018, 69, 196–200. [Google Scholar] [CrossRef]
- Razus, D.; Mitu, M.; Giurcan, V.; Oancea, D. Propagation indices of methane-nitrous oxide flames in the presence of inert additives. J. Loss Prev. Process Ind. 2017, 49, 418–426. [Google Scholar] [CrossRef]
- Razus, D.; Mitu, M.; Giurcan, V.; Oancea, D. Methane-unconventional oxidant flames. Laminar burning velocities of nitrogen-diluted methane-N2O mixtures. Process Saf. Environ. Prot. 2018, 114, 240–250. [Google Scholar] [CrossRef]
- Porpatham, E.; Ramesh, A.; Nagalingam, B. Investigation on the effect of concentration of methane in biogas when used as a fuel for a spark ignition engine. Fuel 2008, 87, 1651–1659. [Google Scholar] [CrossRef]
- Saito, N.; Ogawa, Y.; Saso, Y.; Liao, C.; Sakei, R. Flame-extinguishing concentration and peak concentrations of N2, Ar, CO2 and their mixtures for hydrocarbon fuels. Fire Saf. J. 1996, 27, 185–200. [Google Scholar] [CrossRef]
- Lisochkin, Y.A.; Poznyak, V.I. Inerting of methane–air mixtures by compositions based on carbon dioxide and nitrogen with addition of halocarbons. Combust. Explos. Shock Waves 2005, 41, 504–509. [Google Scholar] [CrossRef]
- Seiser, R.; Seshadri, K. The influence of water on extinction and ignition of hydrogen and methane flames. Proc. Combus. Inst. 2005, 30, 407–414. [Google Scholar] [CrossRef]
- Cohé, C.; Chauveau, C.; Gökalp, I.; Kurtuluş, D.F. CO2 addition and pressure effects on laminar and turbulent lean premixed CH4 air flames. Proc. Combust. Inst. 2009, 32, 1803–1810. [Google Scholar] [CrossRef]
- Patino, M.A.M.; Alviso, D.; dos Santos, R.G. Numerical study of laminar premixed methane/air flames with carbon dioxide dilution. In Proceedings of the 16th Brazilian Congress of Thermal Sciences and Engineering, Vitória, Brazil, 7 November 2016. [Google Scholar]
- Jithin, E.V.; Varghese, R.J.; Velamati, R.K. Experimental and numerical investigation on the effect of hydrogen addition and N2/CO2 dilution on laminar burning velocity of methane/oxygen mixtures. Intern. J. Hydrog. Energy 2020, 45, 16838–16850. [Google Scholar] [CrossRef]
- Ren, F.; Xiang, L.; Chu, H.; Jiang, H.; Ya, Y. Modeling study of the impact of blending N2, CO2, and H2O on characteristics of CH4 laminar premixed combustion. Energy Fuels 2020, 34, 1184–1192. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Osaka, Y.; Bai, Y.; Kobayashi, N.; Chen, Y. Combustion and heat release characteristics of biogas under Hydrogen- and Oxygen-enriched condition. Energies 2017, 10, 1200. [Google Scholar] [CrossRef]
- Acero, M.J.; Pacheco, L.E.; Diaz, C.A. Numerical study of the effect of hydrogen addition on the laminar flame speed and premixed flame structure of biogas. Int. J. Renew. Energy Res. 2018, 8, 1098–1104. [Google Scholar]
- Suhaimi, M.S.; Saat, A.; Abdul Wahid, M.; Rahman, M.M.; Daierobbi, G. Effect of hydrogen addition on biogas combustion and flame propagation. J. Mek. 2018, 41, 17–23. [Google Scholar]
- Acero-Caballero, M.J.; Pacheco-Sandoval, L.E.; Díaz-González, C.A. Effect of hydrogen addition on the laminar flame speed of biogas. Experimental and numerical study. Sci. Tech. 2019, 24, 472–478. [Google Scholar] [CrossRef]
- Wei, Z.; Zhen, H.; Fu, J.; Leung, C.; Cheung, C.; Huang, Z. Experimental and numerical study on the laminar burning velocity of hydrogen enriched biogas mixture. Intern. J. Hydrog. Energy 2019, 44, 22240–22249. [Google Scholar] [CrossRef]
- Zheng, L.; Dou, Z.; Du, D.; Wang, X.; Jin, H.; Yu, M.; Wang, Y. Study on explosion characteristics of premixed hydrogen/biogas/air mixture in a duct. Intern. J. Hydrog. Energy 2019, 44, 27159–27173. [Google Scholar] [CrossRef]
- Nurmukan, D.; Chen, T.J.M.; Hung, Y.M.; Ismadi, M.Z.; Chong, C.T.; Tran, M.V. Enhancement of biogas/air combustion by hydrogen addition at elevated temperatures. Int. J. Energy Res. 2020, 44, 1519–1534. [Google Scholar] [CrossRef]
- Quintino, F.M.; Fernandes, E.C. Numerical investigation of the impact of H2 enrichment on lean biogas/air flames: An analytical modelling approach. Energies 2021, 14, 369. [Google Scholar] [CrossRef]
- Jeong, C.; Kim, T.; Lee, K.; Song, S.; Chung, K.M. Generating efficiency and emissions of a spark-ignition gas engine generator fuelled with biogas–hydrogen blends. Intern. J. Hydrog. Energy 2009, 34, 9620–9627. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Michos, C.N. Generation of combustion irreversibilities in a spark ignition engine under biogas–hydrogen mixtures fuelling. Intern. J. Hydrog. Energy 2009, 34, 4422–4437. [Google Scholar] [CrossRef]
- Bouguessa, R.; Tarabet, L.; Loubar, K.; Belmrabet, T.; Tazerout, M. Experimental investigation on biogas enrichment with hydrogen for improving the combustion in diesel engine operating under dual fuel mode. Intern. J. Hydrog. Energy 2020, 45, 9052–9063. [Google Scholar] [CrossRef]
- Khatri, N.; Khatri, K.K. Hydrogen enrichment on diesel engine with biogas in dual fuel mode. Int. J. Hydrog. Energy 2020, 45, 7128–7140. [Google Scholar] [CrossRef]
- Cacua, K.; Amell, A.; Cadavid, F. Effects of oxygen enriched air on the operation and performance of a diesel-biogas dual fuel engine. Biomass Bioenergy 2012, 45, 159–167. [Google Scholar] [CrossRef]
- Oh, J.; Noh, D. Laminar burning velocity of oxy-methane flames in atmospheric condition. Energy 2012, 45, 669–675. [Google Scholar] [CrossRef]
- Cardona, C.A.; Amell, A.A. Laminar burning velocity and interchangeability analysis of biogas/C3H8/H2 with normal and oxygen-enriched air. Intern. J. Hydrog. Energy 2013, 38, 7994–8001. [Google Scholar] [CrossRef]
- Navarro-Puyuelo, A.; Reyero, I.; Moral, A.; Bimbela, F.; Bañares, M.A.; Gandía, L.M. Effect of oxygen addition, reaction temperature and thermal treatments on syngas production from biogas combined reforming using Rh/alumina catalysts. J. Ind. Eng. Chem. 2019, 80, 217–226. [Google Scholar] [CrossRef]
- Striūgas, N.; Zakarauskas, K.; Paulauskas, R.; Skvorčinskienė, R. Chemiluminescence-based characterization of tail biogas combustion stability under syngas and oxygen-enriched conditions. Exp. Therm. Fluid Sci. 2020, 116, 110133. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; He, Y.; Han, X.; Sun, Z.; Zhu, Y.; Costa, M. Laminar burning velocities of CH4/O2/N2 and oxygen-enriched CH4/O2/CO2 flames at elevated pressures measured using the heat flux method. Fuel 2020, 259, 116152. [Google Scholar] [CrossRef]
- Pizzuti, L.; Martins, C.A.; Lacava, P.T. Laminar burning velocity and flammability limits in biogas: A literature review. Renew. Sustain. Energy Rev. 2016, 62, 856–865. [Google Scholar] [CrossRef]
- Chen, Z.; Burke, M.P.; Ju, Y. Effects of Lewis number and ignition energy on the determination of laminar flame speed using propagating spherical flames. Proc. Combust. Inst. 2009, 32, 1253–1260. [Google Scholar] [CrossRef]
- Pizzuti, L.; Torres, F.A.; Ferreira, R.W.; dos Santos, L.R.; Lacava, P.T.; Martins, C.A. Laminar Burning and Flammability Limits in Biogas: A State of the Art. In Proceedings of the 10th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Orlando, FL, USA, 14–16 July 2014; Available online: http://hdl.handle.net/2263/44642 (accessed on 8 February 2021).
- Rallis, C.J.; Garforth, A.M. The determination of laminar burning velocity. Prog. Energy Combust. Sci. 1980, 6, 303–329. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Hansen, N.; Ju, Y.; Kohse-Höinghaus, K.; Law, C.K.; Qi, F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Prog. Energy Combust. Sci. 2014, 43, 36–67. [Google Scholar] [CrossRef]
- Andrews, G.E.; Bradley, D. Determination of burning velocities: A critical review. Combust Flame 1972, 18, 133–153. [Google Scholar] [CrossRef]
- Forman, A.W. Combustion Theory, 2nd ed.; The Benjamin/Cummings Publishing Copmany: Princeton, NJ, USA, 1984. [Google Scholar]
- Law, C.K. Combustion Physics; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Kuo, K.K. Principles of Combustion, 2nd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Correa, S.M. A Review of NOx Formation Under gas-turbine combustion conditions. Combust. Sci. Technol. 1993, 87, 329–362. [Google Scholar] [CrossRef]
- Flamme, M. New combustion systems for gas turbines (NGT). Appl. Therm. Eng. 2004, 24, 1551–1559. [Google Scholar] [CrossRef]
- Lewis, B.; von Elbe, G. Determination of the speed of flames and the temperature distribution in a spherical bomb from time-pressure explosion records. J. Chem. Phys. 1934, 2, 283–290. [Google Scholar] [CrossRef]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.I.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel + air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Liu, J.; Sun, N. Investigation of laminar flame speeds of CH4/O2/CO2 mixtures at ordinary pressure and kinetic simulation. Energy 2014, 70, 626–634. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q. Effect of the elevated initial temperature on the laminar flame speeds of oxy-methane mixtures. Energy 2018, 147, 876–883. [Google Scholar] [CrossRef]
- Kishore, V.R.; Duhan, N.; Ravi, M.R.; Ray, A. Measurement of adiabatic burning velocity in natural gas-like mixtures. Exp. Therm. Fluid Sci. 2008, 33, 10–16. [Google Scholar] [CrossRef]
- Chan, Y.; Zhu, M.; Zhang, Z.; Liu, P.; Zhang, D. The effect of CO2 dilution on the laminar burning velocity of premixed methane/air flames. Energy Procedia 2015, 75, 3048–3053. [Google Scholar] [CrossRef]
- Nonaka, H.O.B.; Pereira, F.M. Experimental and numerical study of CO2 content effects on the laminar burning velocity of biogas. Fuel 2016, 182, 382–390. [Google Scholar] [CrossRef]
- Park, O.; Veloo, P.S.; Liu, N.; Egolfopoulos, F.N. Combustion characteristics of alternative gaseous fuels. Proc. Combust. Inst. 2011, 33, 887–894. [Google Scholar] [CrossRef]
- Halter, F.; Foucher, F.; Landry, L.; Mounaïm-Rousselle, C. Effect of dilution by nitrogen and/or carbon dioxide on methane and iso-octane air flames. Combust. Sci. Technol. 2009, 181, 813–827. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zhang, M.; Gong, J.; Jin, W.; Huang, Z. Experimental and numerical study on laminar flame characteristics of methane oxy-fuel mixtures highly diluted with CO2. Energy Fuels 2013, 27, 6231–6237. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, Z.; Yu, G.; Yang, Y.; Metghalchi, H. Experimental study of laminar burning speed for premixed biomass/air flame. J. Energy Resour. Technol. 2019, 141, 022206. [Google Scholar] [CrossRef]
- Stone, R.; Clarke, A.; Beckwith, B. Correlations for the laminar burning velocity of methane/diluent/air mixtures obtained in free-fall experiments. Combust. Flame 1998, 114, 546–555. [Google Scholar] [CrossRef]
- Anggono, W.; Wardana, I.N.G.; Lawes, M.; Hughes, K.J.; Wahyudi, S.; Hamidi, N.; Hayakawa, A. The influence of CO2 in biogas flammability limit and laminar burning velocity in spark ignited premix combustion at various pressures. Sustain. Energy Adv. Mater. AIP Conf. Proc. 2016, 1717, 030001-1–030001-7. [Google Scholar] [CrossRef]
- Vagelopoulos, C.M.; Egolfopoulos, F.N. Direct experimental determination of laminar flame speeds. Symp. Int. Combust. 1998, 27, 513–519. [Google Scholar] [CrossRef]
- Law, C.K. Dynamics of stretched flames. Symp. Int. Combust. Proc. 1989, 22, 1381–1402. [Google Scholar] [CrossRef]
- Echekki, T.; Mungal, M.G. Flame speed measurements at the tip of a slot burner: Effects of flame curvature and hydrodynamic stretch. Symp. Int. Combust. Proc. 1991, 23, 455–461. [Google Scholar] [CrossRef]
- Gaydon, A.G.; Wolfhard, H.G. Flames: Their Structure, Radiation, and Temperature, 3rd ed.; Chapman & Hall Ltd.: New York, NY, USA, 1970. [Google Scholar]
- Tsuji, H. Experimental studies of near limit flames using counterflow flame techniques. In Proceedings of the ASME-JSME Thermal Engineering Joint Conference Proceedings, Honolulu, HI, USA, 20–24 March 1983; p. 9. [Google Scholar]
- Wu, C.K.; Law, C.K. On the determination of laminar flame speeds from stretched flames. Symp. Int. Combust. 1985, 20, 1941–1949. [Google Scholar] [CrossRef]
- Zhu, D.L.; Egolfopoulos, F.N.; Law, C.K. Experimental and numerical determination of laminar flame speeds of methane/(Ar, N2, CO2)-air mixtures as function of stoichiometry, pressure, and flame temperature. Symp. Int. Combust. 1989, 22, 1537–1545. [Google Scholar] [CrossRef]
- Chen, Z.; Burke, M.P.; Ju, Y.G. Effects of compression and stretch on the determination of laminar flame speeds using propagating spherical flames. Combust. Theory Modeling 2009, 13, 343–364. [Google Scholar] [CrossRef]
- Faghih, M.; Chen, Z. The constant-volume propagating spherical flame method for laminar flame speed measurement. Sci. Bull. 2016, 61, 1296–1310. [Google Scholar] [CrossRef]
- Xiouris, C.; Ye, T.; Jayachandran, J.; Egolfopoulos, F.N. Laminar flame speeds under engine-relevant conditions: Uncertainty quantification and minimization in spherically expanding flame experiments. Combust Flame 2016, 163, 270–283. [Google Scholar] [CrossRef]
- Mével, R.; Lafosse, F.; Chaumeix, N.; Dupré, G.; Paillard, C.E. Spherical expanding flames in H2-N2O-Ar mixtures: Flame speed measurements and kinetic modelling. Intern. J. Hydrog. Energy 2009, 34, 9007–9018. [Google Scholar] [CrossRef]
- Razus, D.; Oancea, D.; Movileanu, C. Burning velocity evaluation from pressure evolution during the early stage of closed-vessel explosions. J. Loss Prev. Process Ind. 2006, 19, 334–342. [Google Scholar] [CrossRef]
- Zahedi, P.; Yousefi, K. Effects of pressure and carbon dioxide, hydrogen and nitrogen concentration on laminar burning velocities and NO formation of methane-air mixtures. J. Mech. Sci. Technol. 2014, 28, 377–386. [Google Scholar] [CrossRef]
- Goodwin, D.; Moffat, H.; Speth, R. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. 2015. Available online: http://www.cantera.org (accessed on 19 February 2021).
- Boushaki, T.; Zaidaoui, H.; Manseur, F.; Rahib, Y.; Sarh, B. Characteristics of Biogas and Syngas Combustion. In Proceedings of the 7th International Renewable and Sustainable Energy Conference (IRSEC), Agadir, Morocco, 27–30 November 2019. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Goldenberg, B.E.M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C.; Lissianski, V.V.; et al. GRI-Mech 3.0. 1999. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 19 February 2021).
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.N.; Law, C.K. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds. USC Mech, Version II; Combustion Kinetics Laboratory, University of Southern California. 2007. Available online: http://ignis.usc.edu/USC_Mech_II.htm (accessed on 10 May 2021).
- Petrova, M.V.; Williams, F.A. A small detailed chemical kinetic mechanism for hydrocarbon combustion. Combust. Flame 2006, 144, 526–544. [Google Scholar] [CrossRef]
- Qin, Z.; Lissianski, V.V.; Yang, H.; Gardiner, W.C.; Davis, S.G.; Wang, H. Combustion chemistry of propane: A case study of detailed reaction mechanism optimization. Proc. Combust. Inst. 2000, 28, 1663–1669. [Google Scholar] [CrossRef]
- Le Cong, T.; Dagaut, P. Experimental and detailed kinetic modeling of the oxidation of methane and methane/syngas mixtures and effect of carbon dioxide addition. Combust. Sci. Technol. 2008, 180, 2046–2091. [Google Scholar] [CrossRef]
- Le Cong, T.; Dagaut, P.; Dayma, G. Oxidation of natural gas, natural gas/syngas mixtures, and effect of burnt gas recirculation: Experimental and detailed kinetic modeling. J. Eng. Gas Turbine Power 2008, 130, 41502. [Google Scholar] [CrossRef]
- Zhen, H.S.; Leung, C.W.; Cheung, C.S.; Huang, Z.H. Characterization of biogas-hydrogen premixed flames using Bunsen burner. Int. J. Hydrog. Energy 2014, 39, 13292–13299. [Google Scholar] [CrossRef]
- Qin, W.; Egolfopoulos, F.N.; Tsotsis, T.T. Fundamental and environmental aspects of landfill gas utilization for power generation. Chem. Eng. J. 2001, 82, 157–172. [Google Scholar] [CrossRef]
- Ju, Y.; Masuya, G.; Ronney, P.D. Effects of radiative emission and absorbtion on the propagation of premixed gas flames. Symp. Intern. Combust. 1998, 27, 2619–2626. [Google Scholar] [CrossRef]
- Movileanu, C.; Razus, D.; Oancea, D. Additive effects on the burning velocity of ethylene–air mixtures. Energy Fuels 2011, 25, 2444–2451. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Miao, H. Experimental and numerical study on laminar burning velocities and flame instabilities of hydrogen–air mixtures at elevated pressures and temperatures. Intern. J. Hydrog. Energy 2009, 34, 8741–8755. [Google Scholar] [CrossRef]
- Zhen, H.; Leung, C.; Cheung, C. Effects of hydrogen addition on the characteristics of a biogas diffusion flame. Int. J. Hydrog. Energy 2013, 38, 6874–6881. [Google Scholar] [CrossRef]
- Wei, Z.; Leung, C.; Cheung, C.; Huang, Z. Effects of equivalence ratio, H2, and CO2 addition on the heat release characteristics of premixed laminar biogas-hydrogen flame. Intern. J. Hydrog. Energy 2016, 41, 6567–6580. [Google Scholar] [CrossRef]
- Coppens, F.H.V.; Ruyck, J.D.; Konnov, A.A. Effects of hydrogen enrichment on adiabatic burning velocity and NO formation in methane + air flames. Exp. Therm. Fluid. Sci. 2007, 31, 437–444. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Jin, C.; Zheng, J. Experimental and numerical study on laminar burning characteristics of premixed methane-hydrogen-air flame. Intern. J. Hydrog. Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Xu, N.; Yu, S.; Zhang, M.; Huang, Z. Thermal and chemical effects of water addition on laminar burning velocity of syngas. Energy Fuels 2014, 28, 3391–3398. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Lee, Y.; Kim, C.; Lee, S.; Moriyoshi, Y. Performance and emission characteristics of a SI engine fueled by low calorific biogas blended with hydrogen. Int. J. Hydrog. Energy 2011, 36, 10080–10088. [Google Scholar] [CrossRef]
- Mariani, A.; Unich, A.; Minale, U. Combustion of hydrogen enriched methane and biogases containing hydrogen in a controlled auto-ignition engine. Appl. Sci. 2018, 8, 2667. [Google Scholar] [CrossRef]
- Rocha, N.; Quintino, F.; Fernandes, E. H2 enrichment impact on the chemiluminescence of biogas/air premixed flames. Intern. J. Hydrog. Energy 2020, 45, 3233–3250. [Google Scholar] [CrossRef]
- Fu, J.; Tang, C.; Jin, W.; Huang, Z. Effect of preferential diffusion and flame stretch on flame structure and laminar burning velocity of syngas Bunsen flame using OH-PLIF. Intern. J. Hydrog. Energy 2014, 39, 12187–12193. [Google Scholar] [CrossRef]
- Buhre, B.J.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
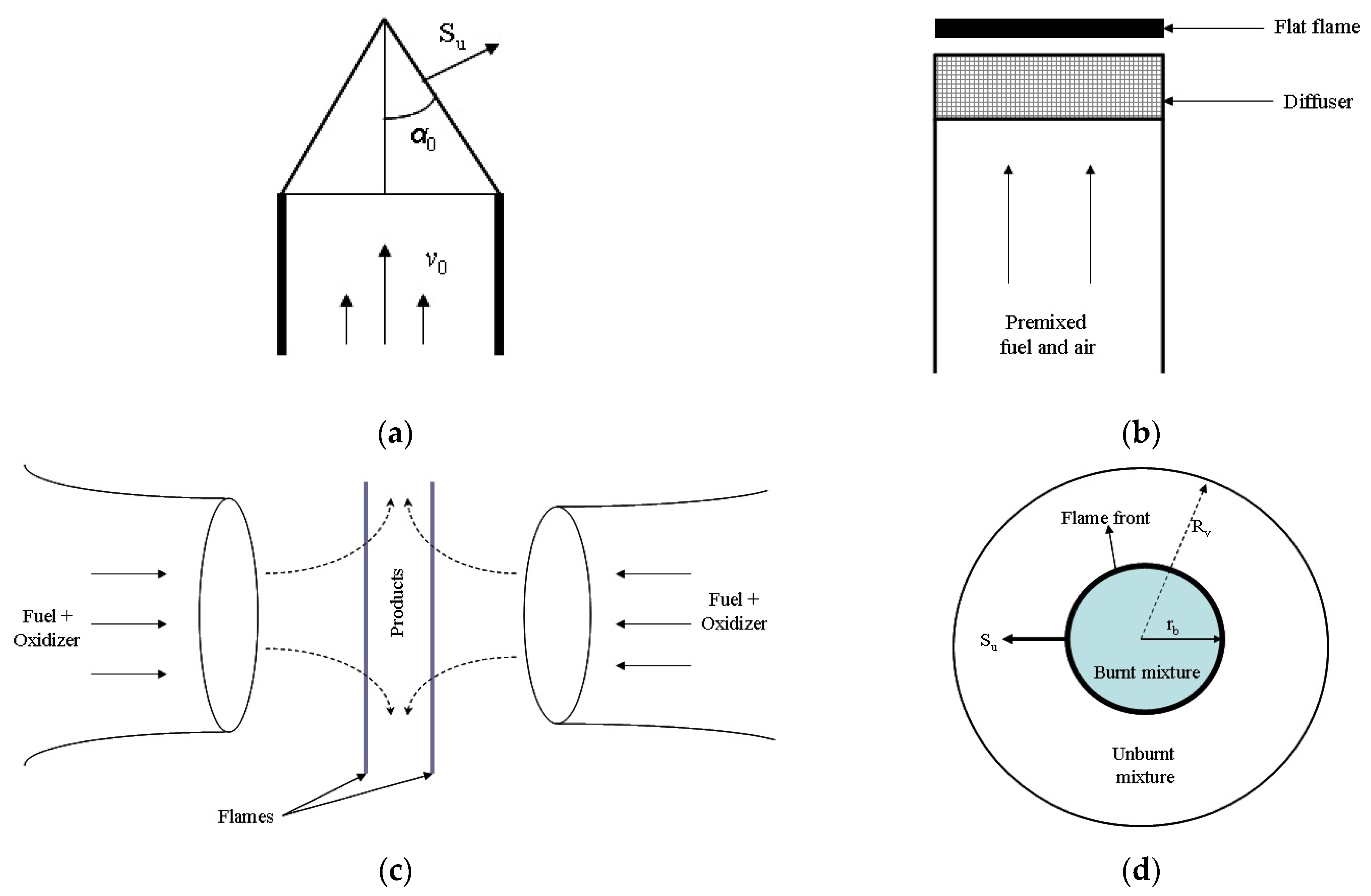




















| Flame Type | Experimental Method | Flame Monitoring | Initial Conditions | |
|---|---|---|---|---|
| Stationary | Burner method | Bunsen burner | Schlieren photography | Various pressures and compositions |
| Flat flame burner | Schlieren photography | Over the whole flammability range | ||
| Counter-flow twin flames | Dopler velocimetry particle Imaging velocimetry | Various pressures and temperatures | ||
| Non-stationary | Spherical flames | Constant volume method | Schlieren photography Pressure transducers Ionization gauges High speed camera | Various compositions, pressures and temperatures |
| Constant pressure method | Schlieren photography Pressure transducers Ionization gauges High speed camera | Various compositions and temperatures | ||
| Method | Advantage | Disadvantage |
|---|---|---|
| Bunsen burner [85] |
|
|
| Flat flame burner [88] |
|
|
| Counter-flow twin flames [89,90,91] |
|
|
| Closed vessel method [93,94,95,96] |
|
|
| Author | Equivalence Ratio, φ | Initial Pressure, p0 | Initial Temperature, T0 | [CO2] % |
|---|---|---|---|---|
| Anggono et al. [20,21,26,84] | 0.5–1.4 | 0.5; 1; 3 bar | 298 K | 30.6 |
| Pizzuti et al. [23] | 0.7–1.1 | 1–5 bar | 298 K | 35–55 |
| Yadav et al. [24] | 0.7–1.4 | 1 bar | 298 K | 5–50 |
| Hinton and Stone [25] | 0.7–1.4 | 1–18 bar | 380–660 K | 0–40 |
| Mitu et al. [29] | 0.6–1.3 | 0.5–2 bar | 298 K | 0–17.5 |
| Cohé et al. [38] | 0.6 | 1–9 bar | 298 K | 0–40 |
| Patino et al. [39] | 0.7–1.3 | 1 bar | 298 K | 0–50 |
| Ren et al. [41] | 0.8–1.2 | 1 bar | 398 K | 0–40 |
| Acero-Caballero et al. [43,45] | 1.0–1.1 | 1 bar | 298 K | 34–40 |
| Suhaimi et al. [44] | 0.4–0.9 | 1 bar | 300 K | 50 |
| Wei et al. [46] | 0.9–1.3 | 1 bar | 298 K | 40–60 |
| Nurmukan et al. [48] | 0.8–1.3 | 1 bar | 298–440 K | 30–40 |
| Quintino et al. [49] | 0.8–1.0 | 1 bar | 298 K | 0–20 |
| Cardona and Amell [56] | 0.6–1.5 | 0.85 bar | 295 K | 34 |
| Hu et al. [74,75] | 0.6–1.4 | 1 bar | 300; 400; 543 K | 25; 35 |
| Kishore et al. [76] | 0.8–1.3 | 1 bar | 307 K | 0–60 |
| Chan et al. [77] | 0.8–1.4 | 1 bar | 298 K | 0–15 |
| Nonaka and Pereira [78] | 0.7–1.4 | 1 bar | 298 K | 0–50 |
| Park et al. [79] | 0.75–1.25 | 1; 2; 4 bar | 298 K | 25; 45 |
| Halter et al. [80] | 1.0 | 1 bar | 300 K | 0–20 |
| Xie et al. [81] | 0.4–1.6 | 1; 2; 3 bar | 300 K | 0–60 |
| Bai et al. [82] | 0.8–1.2 | 0.5–6.9 bar | 298–661 K | 0–60 |
| Stone et al. [83] | 0.6–1.4 | 0.5–10.4 bar | 295–454 K | 0–60 |
| Zahedi and Yousefi [97] | 0.7–1.3 | 1–5 bar | 298 K | 0–20 |
| Boushaki et al. [99] | 0.7–1.3 | 1 bar | 298 K | 0–50 |
| Zhen et al. [106] | 0.8–1.2 | 1 bar | 298 K | 40–60 |
| Qin et al. [107] | 0.65–0.75 | 1 bar | 300 K | 0–50 |
| Type of Method | Method/Model/Mechanism Name | Reference | |
|---|---|---|---|
| Experimental | Bunsen burner | Cohé et al. [38] | |
| Contoured slot burner | Cardona and Amell [56] | ||
| Flat flame burner | Kishore et al. [76] Chan et al. [77] Nonaka and Pereira [78] Zahedi and Yousefi [97] Qin et al. [107] | ||
| Counter-flow twin flames | Park et al. [79] | ||
| Closed vessel | Anggono et al. [20,21,26,84] Pizzuti et al. [23] Hinton and Stone [25] Mitu et al. [29] Halter et al. [80] Bai et al. [82] Stone et al. [83] | ||
| Numerical | CHEMKIN software | GRI-Mech 2.11 | Cardona and Amell [56] |
| GRI-Mech. 3.0 | Anggono et al. [26] Cohé et al. [38] Ren et al. [41] Halter et al. [80] Zahedi and Yousefi [97] | ||
| C1-C3 mechanism | Cardona and Amell [56] | ||
| Le Cong mechanism | Chan et al. [77] | ||
| Cantera package | GRI-Mech. 3.0 | Bai et al. [82] | |
| 1D Premix code | GRI-Mech 2.11 | Qin et al. [107] | |
| GRI-Mech. 3.0 | Pizzuti et al. [23] Kishore et al. [76] | ||
| USC-Mech 2.0 mechanism | Park et al. [79] | ||
| San Diego mechanism | Pizzuti et al. [23] | ||
| Chem1D code | GRI-Mech. 3.0 | Nonaka and Pereira [78] | |
| USC-Mech 2.0 mechanism | Nonaka and Pereira [78] | ||
| San Diego mechanism | Nonaka and Pereira [78] | ||
| Konnov mechanism | Nonaka and Pereira [78] | ||
| REGATH package | GRI-Mech. 3.0 | Patino et al. [39] | |
| COSILAB code | GRI-Mech. 3.0 | Mitu et al. [29] Boushaki et al. [99] | |
| Type of Method | Method/Model/Mechanism Name | Reference | |
|---|---|---|---|
| Experimental | Bunsen burner | Acero-Caballero et al. [45] Nurmukan et al. [48] Zhen et al. [111] | |
| Flat flame burner | Yadav et al. [24] | ||
| Closed vessel | Suhaimi et al. [44] Wei et al. [46] | ||
| Numerical | CHEMKIN software | GRI-Mech 2.11 | Acero et al. [43] Acero-Caballero et al. [45] |
| GRI-Mech. 3.0 | Yadav et al. [24] Acero-Caballero et al. [45] Wei et al. [46] | ||
| San Diego mechanism | Yadav et al. [24] Wei et al. [46] | ||
| ANSYS Fluent software | reduced GRI-Mech 3.0 | Nurmukan et al. [48] | |
| Cantera package | USC-Mech 2.0 mechanism | Quintino et al. [49] | |
| Type of Method | Method/Model/Mechanism Name | Reference | |
|---|---|---|---|
| Experimental | Bunsen burner | Hu et al. [74] | |
| Contoured slot burner | Cardona and Amell [56] | ||
| Flat flame burner | Wang et al. [59] | ||
| Closed vessel | Xie et al. [81] | ||
| Numerical | CHEMKIN software | GRI-Mech. 3.0 | Cardona and Amell [56] Wang et al. [59] Hu et al. [74] Xie et al. [81] |
| HP-Mech mechanism | Wang et al. [59] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giurcan, V.; Movileanu, C.; Musuc, A.M.; Mitu, M. Laminar Burning Velocity of Biogas-Containing Mixtures. A Literature Review. Processes 2021, 9, 996. https://doi.org/10.3390/pr9060996
Giurcan V, Movileanu C, Musuc AM, Mitu M. Laminar Burning Velocity of Biogas-Containing Mixtures. A Literature Review. Processes. 2021; 9(6):996. https://doi.org/10.3390/pr9060996
Chicago/Turabian StyleGiurcan, Venera, Codina Movileanu, Adina Magdalena Musuc, and Maria Mitu. 2021. "Laminar Burning Velocity of Biogas-Containing Mixtures. A Literature Review" Processes 9, no. 6: 996. https://doi.org/10.3390/pr9060996
APA StyleGiurcan, V., Movileanu, C., Musuc, A. M., & Mitu, M. (2021). Laminar Burning Velocity of Biogas-Containing Mixtures. A Literature Review. Processes, 9(6), 996. https://doi.org/10.3390/pr9060996









