Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues
Abstract
1. Introduction
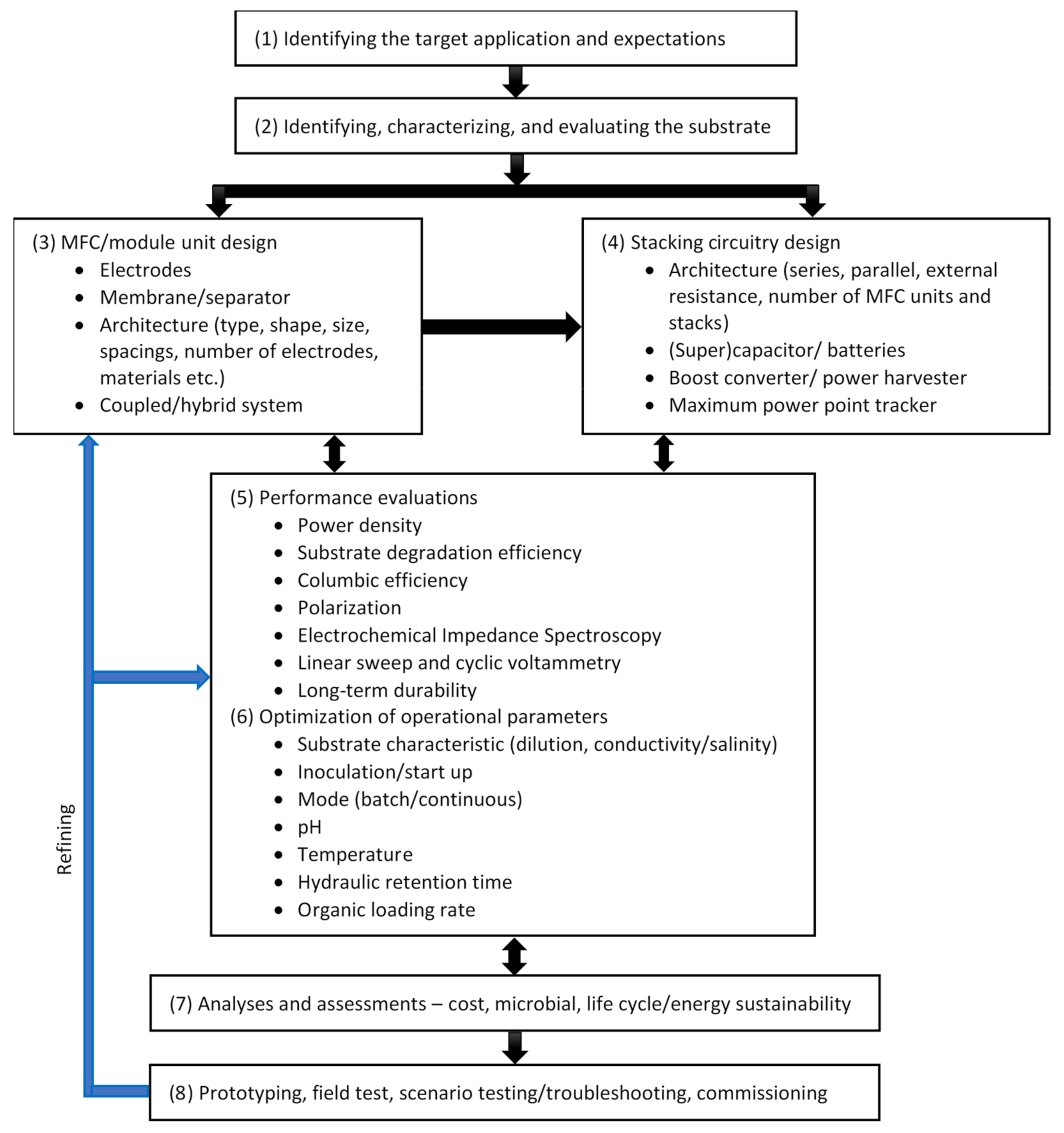
2. Scale up
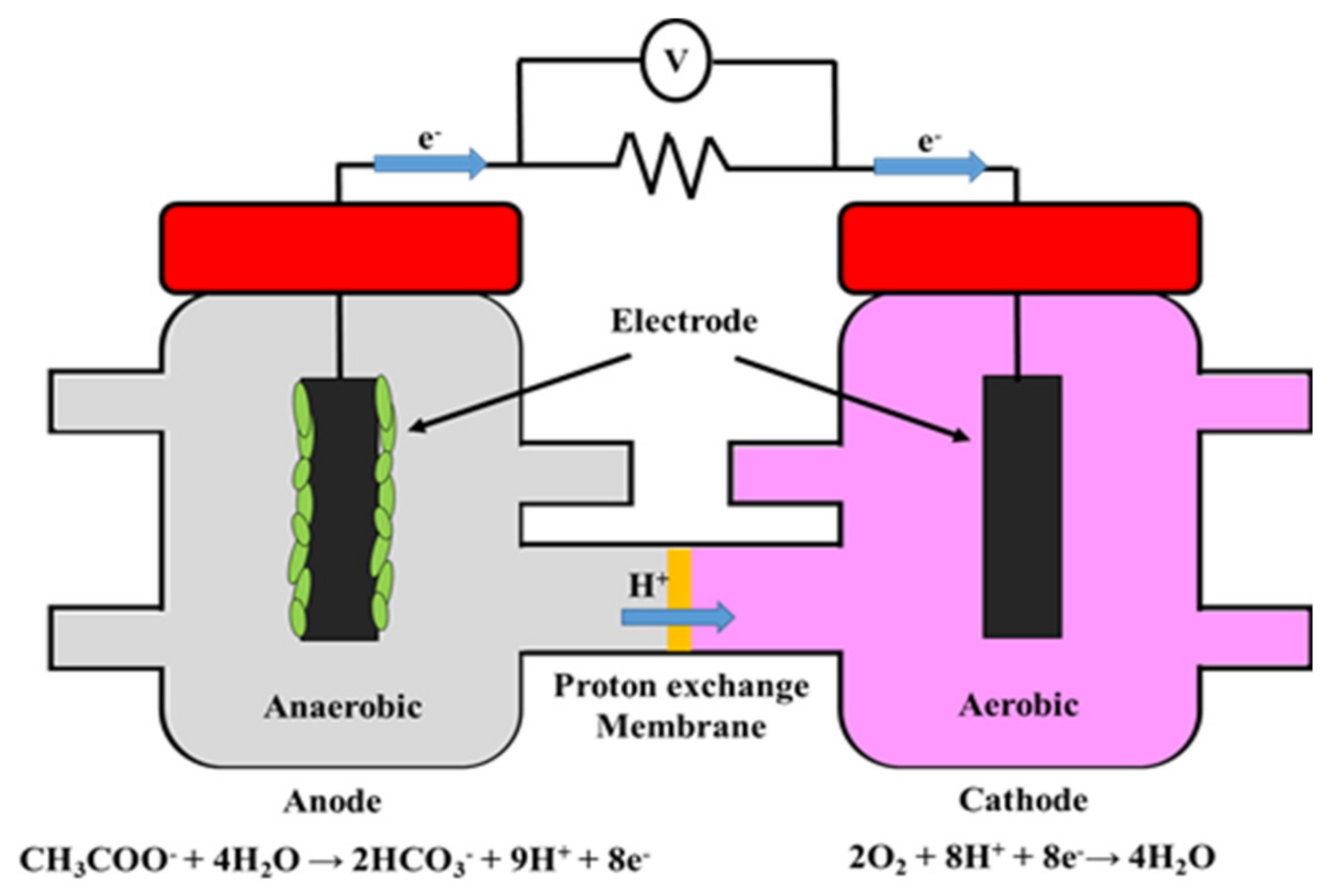
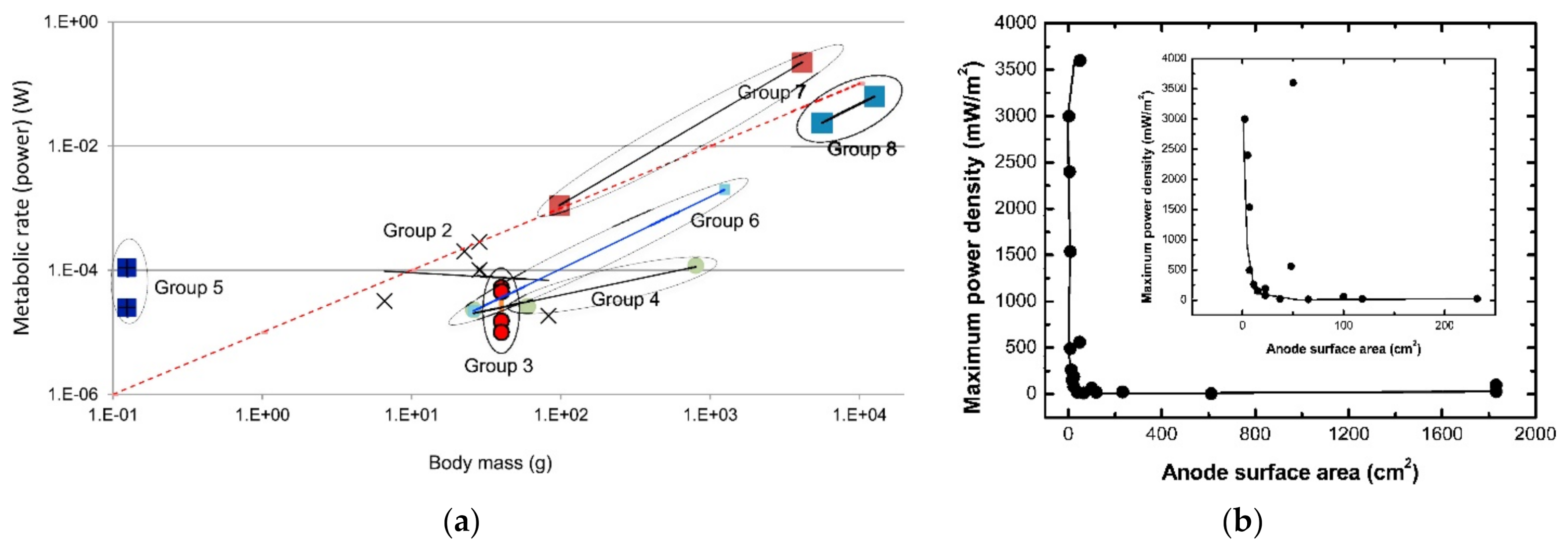
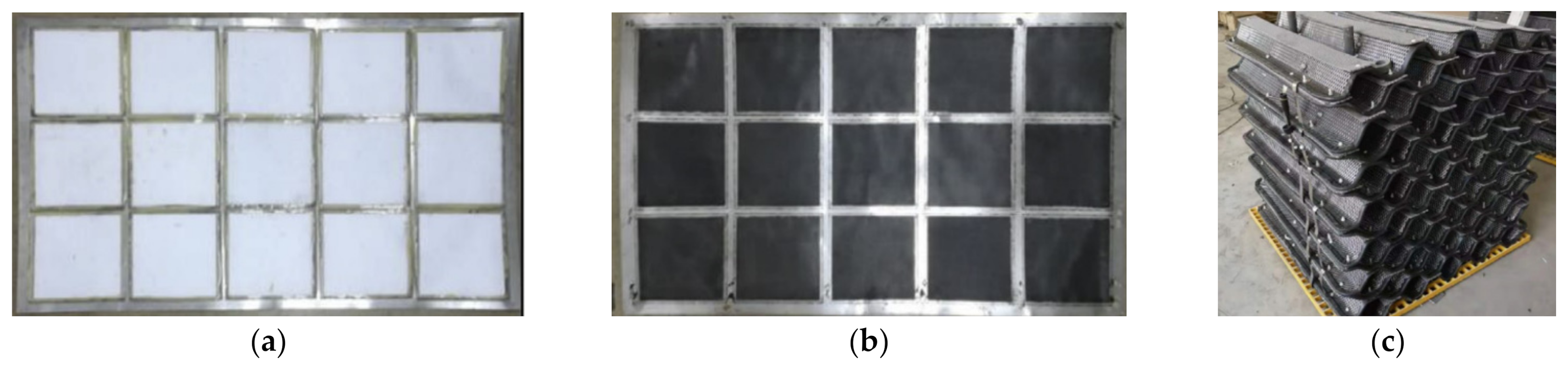
2.1. MFC Architecture
2.2. Electrode Modification
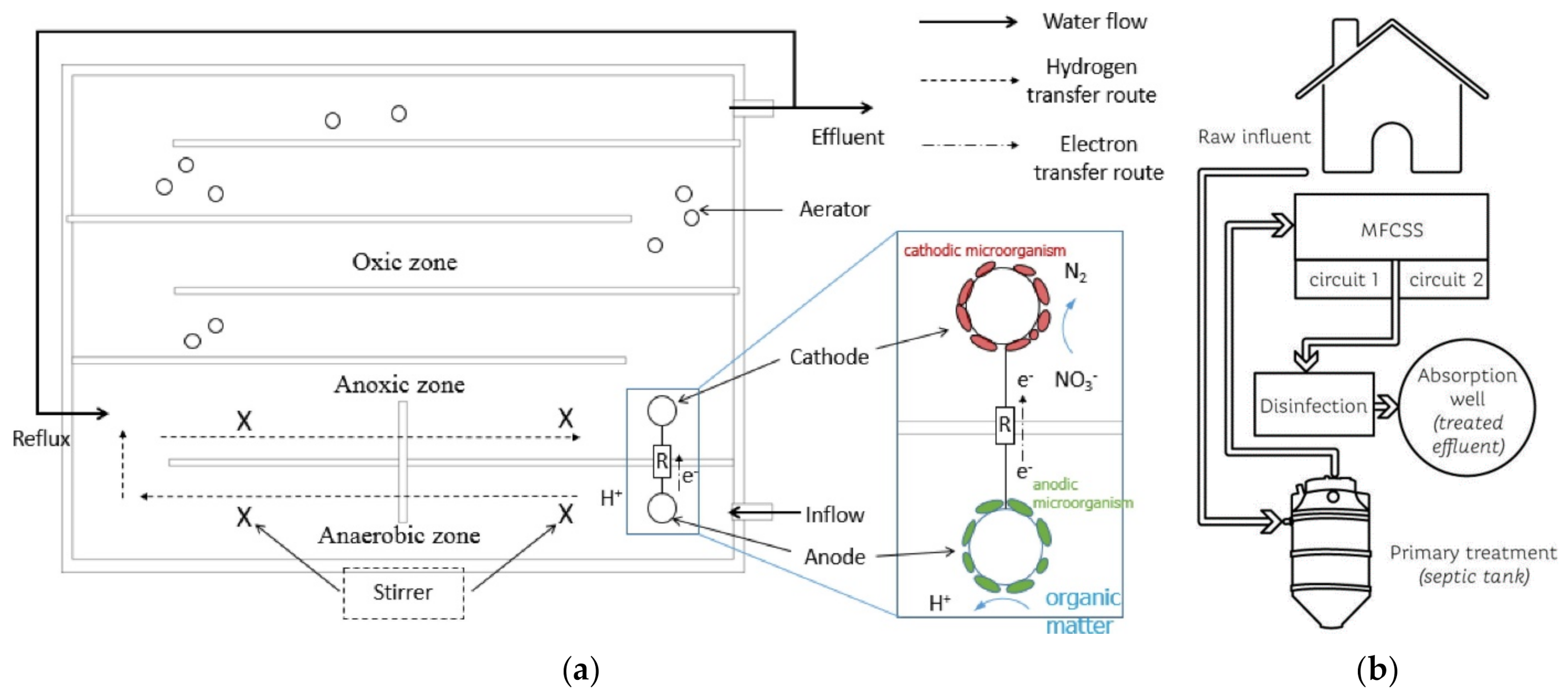
2.3. Application Based
3. Challenges and Future Direction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lewis, C.W. Biomass through the ages. Biomass 1981, 1, 5–15. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Long, H.; Li, X.; Wang, H.; Jia, J. Biomass resources and their bioenergy potential estimation: A review. Renew. Sustain. Energy Rev. 2013, 26, 344–352. [Google Scholar] [CrossRef]
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Scaling up microbial fuel cells. Environ. Sci. Technol. 2008, 42, 7643–7648. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ge, Z.; Zhang, F.; Grimaud, J.; Hurst, J.; He, Z. Long-term investigation of microbial fuel cells treating primary sludge or digested sludge. Bioresour. Technol. 2013, 136, 509–514. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Wahid, Z.A.; Din, M.F.M. Exoelectrogens in microbial fuel cells toward bioelectricity generation: A review. Int. J. Energy Res. 2015, 39, 1048–1067. [Google Scholar] [CrossRef]
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is microbial fuel cell technology ready? An economic answer towards industrial commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Alvarez-Gallego, Y.; Diels, L.; Vanbroekhoven, K. Evaluation of bioelectrogenic potential of four industrial effluents as substrate for low cost Microbial Fuel Cells operation. Environ. Eng. Manag. J. (Eemj) 2016, 15. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhang, S.; Su, Z. Study of operational performance and electrical response on mediator-less microbial fuel cells fed with carbon-and protein-rich substrates. Biochem. Eng. J. 2009, 45, 185–191. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, S.-G.; Zhuang, L.; Zhang, J.-T.; Ni, J.-R. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Walter, X.A.; Stinchcombe, A.; Greenman, J.; Ieropoulos, I. Urine transduction to usable energy: A modular MFC approach for smartphone and remote system charging. Appl. Energy 2017, 192, 575–581. [Google Scholar] [CrossRef]
- Wang, A.-J.; Wang, H.-C.; Cheng, H.-Y.; Liang, B.; Liu, W.-Z.; Han, J.-L.; Zhang, B.; Wang, S.-S. Electrochemistry-stimulated environmental bioremediation: Development of applicable modular electrode and system scale-up. Environ. Sci. Ecotechnol. 2020, 3, 100050. [Google Scholar] [CrossRef]
- Walter, X.A.; Santoro, C.; Greenman, J.; Ieropoulos, I.A. Scalability and stacking of self-stratifying microbial fuel cells treating urine. Bioelectrochemistry 2020, 133, 107491. [Google Scholar] [CrossRef]
- Vilajeliu-Pons, A.; Puig, S.; Salcedo-Dávila, I.; Balaguer, M.; Colprim, J. Long-term assessment of six-stacked scaled-up MFCs treating swine manure with different electrode materials. Environ. Sci. Water Res. Technol. 2017, 3, 947–959. [Google Scholar] [CrossRef]
- Yellappa, M.; Sravan, J.S.; Sarkar, O.; Reddy, Y.V.R.; Mohan, S.V. Modified conductive polyaniline-carbon nanotube composite electrodes for bioelectricity generation and waste remediation. Bioresour. Technol. 2019, 284, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, J.; Pi, J.; Gong, L.; Zhu, G. Long-term electricity generation and denitrification performance of MFCs with different exchange membranes and electrode materials. Bioelectrochemistry 2021, 140, 107748. [Google Scholar] [CrossRef]
- He, W.; Dong, Y.; Li, C.; Han, X.; Liu, G.; Liu, J.; Feng, Y. Field tests of cubic-meter scale microbial electrochemical system in a municipal wastewater treatment plant. Water Res. 2019, 155, 372–380. [Google Scholar] [CrossRef]
- Asensio, Y.; Mansilla, E.; Fernandez-Marchante, C.; Lobato, J.; Cañizares, P.; Rodrigo, M. Towards the scale-up of bioelectrogenic technology: Stacking microbial fuel cells to produce larger amounts of electricity. J. Appl. Electrochem. 2017, 47, 1115–1125. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Domínguez-Maldonado, J.; Rodríguez-Leal, E.; Patrón, G.; Castillo-Hernández, A.; Miranda, A.; Diaz Romero, D.; Moreno-Cervera, R.; Camara-chale, G.; Borroto, C.G.; et al. Scale up of microbial fuel cell stack system for residential wastewater treatment in continuous mode operation. Water 2019, 11, 217. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Effect of sediment microbial fuel cell stacks on 9 V/12 V DC power supply. Int. J. Hydrog. Energy 2021, 46, 14628–14638. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Hernández-Romano, J.; García-Sánchez, L.; Garcés, R.A.G.; Bahena-Bahena, E.O.; Guadarrama-Pérez, O.; Chavez, G.E.M. Domestic wastewater treatment and power generation in continuous flow air-cathode stacked microbial fuel cell: Effect of series and parallel configuration. J. Environ. Manag. 2018, 214, 232–241. [Google Scholar] [CrossRef]
- Fischer, F.; Sugnaux, M.; Savy, C.; Hugenin, G. Microbial fuel cell stack power to lithium battery stack: Pilot concept for scale up. Appl. Energy 2018, 230, 1633–1644. [Google Scholar] [CrossRef]
- Greenman, J.; Ieropoulos, I.A. Allometric scaling of microbial fuel cells and stacks: The lifeform case for scale-up. J. Power Sources 2017, 356, 365–370. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Ieropoulos, I.A. Recent advancements in real-world microbial fuel cell applications. Curr. Opin. Electrochem. 2018, 11, 78–83. [Google Scholar] [CrossRef]
- Liang, P.; Duan, R.; Jiang, Y.; Zhang, X.; Qiu, Y.; Huang, X. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res. 2018, 141, 1–8. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef]
- Hiegemann, H.; Littfinski, T.; Krimmler, S.; Lübken, M.; Klein, D.; Schmelz, K.-G.; Ooms, K.; Pant, D.; Wichern, M. Performance and inorganic fouling of a submergible 255 L prototype microbial fuel cell module during continuous long-term operation with real municipal wastewater under practical conditions. Bioresour. Technol. 2019, 294, 122227. [Google Scholar] [CrossRef]
- Kim, B.; Mohan, S.V.; Fapyane, D.; Chang, I.S. Controlling voltage reversal in microbial fuel cells. Trends Biotechnol. 2020, 38, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wu, Y.; Cao, D.; Zhao, L.; Sun, Q. Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour. Technol. 2009, 100, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Cañizares, P.; Fernandez-Morales, F.J.; Rodrigo, M.A. A critical view of microbial fuel cells: What is the next stage? Chemsuschem 2018, 11, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Blatter, M.; Delabays, L.; Furrer, C.; Huguenin, G.; Cachelin, C.P.; Fischer, F. Stretched 1000-L microbial fuel cell. J. Power Sources 2021, 483, 229130. [Google Scholar] [CrossRef]
- Das, I.; Ghangrekar, M.; Satyakam, R.; Srivastava, P.; Khan, S.; Pandey, H. On-site sanitary wastewater treatment system using 720-L stacked microbial fuel cell: Case study. J. Hazard. ToxicRadioact. Waste 2020, 24, 04020025. [Google Scholar] [CrossRef]
- Babanova, S.; Jones, J.; Phadke, S.; Lu, M.; Angulo, C.; Garcia, J.; Carpenter, K.; Cortese, R.; Chen, S.; Phan, T. Continuous flow, large-scale, microbial fuel cell system for the sustained treatment of swine waste. Water Environ. Res. 2020, 92, 60–72. [Google Scholar] [CrossRef]
- Rossi, R.; Jones, D.; Myung, J.; Zikmund, E.; Yang, W.; Gallego, Y.A.; Pant, D.; Evans, P.J.; Page, M.A.; Cropek, D.M.; et al. Evaluating a multi-panel air cathode through electrochemical and biotic tests. Water Res. 2019, 148, 51–59. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef]
- Hiegemann, H.; Herzer, D.; Nettmann, E.; Lübken, M.; Schulte, P.; Schmelz, K.-G.; Gredigk-Hoffmann, S.; Wichern, M. An integrated 45 L pilot microbial fuel cell system at a full-scale wastewater treatment plant. Bioresour. Technol. 2016, 218, 115–122. [Google Scholar] [CrossRef]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sources 2017, 356, 274–287. [Google Scholar] [CrossRef]
- Liu, R.; Tursun, H.; Hou, X.; Odey, F.; Li, Y.; Wang, X.; Xie, T. Microbial community dynamics in a pilot-scale MFC-AA/O system treating domestic sewage. Bioresour. Technol. 2017, 241, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhao, Y.; Kang, C.; Yang, Y.; Morgan, D.; Xu, L. Towards concurrent pollutants removal and high energy harvesting in a pilot-scale CW-MFC: Insight into the cathode conditions and electrodes connection. Chem. Eng. J. 2019, 373, 150–160. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Qu, Y.; Li, C.; Tian, Y.; Feng, Y. Pilot-scale benthic microbial electrochemical system (BMES) for the bioremediation of polluted river sediment. J. Power Sources 2017, 356, 430–437. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Qu, Y.; Qiu, Y.; Liu, J.; Feng, Y. A pilot-scale benthic microbial electrochemical system (BMES) for enhanced organic removal in sediment restoration. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Tripathi, R.K. Scale-up and control the voltage of sediment microbial fuel cell for charging a cell phone. Biosens. Bioelectron. 2021, 172, 112767. [Google Scholar] [CrossRef] [PubMed]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® urinal II–Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 392, 150–158. [Google Scholar] [CrossRef]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef]
- Bouwman, L.; Van Houtven, D.; Pant, D.; Alvarez Gallego, Y.; Vanbroekhoven, K. Carbon Based Electrode with Large Geometric Dimensions. U.S. Patent 16/753,027, 17 September 2020. [Google Scholar]
- Wilkinson, S. “Gastrobots”—Benefits and challenges of microbial fuel cells in foodpowered robot applications. Auton. Robot. 2000, 9, 99–111. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Melhuish, C.; Greenman, J. Artificial metabolism: Towards true energetic autonomy in artificial life. In Proceedings of the European Conference on Artificial Life, Dortmund, Germany, 14–17 September 2003; pp. 792–799. [Google Scholar]
- Yi, Y.; Xie, B.; Zhao, T.; Li, Z.; Stom, D.; Liu, H. Effect of external resistance on the sensitivity of microbial fuel cell biosensor for detection of different types of pollutants. Bioelectrochemistry 2019, 125, 71–78. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Varjani, S.; Kumar, M. Microbial fuel cell-based biosensor for online monitoring wastewater quality: A critical review. Sci. Total Environ. 2020, 712, 135612. [Google Scholar] [CrossRef]
- Khan, A.; Salama, E.-S.; Chen, Z.; Ni, H.; Zhao, S.; Zhou, T.; Pei, Y.; Sani, R.K.; Ling, Z.; Liu, P. A novel biosensor for zinc detection based on microbial fuel cell system. Biosens. Bioelectron. 2020, 147, 111763. [Google Scholar] [CrossRef]
- Wu, L.-C.; Tsai, T.-H.; Liu, M.-H.; Kuo, J.-L.; Chang, Y.-C.; Chung, Y.-C. A green microbial fuel cell-based biosensor for in situ chromium (VI) measurement in electroplating wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Han, H.; Liu, P.; Xiong, J.; Tian, F.; Li, X. Microbial fuels cell-based biosensor for toxicity detection: A review. Sensors 2017, 17, 2230. [Google Scholar] [CrossRef] [PubMed]
- Arias-Thode, Y.M.; Hsu, L.; Anderson, G.; Babauta, J.; Fransham, R.; Obraztsova, A.; Tukeman, G.; Chadwick, D.B. Demonstration of the SeptiStrand benthic microbial fuel cell powering a magnetometer for ship detection. J. Power Sources 2017, 356, 419–429. [Google Scholar] [CrossRef]
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The role of microbial electrolysis cell in urban wastewater treatment: Integration options, challenges, and prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.; Georgieva, T.; Ter Heijne, A.; Sleutels, T.H.; Jeremiasse, A.W.; Saakes, M.; Buisman, C.J.; Kuntke, P. Ammonia recovery from urine in a scaled-up Microbial Electrolysis Cell. J. Power Sources 2017, 356, 491–499. [Google Scholar] [CrossRef]
- Liang, D.; He, W.; Li, C.; Wang, F.; Crittenden, J.C.; Feng, Y. Remediation of nitrate contamination by membrane hydrogenotrophic denitrifying biofilm integrated in microbial electrolysis cell. Water Res. 2021, 188, 116498. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.; Rabaey, K.; Keller, J.; Buisman, C.J. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Tommasi, T.; Lombardelli, G. Energy sustainability of Microbial Fuel Cell (MFC): A case study. J. Power Sources 2017, 356, 438–447. [Google Scholar] [CrossRef]
- An, J.; Nam, J.; Kim, B.; Lee, H.-S.; Kim, B.H.; Chang, I.S. Performance variation according to anode-embedded orientation in a sediment microbial fuel cell employing a chessboard-like hundred-piece anode. Bioresour. Technol. 2015, 190, 175–181. [Google Scholar] [CrossRef]
- Chen, S.; Patil, S.A.; Brown, R.K.; Schröder, U. Strategies for optimizing the power output of microbial fuel cells: Transitioning from fundamental studies to practical implementation. Appl. Energy 2019, 233, 15–28. [Google Scholar] [CrossRef]
- Boghani, H.C.; Papaharalabos, G.; Michie, I.; Fradler, K.R.; Dinsdale, R.M.; Guwy, A.J.; Ieropoulos, I.; Greenman, J.; Premier, G.C.J.J.o.P.S. Controlling for peak power extraction from microbial fuel cells can increase stack voltage and avoid cell reversal. J. Power Sources 2014, 269, 363–369. [Google Scholar] [CrossRef]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Liberale, A.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Reducing start-up time and minimizing energy losses of Microbial Fuel Cells using Maximum Power Point Tracking strategy. J. Power Sources 2014, 269, 403–411. [Google Scholar] [CrossRef]
- Lobo, F.L.; Wang, X.; Ren, Z.J. Energy harvesting influences electrochemical performance of microbial fuel cells. J. Power Sources 2017, 356, 356–364. [Google Scholar] [CrossRef]
- Song, Y.; An, J.; Chae, K.J. Effect of temperature variation on the performance of microbial fuel cells. Energy Technol. 2017, 5, 2163–2167. [Google Scholar] [CrossRef]
- Kumar, P.; Mungray, A.K. Microbial fuel cell: Optimizing pH of anolyte and catholyte by using taguchi method. Environ. Prog. Sustain. Energy 2016, 36, 120–128. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Ye, D.; Zhang, L.; Zhu, X.; Liao, Q. A hybrid microbial fuel cell stack based on single and double chamber microbial fuel cells for self-sustaining pH control. J. Power Sources 2016, 306, 685–691. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Nghiem, L.D.; Zhang, X.; Wang, J. Effect of organic loading rate on the recovery of nutrients and energy in a dual-chamber microbial fuel cell. Bioresour. Technol. 2019, 281, 367–373. [Google Scholar] [CrossRef]
- Guo, F.; Luo, H.; Shi, Z.; Wu, Y.; Liu, H. Substrate salinity: A critical factor regulating the performance of microbial fuel cells, a review. Sci. Total Environ. 2020, 143021. [Google Scholar] [CrossRef]
- Stefanova, A.; Angelov, A.; Bratkova, S.; Genova, P.; Nikolova, K. Influence of electrical conductivity and temperature in a microbial fuel cell for treatment of mining waste water. Ann. Constantin Brancusi Univ. Targu Jiu-Lett. Soc. Sci. Ser. 2018, 3, 18–24. [Google Scholar]
- Vicari, F.; Mateo, S.; Fernandez-Morales, F.; Cañizares, P.; Galia, A.; Scialdone, O.; Rodrigo, M. Influence of the methodology of inoculation in the performance of air-breathing microbial fuel cells. J. Electroanal. Chem. 2017, 803, 81–88. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, S.; Luo, G.; Liu, Y. Impacts of hydraulic retention time on a continuous flow mode dual-chamber microbial fuel cell for recovering nutrients from municipal wastewater. Sci. Total Environ. 2020, 734, 139220. [Google Scholar] [CrossRef] [PubMed]
- Rau, G. Electrochemical splitting of calcium carbonate to increase solution alkalinity: Implications for mitigation of carbon dioxide and ocean acidity. Environ. Sci. Technol. 2008, 42, 8935–8940. [Google Scholar] [CrossRef] [PubMed]
- Tlili, M.; Benamor, M.; Gabrielli, C.; Perrot, H.; Tribollet, B. Influence of the interfacial pH on electrochemical CaCO3 precipitation. J. Electrochem. Soc. 2003, 150, C765. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, F.; Yu, Q.; Scott, K.; Wang, X.; Diao, G. Durability and regeneration of activated carbon air-cathodes in long-term operated microbial fuel cells. J. Power Sources 2017, 360, 21–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, M.; Yang, H.; Liang, L.; Gu, T. Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: Synergistic effects, mechanisms and challenges. Renew. Sustain. Energy Rev. 2019, 103, 13–29. [Google Scholar] [CrossRef]

| Reactor Type | Substrate | COD, Conductivity, pH | Anode (Number) | Cathode (Number), Catholyte | Membrane | Max P (W/m3, Unless Specified) | COD Removal (%) | CE (%) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Stacked or scale-up MFCs with volume > 10 L for wastewater treatment: | ||||||||||
| 1 | Movable, 1.5 m3 (Primary clarifier- DCMFC-Secondary clarifier) | Primary effluent | 0.26 g/L | GFB (8) | Bio-GFB (7), Anode effluent | Dynamic (microbial) membrane | 0.4 | 91 | - | [21] |
| 2 | DCMFC, 64 units, 1000 L | Municipal ww | 0.2–0.45 g/L pH 7.2 | RVC | RVC, Groundwater | VANA- Dion | 0.2 | 34–95 | 5–15 | [35] |
| 3 | DCMFC, 50 units, 1000 L | Municipal ww | 0.08–0.25 g/L | GAC | GAC, Artificial catholyte | CEM | 60 | 70–90 | 41–75 | [29] |
| 4 | SCMFC, 6 units, 720 L | Sanitary sewage | 1–8.2 g/L | C felt | CoZnFeO or SnCu/C felt | Clayware | 0.085 | 87 | - | [36] |
| 5 | Submergible MFC, 255 L | Municipal ww | 0.205 g/L 540 mS/m pH 7 | GFB (10) | SS-AC (16, VitoCORE®) | Glass fiber separator | 0.3 | 41 | 30 | [31] |
| 6 | DCMFC, 96 units, 200 L | Primary effluent | 0.16 g/L | CFB | C cloth/N-AC, Aerobic effluent | CEM | 1 | 75 | - | [30] |
| 7 | SCMFC, 12 units, 110 L | Swine ww | 1 g/L pH 7.5 | GFB (20) | Gas diffusion cathode | None | 0.36 | 65 | - | [37] |
| 8 | SCMFC, 85 L | Domestic ww | 0.428 g/L | GFB (22) | SS-AC (15, VitoCORE®) | Glass fiber separator | 0.605 | 80 | 27 | [38] |
| 9 | DCMFC, 72 L | Synthetic ww | 0.2–1.2 g/L pH 6.8–7.1 | GAC/Ti mesh | GAC/Ti mesh, Desalination effluent | CEM | 51 | 97 | - | [39] |
| 10 | DCMFC, 6 units, 60 L | Swine manure | 2.47 g/L 830 mS/m pH 8.5 | GG | GR, Ammonium | AEM | 4 | 36 | 17 | [18] |
| SS | SS, Ammonium | AEM | 2 | 40 | 17 | [18] | ||||
| 11 | SCMFC, 45 L | Primary effluent | 0.13 g/L 300–420 mS/m | GFB (8) | Pt/C cloth/SS (2) | None | 0.875 | 14–67 | 10–25 | [40] |
| 12 | SCMFC, 40 units, 16 L | Municipal ww | 0.3 g/L | C felt | Pb/C cloth | CEM | 47 | 84 | - | [25] |
| 13 | DCMFC, 2 units, 20 L | Brewery ww | 3.2 g/L 242 mS/m pH 7 | Modified C cloth | Modified C cloth | Nano- filtration membrane | 0.44 | 95 | 14 | [41] |
| Hybrid/coupled MFC with volume > 10 L: | ||||||||||
| 14 | AD-SCMFC (1 m3) | Pre-treated pharmaceu- tical ww | 0.16–0.36 g/L | C felt/G-SS | C felt/G-SS | Isolation pad | 1.25 A/m2 | 35 | - | [16] |
| 15 | Hybrid AA/O- SCMFC, 1 m3 | Domestic ww | 0.45–0.65 g/L pH 7.5–8 | CFB-SS | CFB-SS | None | 0.0036 | 95 | - | [42] |
| 16 | Septic tank-SCMFC (18 units, 700 L) -Disinfection | Domestic ww | 789 g/L pH 8 | SS-GAC | C cloth | Nafion 117 | 0.00043 | 87 | 22 | [23] |
| 17 | CW-DCMFC, 30 L | Dewatered alum sludge | 0.5 g/L | SS-C felt (4) | SS-C felt, AS | None | 0.448 | 92 | 0.36 | [43] |
| Scale-up MFC as off-grid power source: | ||||||||||
| 18 | Sediment MFC, 350 L | Synthetic ww | - | C mesh | AC | None | 0.0064 | - | - | [44] |
| 19 | Sediment MFC, 195 L | River sediment | 13.5 mS/m pH 6.8–7.4 | C mesh | AC/SS | None | 0.0415 | - | - | [45] |
| 20 | Sediment MFC, 72 units, 72 L | River sediment water | 890 g/L 27.206 mS/m pH 8 | Copper | Zinc | None | 0.0019 | 23 | - | [46] |
| Sediment MFC, 35 units, 35 L | River sediment water | 890 g/L 27.206 mS/m pH 8 | Copper | Zinc | None | 0.0069 | - | - | [24] | |
| 21 | Self-stratifying SCMFC (38 units), 19.2–57.6 L | Urine | 5.6–6.8 g/L pH 8.5–9.2 | C veil fibers | micro-porous C | None | 7.3–9.9 | 48–88 | 1.6–3.8 | [47] |
| 22 | DCMFC, 12 units, 12 L | Synthetic ww | - | RVC | Pt/RVC, PBS | Nafion 117 | 16.2 mW/m2 | - | - | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.H.; Chong, S.; Fang, H.-W.; Pan, K.-L.; Mohamad, M.; Lim, J.W.; Tiong, T.J.; Chan, Y.J.; Huang, C.-M.; Yang, T.C.-K. Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues. Processes 2021, 9, 985. https://doi.org/10.3390/pr9060985
Tan WH, Chong S, Fang H-W, Pan K-L, Mohamad M, Lim JW, Tiong TJ, Chan YJ, Huang C-M, Yang TC-K. Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues. Processes. 2021; 9(6):985. https://doi.org/10.3390/pr9060985
Chicago/Turabian StyleTan, Wei Han, Siewhui Chong, Hsu-Wei Fang, Kuan-Lun Pan, Mardawani Mohamad, Jun Wei Lim, Timm Joyce Tiong, Yi Jing Chan, Chao-Ming Huang, and Thomas Chung-Kuang Yang. 2021. "Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues" Processes 9, no. 6: 985. https://doi.org/10.3390/pr9060985
APA StyleTan, W. H., Chong, S., Fang, H.-W., Pan, K.-L., Mohamad, M., Lim, J. W., Tiong, T. J., Chan, Y. J., Huang, C.-M., & Yang, T. C.-K. (2021). Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues. Processes, 9(6), 985. https://doi.org/10.3390/pr9060985













