A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy
Abstract
1. Introduction
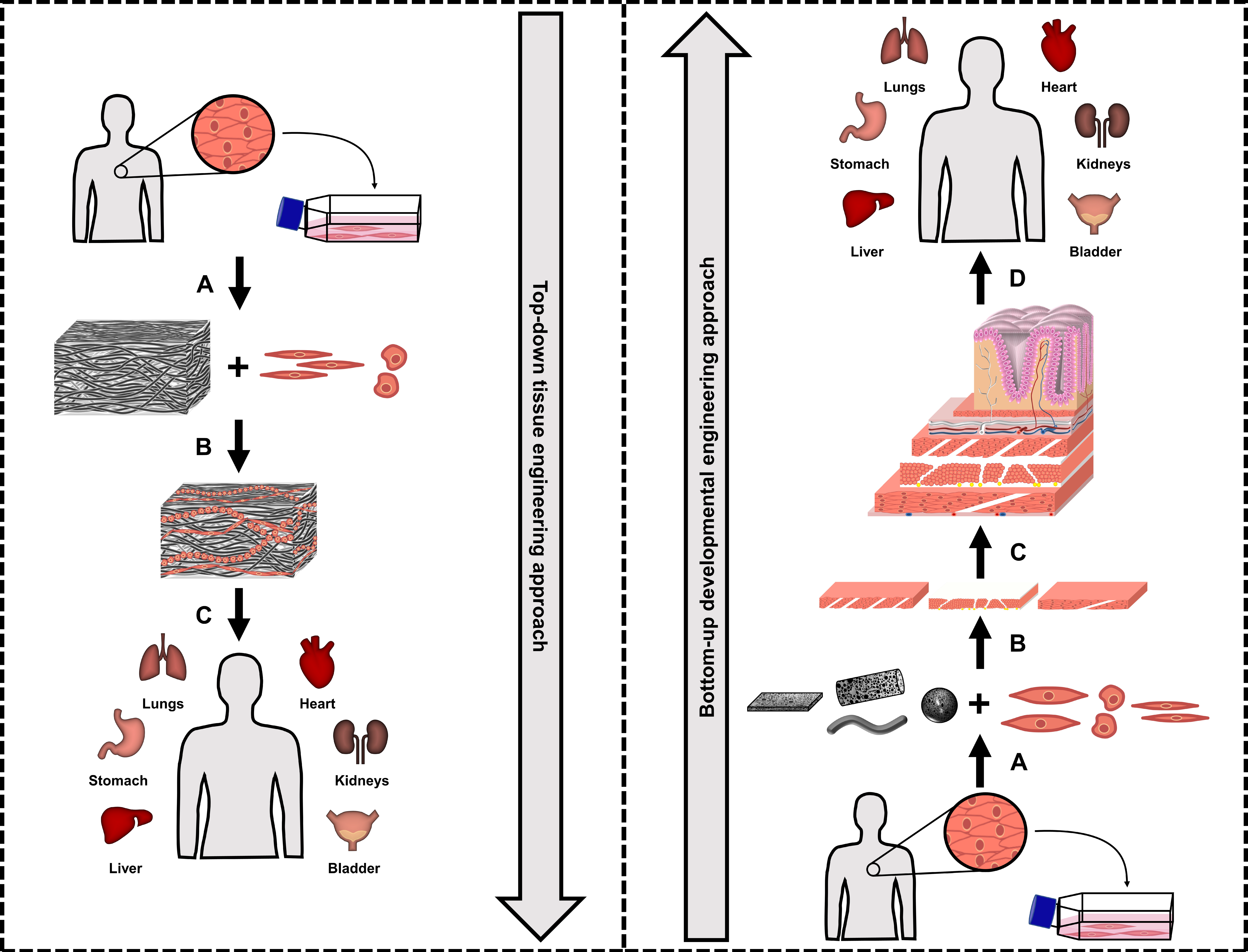
2. Top–Down Tissue Manufacturing Approach
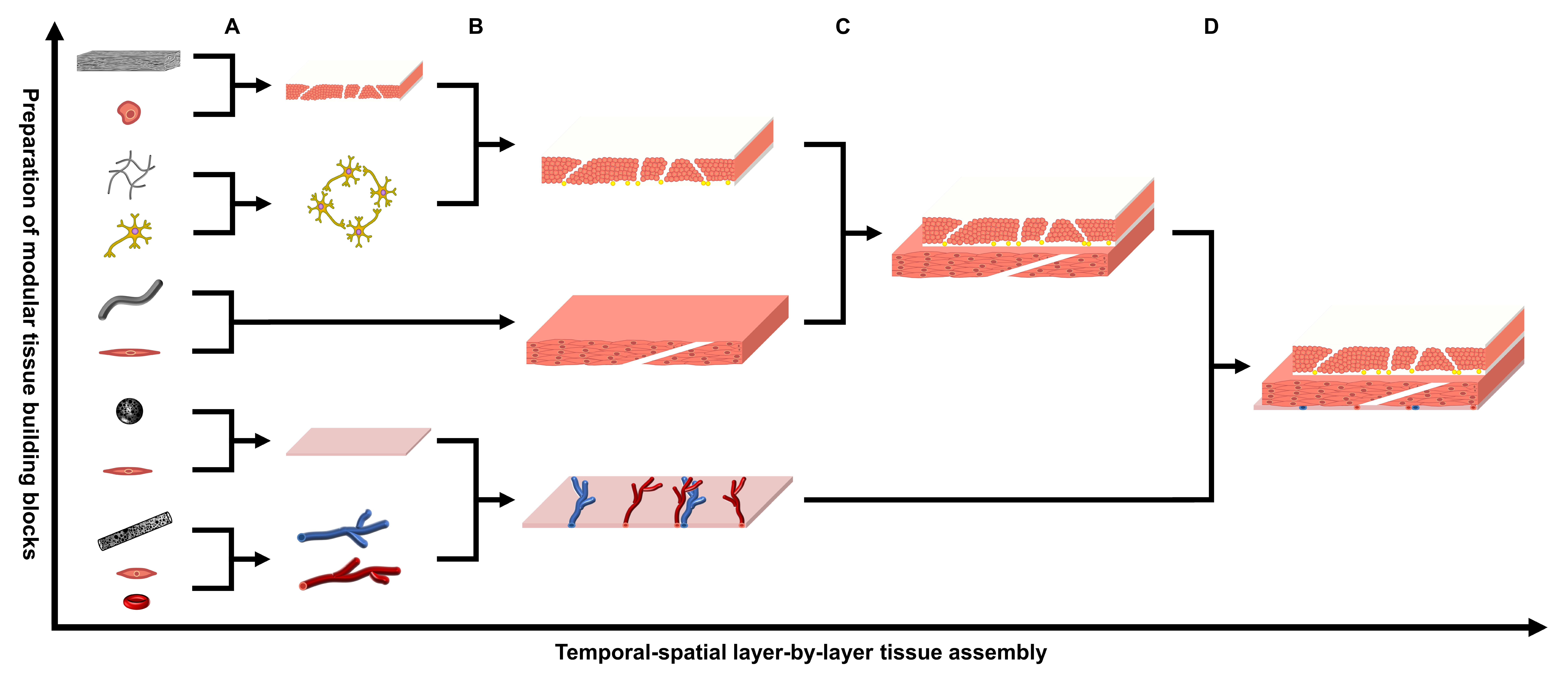
3. Bottom–Up Tissue Manufacturing Approach
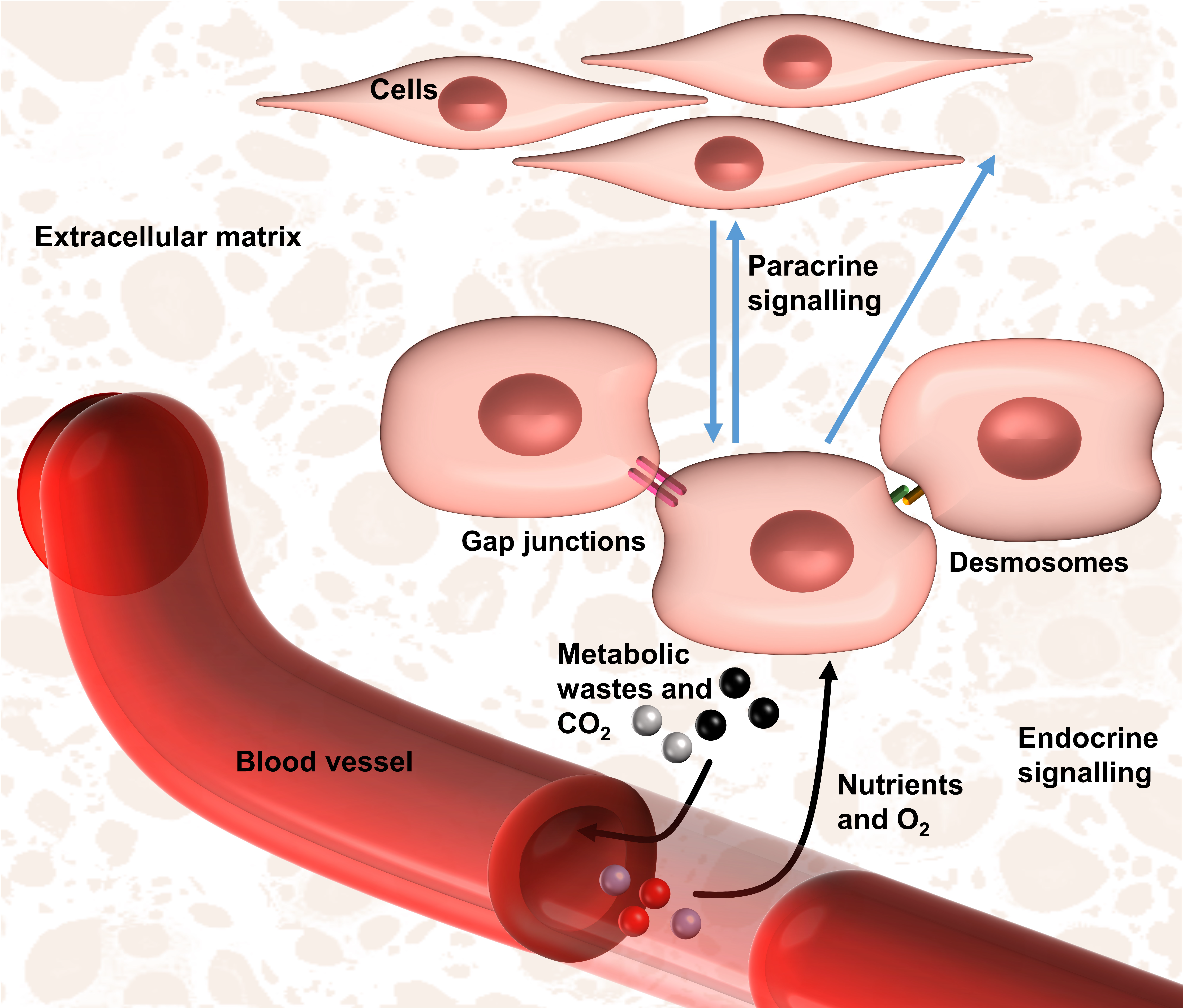
4. Relevant Issues for the Bottom–Up DE Approach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vacanti, C.A. The History of Tissue Engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Birla, R. Introduction to Tissue Engineering: Applications and Challenges, 1st ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 3–4. [Google Scholar]
- Palsson, B.O.; Bhatia, S.N. Tissue Engineerig, Indian ed.; Pearson India Education: Chennai, India, 2016; pp. 4, 21. [Google Scholar]
- Grounds, M.D. Obstacles and Challenges for Tissue Engineering and Regenerative Medicine: Australian Nuances. Clin. Exp. Pharmacol. Physiol. 2017, 45, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, T. A Strategy for the Development of Tissue Engineering Scaffolds That Regulate Cell Behavior. Biomaterials 2003, 24, 2267–2275. [Google Scholar] [CrossRef]
- Sperling, L.E.; Reis, K.P.; Pranke, P.; Wendorff, J.H. Advantages and Challenges Offered by Biofunctional Core-Shell Fiber Systems for Tissue Engineering and Drug Delivery. Drug Discov. Today 2016, 21, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A Tissue Engineering Approach to Bone Repair in Large Animal Models and in Clinical Practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.M.; Solari, R.; Holgate, S.T. Animal Models of Asthma: Value, Limitations and Opportunities for Alternative Approaches. Drug Discov. Today 2011, 16, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Naderi, H.; Matin, M.M.; Bahrami, A.R. Review Paper: Critical Issues in Tissue Engineering: Biomaterials, Cell Sources, Angiogenesis, and Drug Delivery Systems. J. Biomater. Appl. 2011, 26, 383–417. [Google Scholar] [CrossRef]
- Muschler, G.F.; Raut, V.P.; Patterson, T.E.; Wenke, J.C.; Hollinger, J.O. The Design and Use of Animal Models for Translational Research in Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part. B Rev. 2010, 16, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human IPSC-Based Cardiac Microphysiological System For Drug Screening Applications. Sci. Rep. 2015, 5, 8883:1–8883:7. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Hung, C.T.; Kuroki, K.; Stoker, A.M.; Cook, C.R.; Pfeiffer, F.M.; Sherman, S.L.; Stannard, J.P. Animal Models of Cartilage Repair. Bone Joint Res. 2014, 3, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lenas, P.; Moos, M.; Luyten, F.P. Developmental Engineering: A New Paradigm for the Design and Manufacturing of Cell-Based Products. Part I: From Three-Dimensional Cell Growth to Biomimetics of In vivo Development. Tissue Eng. Part. B Rev. 2009, 15, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127:1–127:10. [Google Scholar] [CrossRef] [PubMed]
- Däullary, T.; Fey, C.; Berger, C.; Metzger, M.; Zdzieblo, D. Bioartificial gut—Current state of small intestinal tissue engineering. In Biomaterials for Organ and Tissue Regeneration, 1st ed.; Vrana, N., Knopf-Marques, H., Barthes, J., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 273–297. [Google Scholar]
- Pham, C.; Greenwood, J.; Cleland, H.; Woodruff, P.; Maddern, G. Bioengineered Skin Substitutes for the Management of Burns: A Systematic Review. Burns 2007, 33, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Brenta, F.; Bleve, M.; Pellegatta, T.; Malovini, A.; Faga, A.; Perugini, P. Long-Term in vivo Assessment of Bioengineered Skin Substitutes: A Clinical Study. J. Tissue Eng. Regen. Med. 2015, 9, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86:1–86:18. [Google Scholar] [CrossRef] [PubMed]
- Tiruvannamalai-Annamalai, R.; Armant, D.R.; Matthew, H.W.T. A Glycosaminoglycan Based, Modular Tissue Scaffold System for Rapid Assembly of Perfusable, High Cell Density, Engineered Tissues. PLoS ONE 2014, 9, e84287:1–e84287:15. [Google Scholar] [CrossRef]
- Lenas, P. Developmental Biology in Bioartificial Tissue Design: Manufacturing and Regulatory Considerations. Regen. Med. 2018, 13, 7–11. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40:1–40:22. [Google Scholar] [CrossRef]
- Marga, F.; Neagu, A.; Kosztin, I.; Forgacs, G. Developmental Biology and Tissue Engineering. Birth Defects Res. Part. C Embryo Today Rev. 2007, 81, 320–328. [Google Scholar] [CrossRef]
- Lenas, P.; Ikonomou, L. Developmental Engineering: Design of Clinically Efficacious Bioartificial Tissues through Developmental and Systems Biology. Sci. China Life Sci. 2018, 61, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, K.; Kwapiszewski, R.; Brzózka, Z. Microfluidic Devices as Tools for Mimicking the in vivo Environment. New J. Chem. 2011, 35, 979–990. [Google Scholar] [CrossRef]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in Keratinocyte Delivery in Burn Wound Care. Adv. Drug Deliv. Rev. 2018, 123, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular Cartilage: From Formation to Tissue Engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef]
- Keeney, M.; Lai, J.H.; Yang, F. Recent Progress in Cartilage Tissue Engineering. Curr. Opin. Biotechnol. 2011, 22, 734–740. [Google Scholar] [CrossRef]
- Van Blitterswijk, C.A.; de Boer, J. Tissue Engineering, 2nd ed.; Academic Press: London, UK, 2015; pp. 227–242, 261, 300–305, 397–400, 471–475. [Google Scholar]
- Das, S.; Gordián-Vélez, W.J.; Ledebur, H.C.; Mourkioti, F.; Rompolas, P.; Chen, H.I.; Serruya, M.D.; Cullen, D.K. Innervation: The Missing Link for Biofabricated Tissues and Organs. NPJ Regen. Med. 2020, 5, 11:1–11:19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Brockes, J.P. Nerve Dependence in Tissue, Organ, and Appendage Regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Moniz, T.; Costa Lima, S.A.; Reis, S. Human Skin Models: From Healthy to Disease-Mimetic Systems; Characteristics and Applications. Br. J. Pharmacol. 2020, 177, 4314–4329. [Google Scholar] [CrossRef] [PubMed]
- Paolo Macchiarini: A Surgeon’s Downfall. Available online: https://www.bbc.com/news/magazine-37311038 (accessed on 19 October 2020).
- Pangarkar, N.; Pharoah, M.; Nigam, A.; Hutmacher, D.W.; Champ, S. Advanced Tissue Sciences Inc.: Learning from the Past, a Case Study for Regenerative Medicine. Regen. Med. 2010, 5, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Polykandriotis, E.; Popescu, L.M.; Horch, R.E. Regenerative Medicine: Then and Now-an Update of Recent History into Future Possibilities. J. Cell. Mol. Med. 2010, 14, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Lenas, P.; Moos, M.; Luyten, F.P. Developmental Engineering: A New Paradigm for the Design and Manufacturing of Cell-Based Products. Part II. From Genes to Networks: Tissue Engineering from the Viewpoint of Systems Biology and Network Science. Tissue Eng. Part. B Rev. 2009, 15, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ Printing: Tissue Spheroids as Building Blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, Tunable Constructs for Tissue Regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kumar Meena, L.; Rather, H.; Kedaria, D.; Vasita, R. Polymeric Microgels for Bone Tissue Engineering Applications—A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 381–397. [Google Scholar] [CrossRef]
- Shekaran, A.; Lam, A.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.P.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-Coated PCL Microcarriers Support Scalable Human Early MSC Expansion and in vivo Bone Formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Kornmuller, A.; Brown, C.F.C.; Yu, C.; Flynn, L.E. Fabrication of Extracellular Matrix-Derived Foams and Microcarriers as Tissue-Specific Cell Culture and Delivery Platforms. J. Vis. Exp. 2017, e55436:1–e55436:11. [Google Scholar] [CrossRef]
- Ozbolat, I.T. The Bioink. In 3D Bioprinting Fundamentals, Principles and Applications, 1st ed.; Academic Press: London, UK, 2016; pp. 41–92. [Google Scholar]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. Part. B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Eriberto, B.; Barbara, Z. Nanostructured Biomaterials for Tissue Engineered Bone Tissue Reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Pérez, R.A.; Jin, G.-Z.; Choi, S.-J.; Kim, H.-W.; Wall, I.B. Microcarriers Designed for Cell Culture and Tissue Engineering of Bone. Tissue Eng. Part. B Rev. 2013, 19, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Kazerooni, H.; Hassanpoor, H.; Akbarzadeh, A.; Pazoki-Toroudi, H. Tailoring Synthetic Polymeric Biomaterials towards Nerve Tissue Engineering: A Review. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3524–3539. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The Influence of Surface Nanoroughness of Electrospun PLGA Nanofibrous Scaffold on Nerve Cell Adhesion and Proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.H.; Shen, C.Y.; You, M.L.; Xiao, J.F.; Chen, G.Q. Differentiation of Human Bone Marrow Mesenchymal Stem Cells Grown in Terpolyesters of 3-Hydroxyalkanoates Scaffolds into Nerve Cells. Biomaterials 2010, 31, 1691–1698. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, M.-K.; El-Fiqi, A.; Seo, S.-J.; Lee, E.-J.; Kim, J.-H.; Kim, H.-W. Bioactive and Porous-Structured Nanocomposite Microspheres Effective for Cell Delivery: A Feasibility Study for Bone Tissue Engineering. RSC Adv. 2014, 4, 29062–29071. [Google Scholar] [CrossRef]
- Pedram, P.; Mazio, C.; Imparato, G.; Netti, P.A.; Salerno, A. Bioinspired Design of Novel Microscaffolds for Fibroblast Guidance toward in vitro Tissue Building. ACS Appl. Mater. Interfaces 2021, 13, 9589–9603. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch, K.; Manhardt, M.; Loth, T.; Bernhardt, R.; Schulz-Siegmund, M.; Hacker, M.C. Open Porous Microscaffolds for Cellular and Tissue Engineering by Lipid Templating. Acta Biomater. 2012, 8, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cai, S.; Xu, L.; Yang, Y. Water-Stable Three-Dimensional Ultrafine Fibrous Scaffolds from Keratin for Cartilage Tissue Engineering. Langmuir 2014, 30, 8461–8470. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, H.; He, T. Preparation of Porous Biodegradable Polymer and Its Nanocomposites by Supercritical CO2 Foaming for Tissue Engineering. J. Nanomater. 2012, 2012, 836394:1–836394:12. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and Characterization of Chitosan-Gelatin Blend Nanofibers for Skin Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94B, 264–272. [Google Scholar] [CrossRef]
- Diban, N.; Stamatialis, D.F. Functional Polymer Scaffolds for Blood Vessel Tissue Engineering. Macromol. Symp. 2011, 309/310, 93–99. [Google Scholar] [CrossRef]
- Gui, L.; Dash, B.C.; Luo, J.; Qin, L.; Zhao, L.; Yamamoto, K.; Hashimoto, T.; Wu, H.; Dardik, A.; Tellides, G.; et al. Implantable Tissue-Engineered Blood Vessels from Human Induced Pluripotent Stem Cells. Biomaterials 2016, 102, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Quint, C.; Kondo, Y.; Manson, R.J.; Lawson, J.H.; Dardik, A.; Niklason, L.E. Decellularized Tissue-Engineered Blood Vessel as an Arterial Conduit. Proc. Natl. Acad. Sci. USA 2011, 108, 9214–9219. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Jun, I.D.; Choi, M.J.; Nho, Y.C.; Lee, Y.M.; Shin, H. Development of Electroactive and Elastic Nanofibers That Contain Polyaniline and Poly(L-Lactide-Co-ε-Caprolactone) for the Control of Cell Adhesion. Macromol. Biosci. 2008, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of Conductive Polymers, Scaffolds and Electrical Stimulation for Nerve Tissue Engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xiao, A.; Zhao, Y.; Chen, F.; Ke, M.; Zhang, Q.; Zhang, J.; Shi, X.; He, X.; Chen, Y. An Implantable and Versatile Piezoresistive Sensor for the Monitoring of Human-Machine Interface Interactions and the Dynamical Process of Nerve Repair. Nanoscale 2019, 11, 21103–21118. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; De Laporte, L.; Tortelli, F.; Spedden, E.; Staii, C.; Atherton, T.J.; Hubbell, J.A.; Kaplan, D.L. Silk Hydrogels as Soft Substrates for Neural Tissue Engineering. Adv. Funct. Mater. 2013, 23, 5140–5149. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-Induced Gelation of Silk Fibroin for Cell Encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef]
- Chao, P.-H.G.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk Hydrogel for Cartilage Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95B, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk Scaffolds in Bone Tissue Engineering: An Overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Low, S.; Choon, A.T.; Sampath Kumar, T.S.; Ramakrishna, S. Mineralization of Osteoblasts with Electrospun Collagen/Hydroxyapatite Nanofibers. J. Mater. Sci. Mater. Med. 2008, 19, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, Present, and Future of Microcarrier-Based Tissue Engineering. J. Orthop. Translat. 2015, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Frondoza, C.G. Microcarriers in the Engineering of Cartilage and Bone. Trends Biotechnol. 2006, 24, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Yang, Y.P. Additive Manufacturing of Vascular Grafts and Vascularized Tissue Constructs. Tissue Eng. Part. B Rev. 2017, 23, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Thottappillil, N.; Nair, P.D. Scaffolds in Vascular Regeneration: Current Status. Vasc. Health Risk Manag. 2015, 11, 79–91. [Google Scholar] [CrossRef]
- Quint, C.; Arief, M.; Muto, A.; Dardik, A.; Niklason, L.E. Allogeneic Human Tissue-Engineered Blood Vessel. J. Vasc. Surg. 2012, 55, 790–798. [Google Scholar] [CrossRef]
- Rayatpisheh, S.; Heath, D.E.; Shakouri, A.; Rujitanaroj, P.-O.; Chew, S.Y.; Chan-Park, M.B. Combining Cell Sheet Technology and Electrospun Scaffolding for Engineered Tubular, Aligned, and Contractile Blood Vessels. Biomaterials 2014, 35, 2713–2719. [Google Scholar] [CrossRef]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-Dimensional Printed Bone Scaffolds: The Role of Nano/Micro-Hydroxyapatite Particles on the Adhesion and Differentiation of Human Mesenchymal Stem Cells. Proc. Inst. Mech. Eng. H. 2017, 231, 555–564. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.-C.; Catros, S.; Le Nihouannen, D. Characterization of Printed PLA Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. A 2018, 106, 887–894. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer Fiber Scaffolds for Bone and Cartilage Tissue Engineering. Adv. Funct. Mater. 2019, 29, 1903279:1–1903279:20. [Google Scholar] [CrossRef]
- Shi, X.; Sun, L.; Jiang, J.; Zhang, X.; Ding, W.; Gan, Z. Biodegradable Polymeric Microcarriers with Controllable Porous Structure for Tissue Engineering. Macromol. Biosci. 2009, 9, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Kreijveld, E.; Temenoff, J.S.; Van Blitterswijk, C.A.; Riesle, J. Expansion of Human Nasal Chondrocytes on Macroporous Microcarriers Enhances Redifferentiation. Biomaterials 2003, 24, 5153–5161. [Google Scholar] [CrossRef]
- Tayton, E.; Purcell, M.; Aarvold, A.; Smith, J.O.; Briscoe, A.; Kanczler, J.M.; Shakesheff, K.M.; Howdle, S.M.; Dunlop, D.G.; Oreffo, R.O.C. A Comparison of Polymer and Polymer-Hydroxyapatite Composite Tissue Engineered Scaffolds for Use in Bone Regeneration. An in vitro and in vivo Study. J. Biomed. Mater. Res. A 2014, 102A, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Dev, V.R.G.; Senthilram, T.; Sathiskumar, D.; Gupta, D.; Ramakrishna, S. Osteoblasts Mineralization with Composite Nanofibrous Substrate for Bone Tissue Regeneration. Cell Biol. Int. 2011, 35, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.; Venugopal, J.; Prabhakaran, M.P.; Thavasi, V.; Marsano, E.; Ramakrishna, S. Simultaneous Electrospin-Electrosprayed Biocomposite Nanofibrous Scaffolds for Bone Tissue Regeneration. Acta Biomater. 2010, 6, 4100–4109. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Sun, L.; Kim, H.J.; Hu, X.; Rice, W.; Kluge, J.; Reis, R.L.; Kaplan, D.L. Aligned Silk-Based 3-D Architectures for Contact Guidance in Tissue Engineering. Acta Biomater. 2012, 8, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Streubel, P.N.; Duan, B. Living Nanofiber Yarn-Based Woven Biotextiles for Tendon Tissue Engineering Using Cell Tri-Culture and Mechanical Stimulation. Acta Biomater. 2017, 62, 102–115. [Google Scholar] [CrossRef]
- Senuma, Y.; Franceschin, S.; Hilborn, J.G.; Tissières, P.; Bisson, I.; Frey, P. Bioresorbable Microspheres by Spinning Disk Atomization as Injectable Cell Carrier: From Preparation to in vitro Evaluation. Biomaterials 2000, 21, 1135–1144. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering Approaches for Bladder Regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef]
- Engelhardt, E.-M.; Micol, L.A.; Houis, S.; Wurm, F.M.; Hilborn, J.; Hubbell, J.A.; Frey, P. A Collagen-Poly(Lactic Acid-Co-ɛ-Caprolactone) Hybrid Scaffold for Bladder Tissue Regeneration. Biomaterials 2011, 32, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Herman, C.; Razdan, S.; Abedin, M.R.; Van Stoecker, W.; Barua, S. Microparticles for Suspension Culture of Mammalian Cells. ACS Appl. Bio Mater. 2019, 2, 2791–2801. [Google Scholar] [CrossRef]
- Aghmiuni, A.I.; Baei, M.S.; Keshel, S.H.; Khiyavi, A.A. Design of Novel 3D-Scaffold as a Potential Material to Induct Epidermal-Dermal Keratinocytes of Human-Adipose-Derived Stem Cells and Promote Fibroblast Cells Proliferation for Skin Regeneration. Fibers Polym. 2020, 21, 33–44. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S. Preparation, Characteristics and Assessment of a Novel Gelatin-Chitosan Sponge Scaffold as Skin Tissue Engineering Material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical Stimulation of Nerve Cells Using Conductive Nanofibrous Scaffolds for Nerve Tissue Engineering. Tissue Eng. Part. A 2009, 15, 3605–3619. [Google Scholar] [CrossRef] [PubMed]
- Farkhondehnia, H.; Amani Tehran, M.; Zamani, F. Fabrication of Biocompatible PLGA/PCL/PANI Nanofibrous Scaffolds with Electrical Excitability. Fibers Polym. 2018, 19, 1813–1819. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019, 7, 266:1–266:14. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA Macroporous Hydrogels For Nerve Regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shi, X.; Gan, Z.; Wang, F. Modification of Porous PLGA Microspheres by Poly-L-Lysine for Use as Tissue Engineering Scaffolds. Colloids Surf. B Biointerfaces 2018, 161, 162–168. [Google Scholar] [CrossRef]
- Huang, W.; Shi, X.; Ren, L.; Du, C.; Wang, Y. PHBV Microspheres–PLGA Matrix Composite Scaffold for Bone Tissue Engineering. Biomaterials 2010, 31, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Di Lisa, D.; de Rutte, J.; Sakib, M.N.; Alves e Silva, A.R.; Tamayol, A.; Di Carlo, D.; Khademhosseini, A.; Sheikhi, A. In Situ Forming Microporous Gelatin Methacryloyl Hydrogel Scaffolds from Thermostable Microgels for Tissue Engineering. Bioeng. Transl. Med. 2020, 5, e10180:1–e10180:12. [Google Scholar] [CrossRef] [PubMed]
- Jgamadze, D.; Liu, L.; Vogler, S.; Chu, L.-Y.; Pautot, S. Thermoswitching Microgel Carriers Improve Neuronal Cell Growth and Cell Release for Cell Transplantation. Tissue Eng. Part. C Methods 2015, 21, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, A.; de Rutte, J.; Haghniaz, R.; Akouissi, O.; Sohrabi, A.; Di Carlo, D.; Khademhosseini, A. Modular Microporous Hydrogels Formed from Microgel Beads with Orthogonal Thermo-Chemical Responsivity: Microfluidic Fabrication and Characterization. MethodsX 2019, 6, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Blüml, G. Microcarrier Cell Culture Technology. In Animal Cell Biotechnology: Methods and Protocols, 1st ed.; Pörtner, R., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 149–178. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602:1–290602:19. [Google Scholar] [CrossRef]
- Remya, N.S.; Syama, S.; Gayathri, V.; Varma, H.K.; Mohanan, P.V. An in vitro Study on the Interaction of Hydroxyapatite Nanoparticles and Bone Marrow Mesenchymal Stem Cells for Assessing the Toxicological Behaviour. Colloids Surf. B Biointerfaces 2014, 117, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Beaune, G.; Nagarajan, U.; Brochard-Wyart, F.; Winnik, F.M. Polymeric Nanoparticles Limit the Collective Migration of Cellular Aggregates. Langmuir 2019, 35, 7396–7404. [Google Scholar] [CrossRef]
- Salem, A.K.; Stevens, R.; Pearson, R.G.; Davies, M.C.; Tendler, S.J.B.; Roberts, C.J.; Williams, P.M.; Shakesheff, K.M. Interactions of 3T3 Fibroblasts and Endothelial Cells with Defined Pore Features. J. Biomed. Mater. Res. 2002, 61, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic, M.; Absenger, M.; Riedl, R.; Smole, C.; Roblegg, E.; Fröhlich, L.F.; Fröhlich, E. Assessment of Long-Term Effects of Nanoparticles in a Microcarrier Cell Culture System. PLoS ONE 2013, 8, e56791:1–e56791:10. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Yu, H.-S.; Kim, H.-W. Tissue Engineering Polymeric Microcarriers with Macroporous Morphology and Bone-Bioactive Surface. Macromol. Biosci. 2009, 9, 639–645. [Google Scholar] [CrossRef]
- Hong, Y.; Gao, C.; Xie, Y.; Gong, Y.; Shen, J. Collagen-Coated Polylactide Microspheres as Chondrocyte Microcarriers. Biomaterials 2005, 26, 6305–6313. [Google Scholar] [CrossRef] [PubMed]
- Thissen, H.; Chang, K.-Y.; Tebb, T.A.; Tsai, W.-B.; Glattauer, V.; Ramshaw, J.A.M.; Werkmeister, J.A. Synthetic Biodegradable Microparticles for Articularcartilage Tissue Engineering. J. Biomed. Mater. Res. A 2006, 77, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Curran, S.J.; Curran, J.M.; Hunt, J.A. The Use of Poly(l-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engineering Cartilage. Biomaterials 2006, 27, 4453–4460. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-W.; Yoon, J.-R.; Lee, J.-S.; Kim, H.J.; Lim, H.-W.; Lim, H.C.; Park, J.-H.; Kim, B.-S. The Use of Poly(Lactic-Co-Glycolic Acid) Microspheres as Injectable Cell Carriers for Cartilage Regeneration in Rabbit Knees. J. Biomater. Sci. Polym. Ed. 2006, 17, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Mercier, N.R.; Costantino, H.R.; Tracy, M.A.; Bonassar, L.J. Poly(Lactide-Co-Glycolide) Microspheres as a Moldable Scaffold for Cartilage Tissue Engineering. Biomaterials 2005, 26, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Mercier, N.R.; Costantino, H.R.; Tracy, M.A.; Bonassar, L.J. A Novel Injectable Approach for Cartilage Formation in vivo Using PLG Microspheres. Ann. Biomed. Eng. 2004, 32, 418–429. [Google Scholar] [CrossRef]
- Ki, W.C.; Hyuk, S.Y.; Jun, J.Y.; Tae, G.P. Biodegradable PLGA Microcarriers for Injectable Delivery of Chondrocytes: Effect of Surface Modification on Cell Attachment and Function. Biotechnol. Prog. 2004, 20, 1797–1801. [Google Scholar] [CrossRef]
- Tan, H.; Wu, J.; Huang, D.; Gao, C. The Design of Biodegradable Microcarriers for Induced Cell Aggregation. Macromol. Biosci. 2010, 10, 156–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Jiang, J.; Zhang, X.; Ding, W.; Gan, Z. Biodegradation-Induced Surface Change of Polymer Microspheres and Its Influence on Cell Growth. Polym. Degrad. Stab. 2010, 95, 1356–1364. [Google Scholar] [CrossRef]
- Chan, B.P.; Hui, T.Y.; Wong, M.Y.; Yip, K.H.K.; Chan, G.C.F. Mesenchymal Stem Cell-Encapsulated Collagen Microspheres for Bone Tissue Engineering. Tissue Eng. Part. C Methods 2010, 16, 225–235. [Google Scholar] [CrossRef]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-Low Molecular Weight Hyaluronic Acid Semi-Interpenetrating Network Loaded with Gelatin Microspheres for Cell and Growth Factor Delivery for Nucleus Pulposus Regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Taek, K.K.; Jun, J.Y.; Doo, S.L.; Park, T.G. Gas Foamed Open Porous Biodegradable Polymeric Microspheres. Biomaterials 2006, 27, 152–159. [Google Scholar] [CrossRef]
- Botchwey, E.A.; Pollack, S.R.; Levine, E.M.; Laurencin, C.T. Bone Tissue Engineering in a Rotating Bioreactor Using a Microcarrier Matrix System. J. Biomed. Mater. Res. 2001, 55, 242–253. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Injectable Cellular Aggregates Prepared from Biodegradable Porous Microspheres for Adipose Tissue Engineering. Tissue Eng. Part. A 2009, 15, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Systematic Microcarrier Screening and Agitated Culture Conditions Improve Human Mesenchymal Stem Cell Yield in Bioreactors. Biotechnol. J. 2016, 11, 473–486. [Google Scholar] [CrossRef]
- Ma, S.; Natoli, M.; Liu, X.; Neubauer, M.P.; Watt, F.M.; Fery, A.; Huck, W.T.S. Monodisperse Collagen-Gelatin Beads as Potential Platforms for 3D Cell Culturing. J. Mater. Chem. B 2013, 1, 5128–5136. [Google Scholar] [CrossRef]
- Leslie, S.K.; Cohen, D.J.; Sedlaczek, J.; Pinsker, E.J.; Boyan, B.D.; Schwartz, Z. Controlled Release of Rat Adipose-Derived Stem Cells from Alginate Microbeads. Biomaterials 2013, 34, 8172–8184. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhang, K.; Gong, Y.; Li, G.; Yan, S.; Yin, J. Injectable Stem Cell Laden Open Porous Microgels That Favor Adipogenesis: In vitro and in vivo Evaluation. ACS Appl. Mater. Interfaces 2017, 9, 34751–34761. [Google Scholar] [CrossRef] [PubMed]
- Nisal, A.; Sayyad, R.; Dhavale, P.; Khude, B.; Deshpande, R.; Mapare, V.; Shukla, S.; Venugopalan, P. Silk Fibroin Micro-Particle Scaffolds with Superior Compression Modulus and Slow Bioresorption for Effective Bone Regeneration. Sci. Rep. 2018, 8, 7235:1–7235:10. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Wyman, O.M.; Alge, D.L. Assembly of PEG Microgels into Porous Cell-Instructive 3D Scaffolds via Thiol-Ene Click Chemistry. Adv. Healthc. Mater. 2018, 7, 1800160:1–1800160:7. [Google Scholar] [CrossRef]
- Kook, Y.-M.; Jeong, Y.; Lee, K.; Koh, W.-G. Design of Biomimetic Cellular Scaffolds for Co-Culture System and Their Application. J. Tissue Eng. 2017, 8, 2041731417724640:1–2041731417724640:17. [Google Scholar] [CrossRef]
- Battiston, K.G.; Cheung, J.W.C.; Jain, D.; Santerre, J.P. Biomaterials in Co-Culture Systems: Towards Optimizing Tissue Integration and Cell Signaling within Scaffolds. Biomaterials 2014, 35, 4465–4476. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D Printed Bone Scaffolds by Bioactive Hydrogels and Cell Co-Culture. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106B, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Stoppato, M.; Stevens, H.Y.; Carletti, E.; Migliaresi, C.; Motta, A.; Guldberg, R.E. Effects of Silk Fibroin Fiber Incorporation on Mechanical Properties, Endothelial Cell Colonization and Vascularization of PDLLA Scaffolds. Biomaterials 2013, 34, 4573–4581. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.-T.; Leung, M.; Chang, J.Y.-F.; Zhang, M. A Simple Material Model to Generate Epidermal and Dermal Layers in vitro for Skin Regeneration. J. Mater. Chem. B 2014, 2, 5256–5264. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Sokic, S.; Schardt, J.S.; Raiker, R.S.; Lin, J.W.; Jay, S.M. Emerging Roles for Extracellular Vesicles in Tissue Engineering and Regenerative Medicine. Tissue Eng. Part. B Rev. 2015, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Wong, S.; Jean, J.S.; Slavcev, R. Bacteriophage Interactions with Mammalian Tissue: Therapeutic Applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef]
- Bodner, K.; Melkonian, A.L.; Covert, M.W. The Enemy of My Enemy: New Insights Regarding Bacteriophage–Mammalian Cell Interactions. Trends Microbiol. 2020, 1896:1–1896:14. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Xie, A.W.; Yamato, M.; Okano, T.; Wong, J.Y. Stacking of Aligned Cell Sheets for Layer-by-Layer Control of Complex Tissue Structure. Biomaterials 2011, 32, 5625–5632. [Google Scholar] [CrossRef] [PubMed]
- Galliger, Z.; Vogt, C.D.; Panoskaltsis-Mortari, A. 3D Bioprinting for Lungs and Hollow Organs. Transl. Res. 2019, 211, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Leber, J.; Barekzai, J.; Blumenstock, M.; Pospisil, B.; Salzig, D.; Czermak, P. Microcarrier Choice and Bead-to-Bead Transfer for Human Mesenchymal Stem Cells in Serum-Containing and Chemically Defined Media. Process. Biochem. 2017, 59, 255–265. [Google Scholar] [CrossRef]
| Engineered Tissue | Cell Types | Scaffold Morphologies | Common Polymers | References |
|---|---|---|---|---|
| Vascular tissue (e.g., blood vessel) | Human mesenchymal stem cells (hMSCs) | Tubes | PCL 1, PLLA 2 | [71,72] |
| Human turbinate mesenchymal stromal cells | Tubes | PEG 3 | [71] | |
| Human umbilical vein endothelial cells (HUVECs) | Tubes | PPO-PEO 4 | [71] | |
| Human smooth muscle cells | Mesh tubes | PGA 5 | [59,73] | |
| Human smooth muscle cells | Tubes | PGA | [58] | |
| Vascular smooth muscle cells from human induced pluripotent stem cells (hiPSCs-VSMCs) | Tubes | pNIPAm-grafted PDMS 6 | [74] | |
| Bone tissue (e.g., tendon, cartilage) | hMSCs | Three-dimensional (3D) porous scaffolds | PCL/HA 7 | [75] |
| Human bone marrow stromal cells (hBMSCs) | Membrane/3D porous scaffolds | PHA 8 | [50] | |
| hBMSCs | 3D porous scaffolds | PLA 9 | [76] | |
| Human placenta-derived mesenchymal stem cells (hPMSCs) | Fibres | PLA, PLGA 10 | [77] | |
| MG-63 human osteoblast-like cells | Porous microspheres | PLA | [78] | |
| Human nasal chondrocytes | Porous microspheres | PEGT/PBT 11 | [79] | |
| Human skeletal stems cells (hSSCs) | Sponges | PLA/HA 12, PLGA/HA 13 | [80] | |
| Human fetal osteoblasts (hFOBs) | Nanofibres | Chitosan/HA 14 | [81] | |
| hFOBs | Nanofibres | Gelatin/HA 15 | [82] | |
| hFOBs | Nanofibres | Collagen/HA 16 | [68] | |
| Adipose-derived mesenchymal stem cells (hADMSCs) | 3D fibrous scaffolds | Keratin | [54] | |
| Human bone marrow derived mesenchymal stem cells | Hydrogels | Silk fibroin | [64] | |
| Human bone marrow stem cells | Sponges | Silk | [83] | |
| hADMSCs, Human tenocytes (HT), HUVECs | Non-woven meshes, nanofibrous woven fabrics | PCL | [84] | |
| Urinary tissue (e.g., bladder, urethra, ureter) | Human bladder smooth muscle cells (hBSMCs), urothelial cells (UCs) | Porous microspheres, meshes, nanofibres | HA 17, PGA, PLGA, PLLA | [85,86] |
| hBSMCs, UCs | Meshes | PLAC 18 copolymer | [87] | |
| Dermal tissue | HUVECs | Non-porous microspheres | PLGA | [88] |
| Human Skin Fibroblast cells (HSFs) | 3D porous scaffolds | CPCP 19 composite | [89] | |
| Human dermal fibroblasts (HDFs) | Porous microspheres | PCL | [52] | |
| Human keratinocytes (HaCaTs) | Hydrogels | Gelatin | [90] | |
| HSFs, HaCaTs | Sponges | Gelatin–chitosan | [91] | |
| Nerve tissue | Nerve stem cells (NSCs) | Nanofibres | PANI/PG(PCL&Gelatin) 20 | [92] |
| Human glioma cells (A-172 cells) | Nanofibres | PLGA | [49] | |
| Human glioma cells (A-172 cells) | Nanofibres | PLGA/PCL/PANI 21 | [93] | |
| Human embryonic stem cell-derived neural crest stem cells (hESC-NCSCs) | Porous scaffold 3D printed from fibres | PPy-b-PCL 22 | [94] | |
| hMSCs | Macroporous hydrogels | PANI/PEGDA 23 | [95] |
| Microcarriers | Polymers | Sizes (μm) | Cell Types | References |
|---|---|---|---|---|
| Non-Porous Spheres | PCL 1 | 261 ± 71 | Rat bone-marrow-derived stromal cells (rBMSCs) | [107] |
| PLA 2 | 180–280 | Rabbit chondrocytes | [108] | |
| PLGA 3 | 47–210 | Sheep articular cartilage chondrocytes | [109] | |
| PLLA 4 | 100–200 | Human OUMS-27 chondrosarcoma cells | [110] | |
| PLGA | 30–80 | White rabbit chondrocytes | [111] | |
| PLG 5 | 52–68 | Calves chondrocytes | [112] | |
| PLG | 52–199 | Calves chondrocytes | [113] | |
| PLGA | 80–90 | Bovine chondrocytes | [114] | |
| PLLA | 80–120 | Rabbit ear chondrocytes | [115] | |
| PCL-b-PEO 6 or PCL | 100–150 | MG-63 human osteosarcoma cells | [116] | |
| PLGA | 165 ± 40.4 | Human umbilical vein endothelial cells (HUVECs) | [99] | |
| Collagen | ≈250 | Human and rat bone marrow-derived mesenchymal stem cells (hMSCs/rMSCs) | [117] | |
| Gelatin | 260 ± 50 | Human mesenchymal stem cells (hMSCs) and nasal chondrocytes (hNCs) | [118] | |
| Porous Spheres | PCL | 168–220 | rBMSCs | [107] |
| Blend of PCL and PLA | 50–100 | rBMSCs | [51] | |
| PLA | 160–320 | Rat bladder smooth muscle cells | [96] | |
| PEGT/PBT 7 | 130–180 | Human nasal chondrocytes | [90] | |
| PLA | 150–250 | MG-63 human osteoblast-like cells | [89] | |
| PLGA | 343 ± 60 | NIH 3T3 mouse embryo fibroblasts | [119] | |
| PLAGA 5 | 500–860 | Human SaOS-2 line HTB-85 | [120] | |
| PLGA | ≈50 | 3T3 L1 mouse preadipocyte cells | [121] | |
| PCL | 100–600 | Human dermal fibroblasts (HDFs) | [52] | |
| PLGA | 500–800 | L929 fibroblasts and rat adipose-derived stromal cells (ADSC) | [53] | |
| Non-Porous Microgels | pNIPAM 8 | 120 ± 15 | Rat hippocampal neuronal cells | [74] |
| Collagen coated PS 9 | 125–212 | Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | [122] | |
| Collagen-gelatin | 80 | 3T3 fibroblast cells | [123] | |
| Alginate | <200 | Rat adipose-derived stem cells (rASCs) | [124] | |
| Porous Microgels | GelMA 10 | ≈90 | NIH 3T3 mouse embryo fibroblasts/HUVECs | [73,75] |
| PLGA-g-HEMA and MCS 11 | 200–300 | Human adipose stem cells (hASCs) | [125] | |
| Silk fibroin | 503 | MG-63 human osteoblast-like cells | [126] | |
| PEG 12 | ≈200 | hMSCs | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, T.; Xiang, Y.; Bao, X.; Sun, T. A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy. Processes 2021, 9, 935. https://doi.org/10.3390/pr9060935
Schmidt T, Xiang Y, Bao X, Sun T. A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy. Processes. 2021; 9(6):935. https://doi.org/10.3390/pr9060935
Chicago/Turabian StyleSchmidt, Theresa, Yu Xiang, Xujin Bao, and Tao Sun. 2021. "A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy" Processes 9, no. 6: 935. https://doi.org/10.3390/pr9060935
APA StyleSchmidt, T., Xiang, Y., Bao, X., & Sun, T. (2021). A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy. Processes, 9(6), 935. https://doi.org/10.3390/pr9060935








