Curcumin and Freshwater Clam Extracts Alleviate the Progression of Osteoarthritis by Reducing Synovial Inflammation and Allowing Cartilage Regeneration
Abstract
1. Introduction
2. Materials and Methods
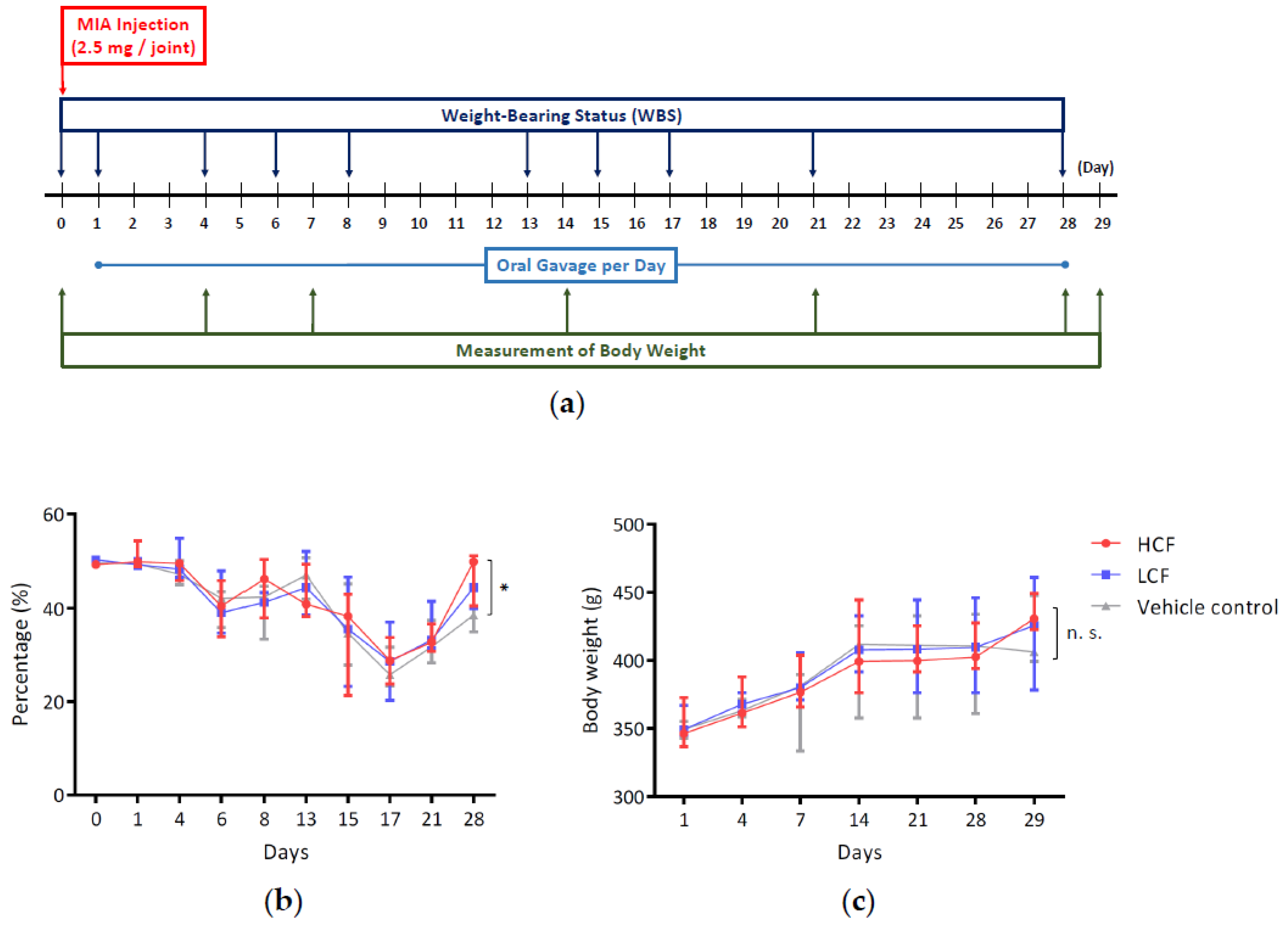
2.1. MIA-Induced OA Animal Model for Cartilage Regeneration and Synovial Repair Evaluation
2.2. Measuring Hindlimb Weight-Bearing Distribution
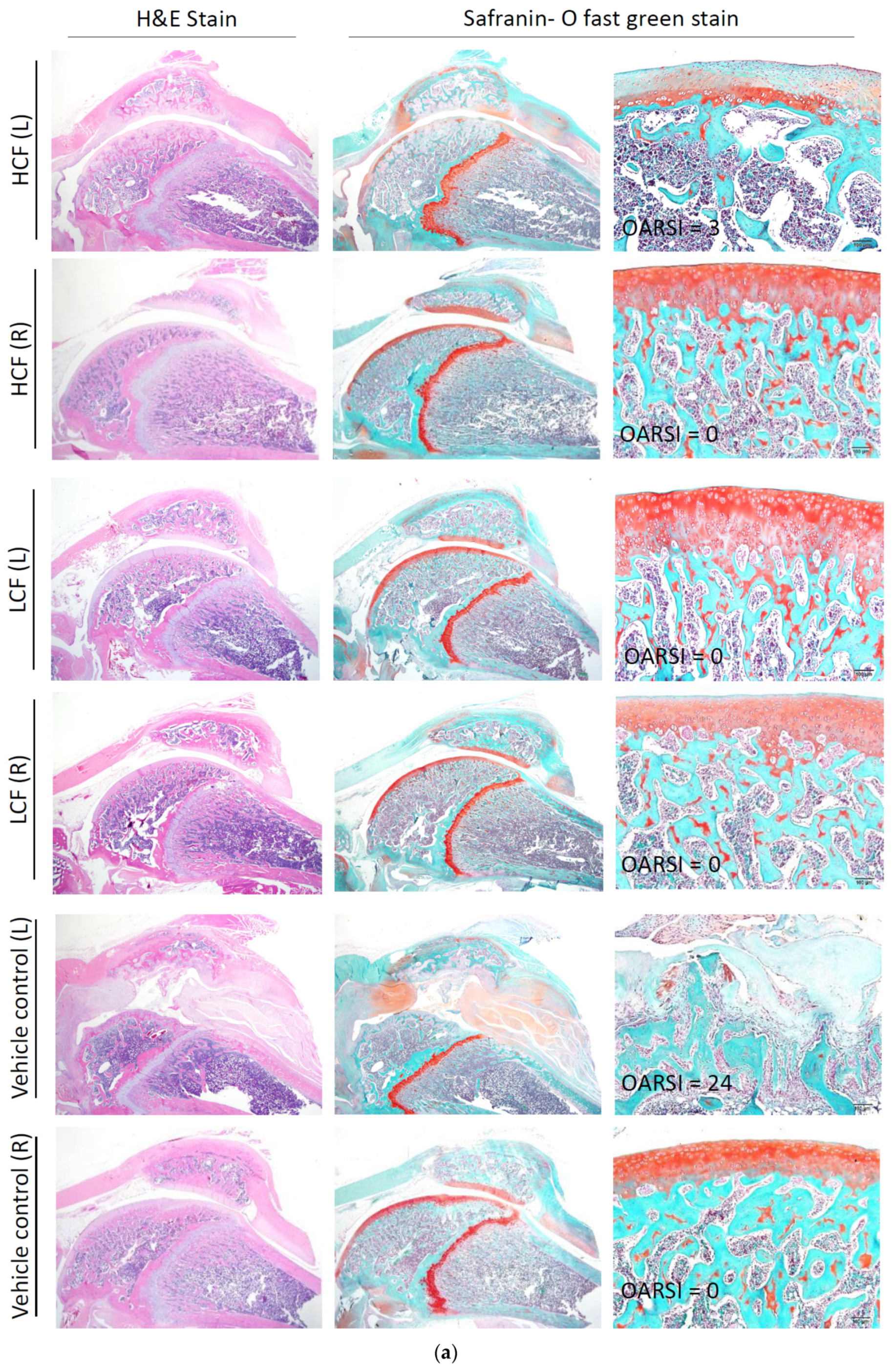
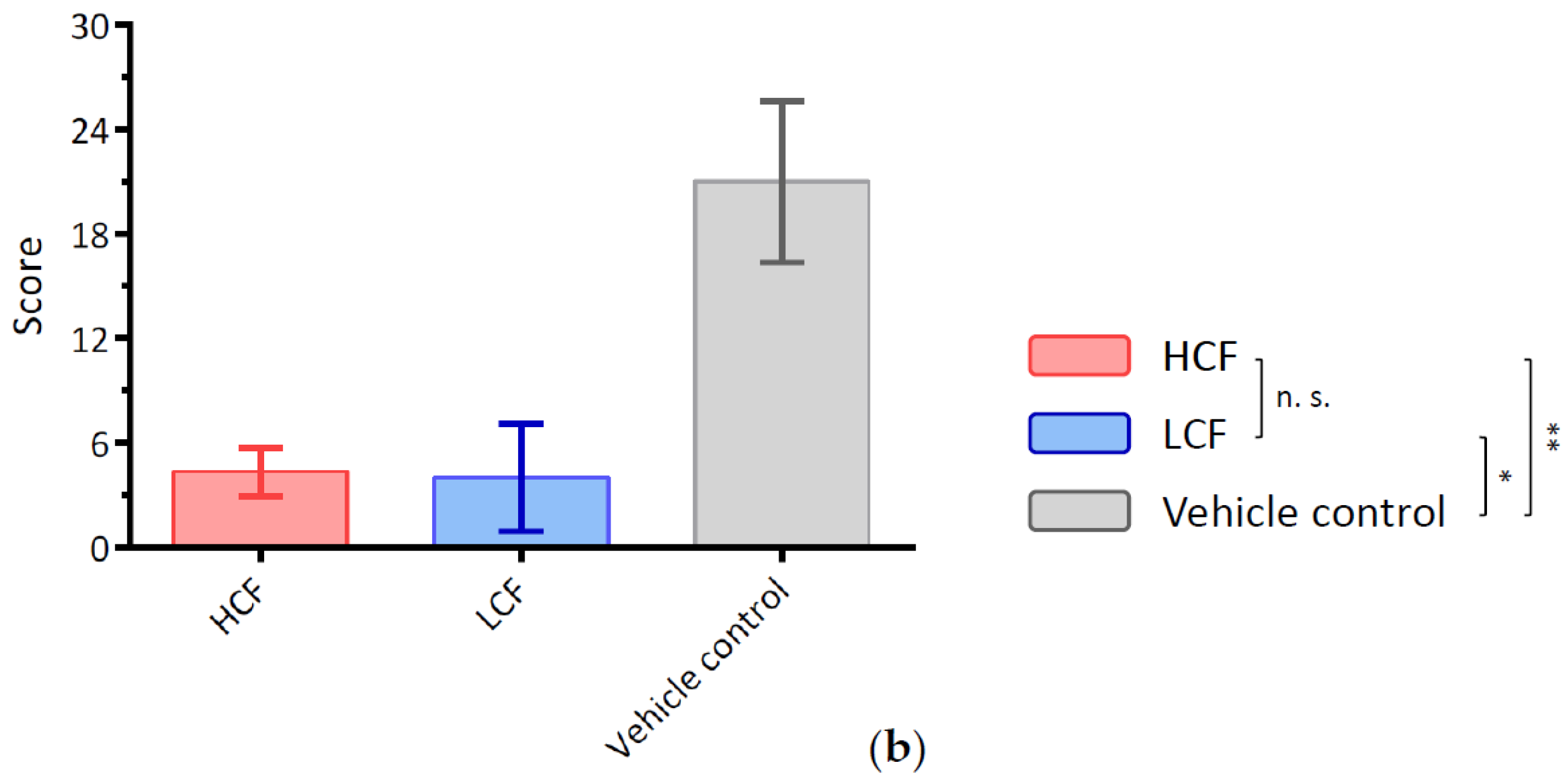
2.3. Histopathological Examination for Synovial Inflammation and Cartilage Regeneration
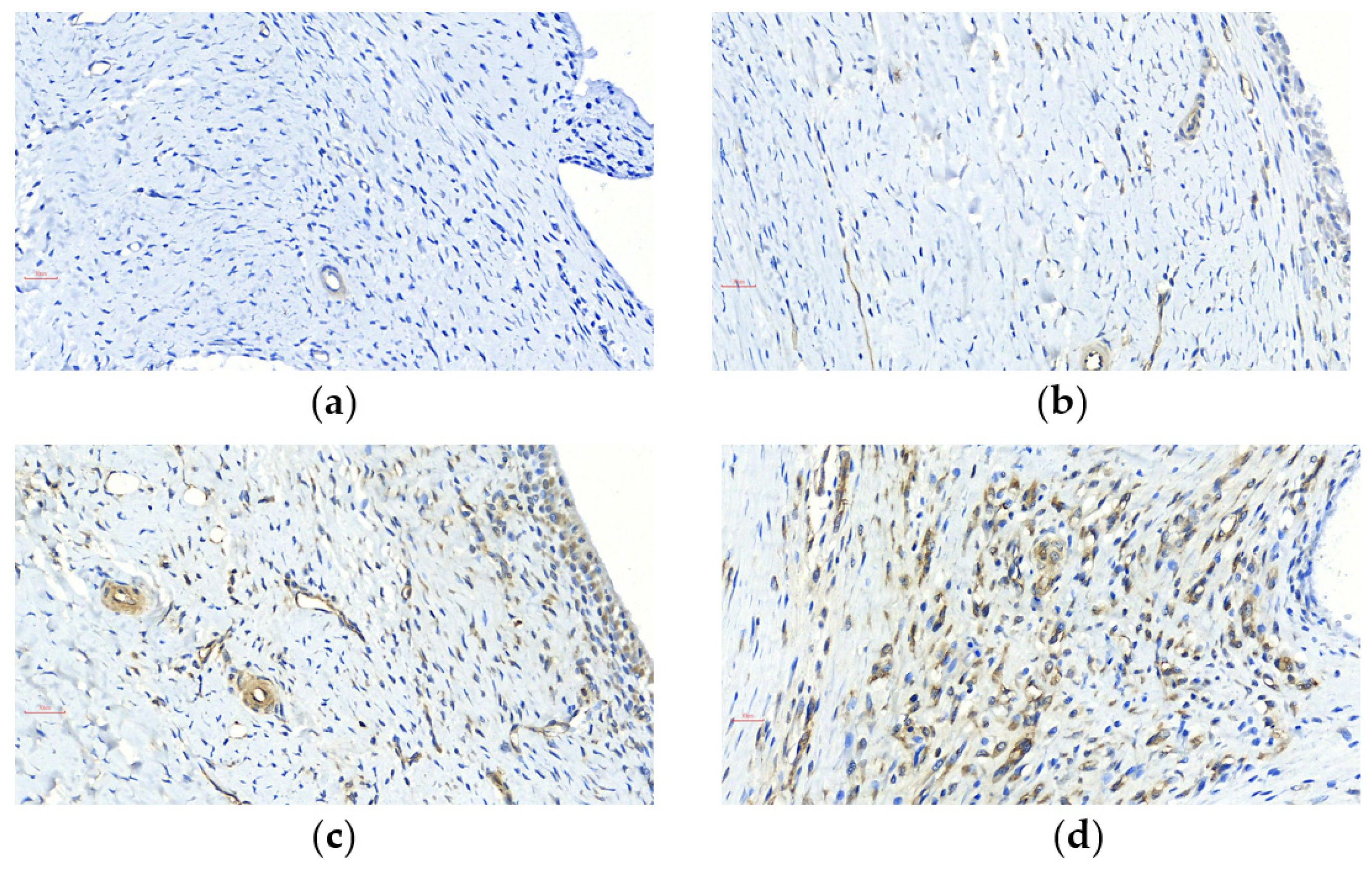
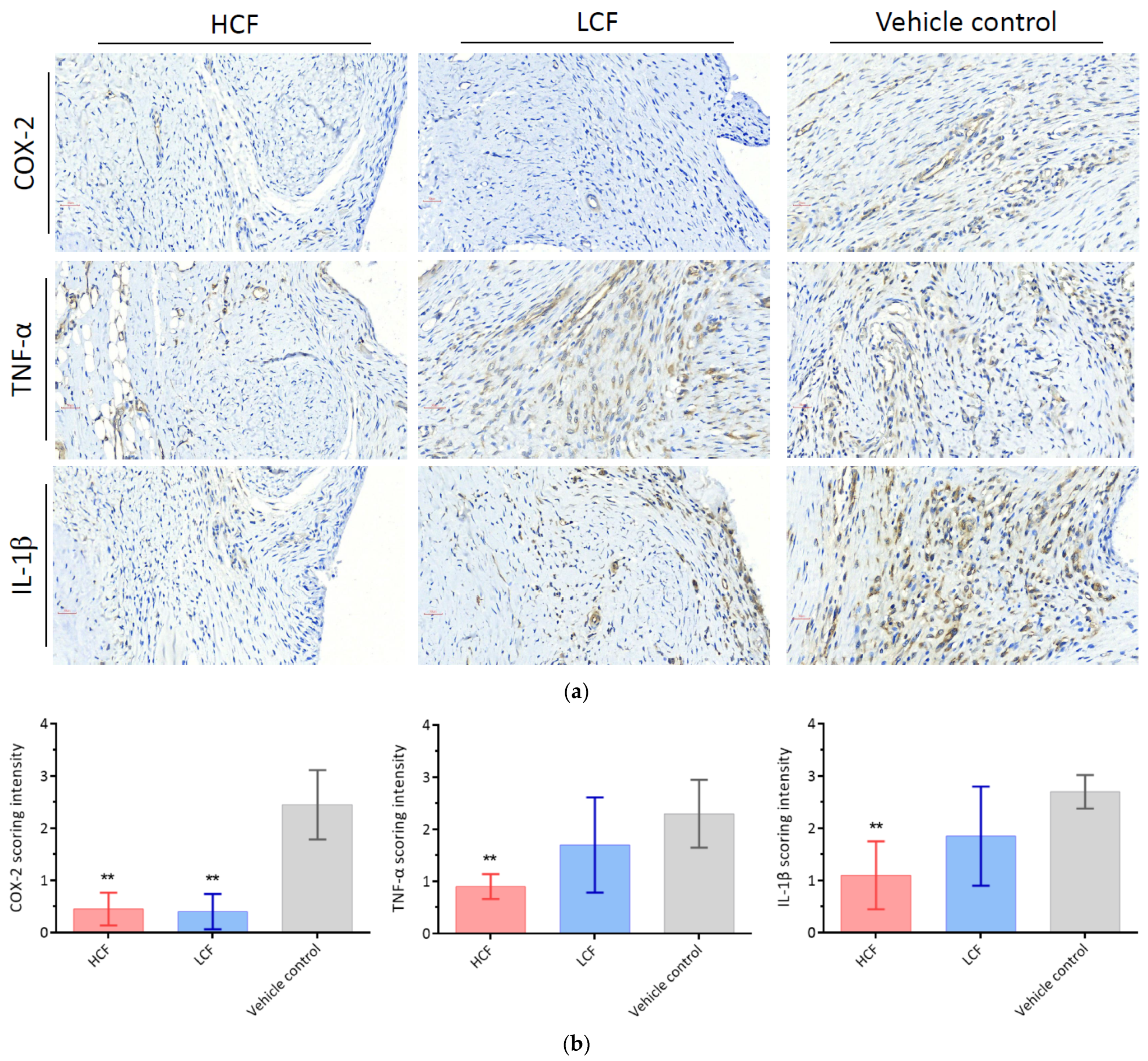
2.4. Immunohistochemical Staining and Scoring System
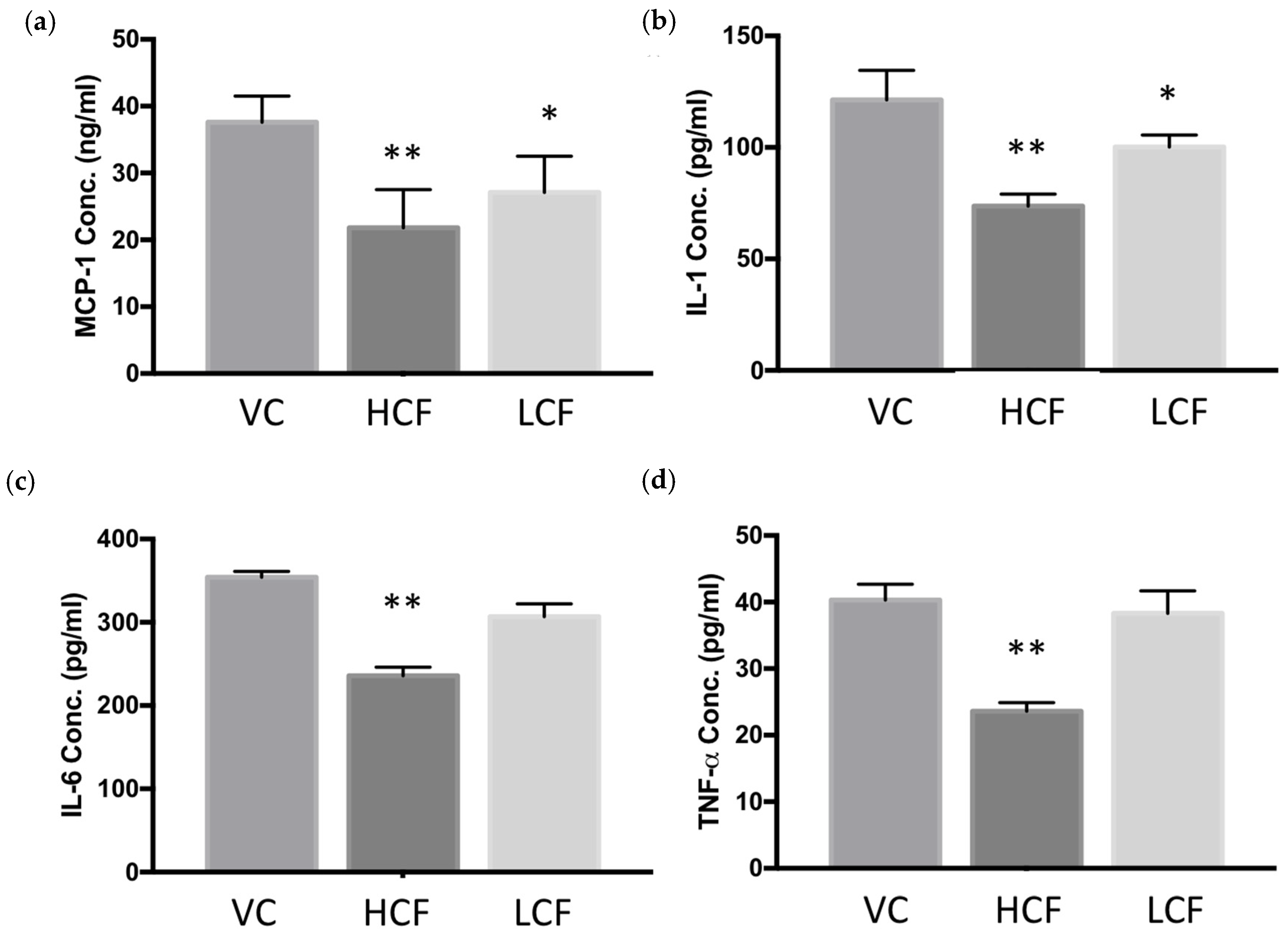
2.5. ELISA
2.6. Statistical Analysis
3. Results
3.1. High-Dosage Curcumin and FCE Can Effectively Relieve Clinical Symptoms Safely
3.2. Curcumin and FCE Simultaneously Alleviated Local and Systemic Inflammatory Responses
3.3. Curcumin and FCE Can Significantly Promote Cartilage Regeneration in High and Low Doses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Yu, S.P.; Hunter, D.J. Managing osteoarthritis. Aust. Prescr. 2015, 38, 115–119. [Google Scholar] [CrossRef]
- Udo, M.; Muneta, T.; Tsuji, K.; Ozeki, N.; Nakagawa, Y.; Ohara, T.; Saito, R.; Yanagisawa, K.; Koga, H.; Sekiya, I. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: Proposed model-specific scoring systems. Osteoarthr. Cartil. 2016, 24, 1284–1291. [Google Scholar] [CrossRef]
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, H.; Bai, F.; Zhang, M.; Yang, L.; Deng, J.; Xiong, L. A rat model of knee osteoarthritis suitable for electroacupuncture study. Exp. Anim. 2018, 67, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Losina, E.; Katz, J.N. Total joint replacement outcomes in patients with concomitant comorbidities: A glass half empty or half full? Arthritis Rheum. 2013, 65, 1157. [Google Scholar] [CrossRef] [PubMed]
- Legg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham III., C.O.; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef]
- Savvidou, O.; Milonaki, M.; Goumenos, S.; Flevas, D.; Papagelopoulos, P.; Moutsatsou, P. Glucocorticoid signaling and osteoarthritis. Mol. Cell Endocrinol. 2019, 480, 153–166. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef]
- Cheragh-Birjandi, S.; Moghbeli, M.; Haghighi, F.; Safdari, M.R.; Baghernezhad, M.; Akhavan, A.; Ganji, R. Impact of resistance exercises and nano-curcumin on synovial levels of collagenase and nitric oxide in women with knee osteoarthritis. Transl. Med. Commun. 2020, 5, 3. [Google Scholar] [CrossRef]
- Green, J.A.; Hirst-Jones, K.L.; Davidson, R.K.; Jupp, O.; Bao, Y.; MacGregor, A.J.; Donell, S.T.; Cassidy, A.; Clark, I.M. The potential for dietary factors to prevent or treat osteoarthritis. Proc. Nutr. Soc. 2014, 73, 278–288. [Google Scholar] [CrossRef]
- Kuo, I.M.; Lee, J.J.; Wang, Y.S.; Chiang, H.C.; Huang, C.C.; Hsieh, P.J.; Han, W.; Ke, C.H.; Liao, A.T.C.; Lin, C.S. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro- hyperthermia. BMC Cancer 2020, 20, 603. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, R.; Bakhshinejad, B.; Babashah, S.; Baghi, N.; Sadeghizadeh, M. Dendrosomal nanocurcumin and p53 overexpression synergistically trigger apoptosis in glioblastoma cells. Iran. J. Basic Med. Sci. 2016, 19, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complementary Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ying, Y. The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia. Front. Cell Dev. Biol. 2020, 8, 479. [Google Scholar] [CrossRef]
- Huang, K.-C.; Wu, W.-T.; Yang, F.-L.; Chiu, Y.-H.; Peng, T.-C.; Hsu, B.-G.; Liao, K.-W.; Lee, R.-P. Effects of freshwater clam extract supplementation on time to exhaustion, muscle damage, pro/anti-inflammatory cytokines, and liver injury in rats after exhaustive exercise. Molecules 2013, 18, 3825–3838. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Subeq, Y.M.; Lee, C.J.; Lee, C.C.; Tsai, C.J.; Chang, F.M.; Lee, R.P. Freshwater clam extract ameliorates acute liver injury induced by hemorrhage in rats. Am. J. Chin. Med. 2008, 36, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Hsu, C.C.; Yen, G.C. Hepatoprotection by freshwater clam extract against CCl4-induced hepatic damage in rats. Am. J. Chin. Med. 2010, 38, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lin, Y.L.; Tsai, N.M.; Wu, H.Y.; Ho, S.Y.; Chen, C.H.; Liu, Y.K.; Chiu, Y.H.; Ho, L.P.; Lee, R.P.; et al. Inhibitory effects of chloroform extracts derived from Corbicula fluminea on the release of pro-inflammatory cytokines. J. Agric. Food Chem. 2012, 60, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.Y.; Choi, H.J.; Kim, M.G.; Jeong, D.G.; Lee, D.G.; Yoon, J.M.; Kang, D.J.; Park, S.; Ji, J.G.; Joo, I.H.; et al. Effects of ID-CBT5101 in Preventing and Alleviating Osteoarthritis Symptoms in a Monosodium Iodoacetate-Induced Rat Model. J. Microbiol. Biotechnol. 2018, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS ONE 2018, 13, e0196625. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Grevenstein, D.; Heilig, J.; Dargel, J.; Oppermann, J.; Eysel, P.; Brochhausen, C.; Niehoff, A. COMP in the Infrapatellar Fat Pad-Results of a Prospective Histological, Immunohistological, and Biochemical Case-Control Study. J. Orthop. Res. 2020, 38, 747–758. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- He, Q.; Sun, C.; Lei, W.; Ma, J. SOCS1 Regulates Apoptosis and Inflammation by Inhibiting IL-4 Signaling in IL-1β-Stimulated Human Osteoarthritic Chondrocytes. BioMed Res. Int. 2017, 2017, 4601959. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, M.; Wang, K. Taurine protects against knee osteoarthritis development in experimental rat models. Knee 2018, 25, 374–380. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis-chondrocytes in focus. Cell Signal 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Lee, E.K.; Choi, Y.J.; Kim, J.M.; Kim, D.H.; Zou, Y.; Kim, C.H.; Lee, J.; Kim, H.S.; Kim, N.D.; et al. Molecular Inflammation as an Underlying Mechanism of the Aging Process and Age-related Diseases. J. Dent. Res. 2011, 90, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Deby-Dupont, G.; Deby, C.; De Bruyn, M.; Lamy, M.; Franchimont, P. Production of active oxygen species by isolated human chondrocytes. Br. J. Rheumatol. 1993, 32, 562–567. [Google Scholar] [CrossRef]
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 48–56. [Google Scholar] [PubMed]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 2009, 58, 899–908. [Google Scholar] [CrossRef]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, S.; Mao, X.; Wang, W.; Tai, H. Inhibition effect of curcumin on TNF-α and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology 2013, 91, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.F.; Chen, G.M.; Ho, C.L.; Chen, M.C.; Chen, Y.L.; Chen, W.J.; Huang, J.F.; Perng, Y.S.; Lin, C.C. Freshwater Clam Extract Inhibits Inflammatory Responses in LPS-Activated Macrophages by Reducing the Activation of Mitogen-Activated Protein Kinases and NF-κB. Nat. Prod. Commun. 2012, 7, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
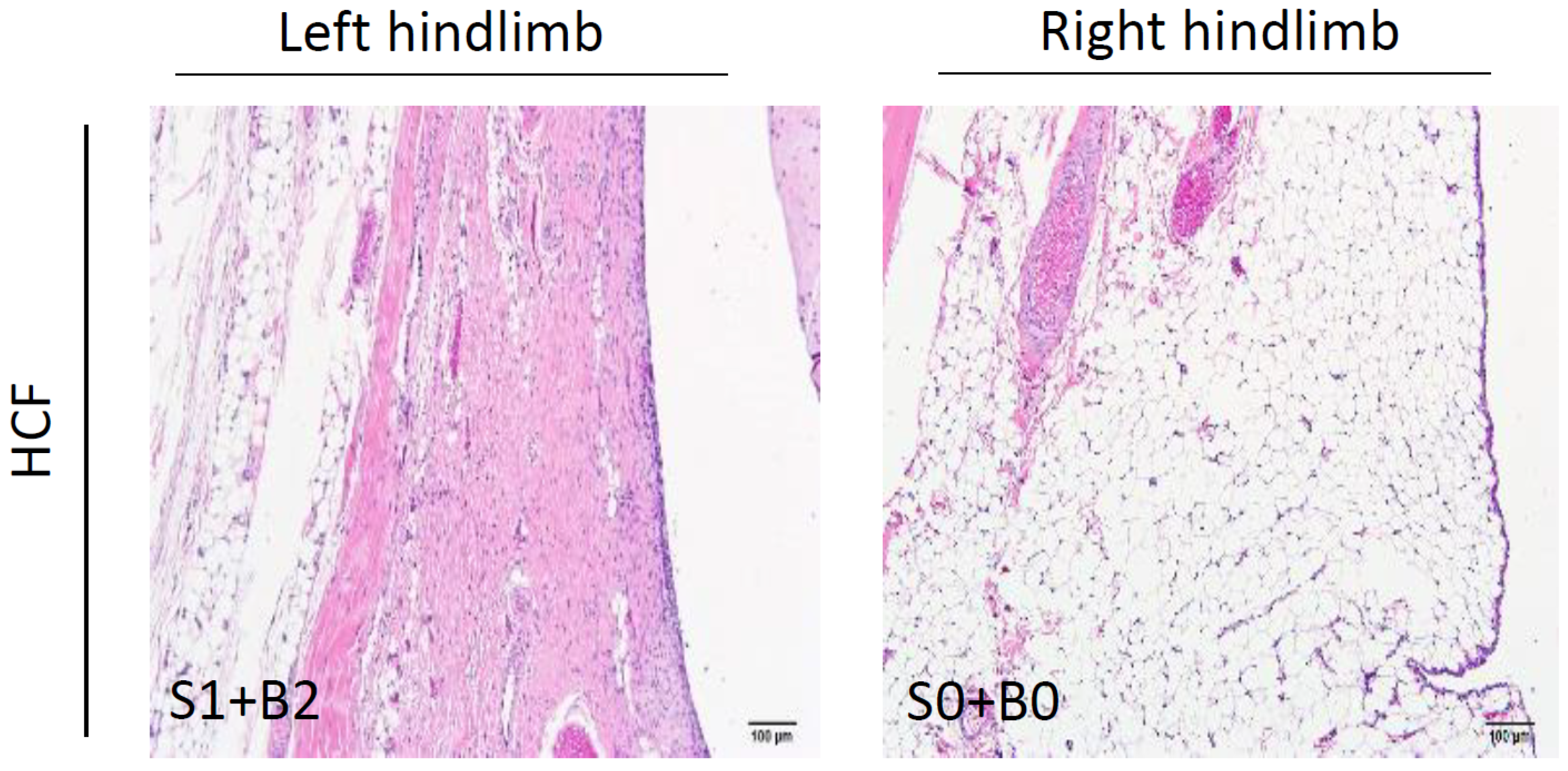
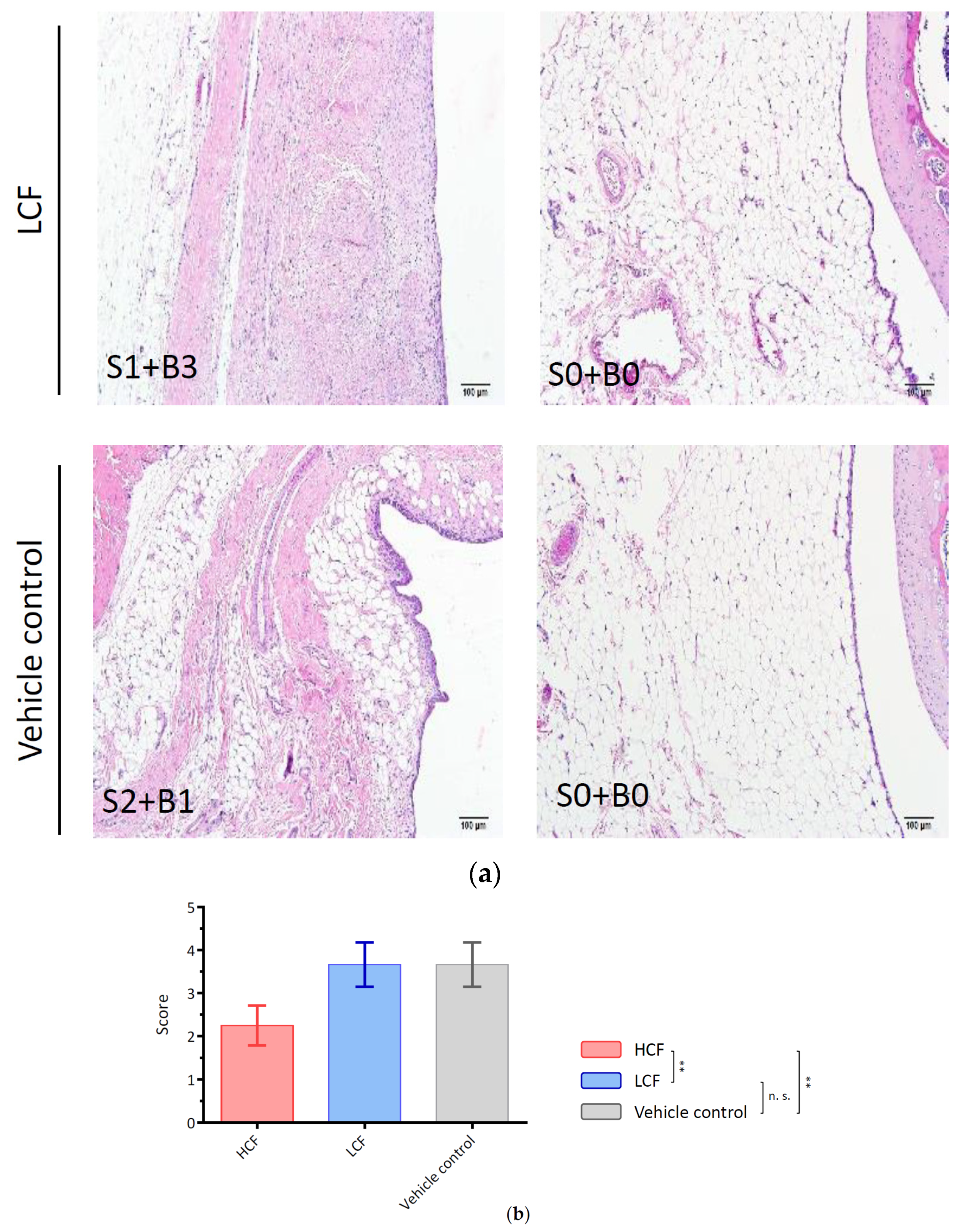
| Points | Histological Features |
| Cell infiltration at the surface of IFP | |
| 0 | Normal |
| 1 | Cellularity is increased, multinucleated cells present |
| 2 | Thickened lining cells, low (<three-fold thickness of the normal synovium) |
| 3 | Thickened lining cells, high (>three-fold thickness of the normal synovium) |
| Points | Histological Features |
| Fibrosis in the body of the IFP | |
| 0 | No fibrotic lesion |
| 1 | Fibrotic lesion in infrapatellar fat pad present, low |
| 2 | Fibrotic lesion increased, high |
| 3 | Infrapatellar fat pad filled with the fibrotic lesion and fat cells absent |
| Days | Vehicle Control Group | HCF Group | LCF Group |
|---|---|---|---|
| 0 (Baseline) | 49.75 ± 0.45 | 49.38 ± 0.50 | 50.38 ± 0.18 |
| 1 (MIA injection) | 49.50 ± 0.54 | 50.88 ± 2.15 | 49.50 ± 0.93 |
| 4 | 47.50 ± 1.90 | 48.95 ± 1.74 | 49.63 ± 3.18 |
| 6 | 40.95 ± 3.02 | 40.23 ± 5.51 | 40.25 ± 5.17 |
| 8 | 40.78 ± 4.37 | 45.25 ± 4.88 | 40.98 ± 1.99 |
| 13 | 46.83 ± 3.17 | 42.40 ± 4.37 | 44.93 ± 4.95 |
| 15 | 35.65 ± 6.22 | 35.28 ± 8.81 | 35.33 ± 8.35 |
| 17 | 26.68 ± 3.32 | 28.83 ± 3.54 | 28.68 ± 5.96 |
| 21 | 32.38 ± 3.32 | 33.28 ± 2.20 | 34.88 ± 3.96 |
| 28 | 39.21 ± 3.59 * | 47.96 ± 4.33 * | 43.54 ± 2.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, C.-H.; Hsu, C.-H.; Lin, Y.-L.; Huang, W.-H.; Weng, H.-P.; Ke, Y.-T.; Lin, C.-S. Curcumin and Freshwater Clam Extracts Alleviate the Progression of Osteoarthritis by Reducing Synovial Inflammation and Allowing Cartilage Regeneration. Processes 2021, 9, 931. https://doi.org/10.3390/pr9060931
Ke C-H, Hsu C-H, Lin Y-L, Huang W-H, Weng H-P, Ke Y-T, Lin C-S. Curcumin and Freshwater Clam Extracts Alleviate the Progression of Osteoarthritis by Reducing Synovial Inflammation and Allowing Cartilage Regeneration. Processes. 2021; 9(6):931. https://doi.org/10.3390/pr9060931
Chicago/Turabian StyleKe, Chiao-Hsu, Chia-Hui Hsu, Yu-Ling Lin, Wei-Hsiang Huang, Hsin-Pei Weng, Yi-Tzu Ke, and Chen-Si Lin. 2021. "Curcumin and Freshwater Clam Extracts Alleviate the Progression of Osteoarthritis by Reducing Synovial Inflammation and Allowing Cartilage Regeneration" Processes 9, no. 6: 931. https://doi.org/10.3390/pr9060931
APA StyleKe, C.-H., Hsu, C.-H., Lin, Y.-L., Huang, W.-H., Weng, H.-P., Ke, Y.-T., & Lin, C.-S. (2021). Curcumin and Freshwater Clam Extracts Alleviate the Progression of Osteoarthritis by Reducing Synovial Inflammation and Allowing Cartilage Regeneration. Processes, 9(6), 931. https://doi.org/10.3390/pr9060931







