Apparent Pyrolysis Kinetics and Index-Based Assessment of Pretreated Peach Seeds
Abstract
1. Introduction
Scope and Objective
- (1)
- Pretreatment of peach seeds by hexane to recover the oils, in the biorefinery approach.
- (2)
- Pyrolysis of the remaining solid, after extraction of lipids, in a laboratory plug flow reactor (PFR) for the parametric study of the effect of temperature, heating rate and the carrier gas flow on the pyrolysis products yield.
- (3)
- Kinetic study of the process by using the non-isothermal model proposed by Coats and Redfern fitting the model [32] on the experimental results to estimate the reaction order (n), specific rate constant (k) and activation energy (Ea).
- (4)
- Estimation of quality indexes based on isothermal TGA data for assessing the appropriateness of the pretreated peach seeds for the pyrolysis process.
2. Materials and Methods
2.1. The Material-Feedstock
2.2. Protocol of Feedstock Extraction with Hexane
2.3. Pyrolysis Experimentation Protocol
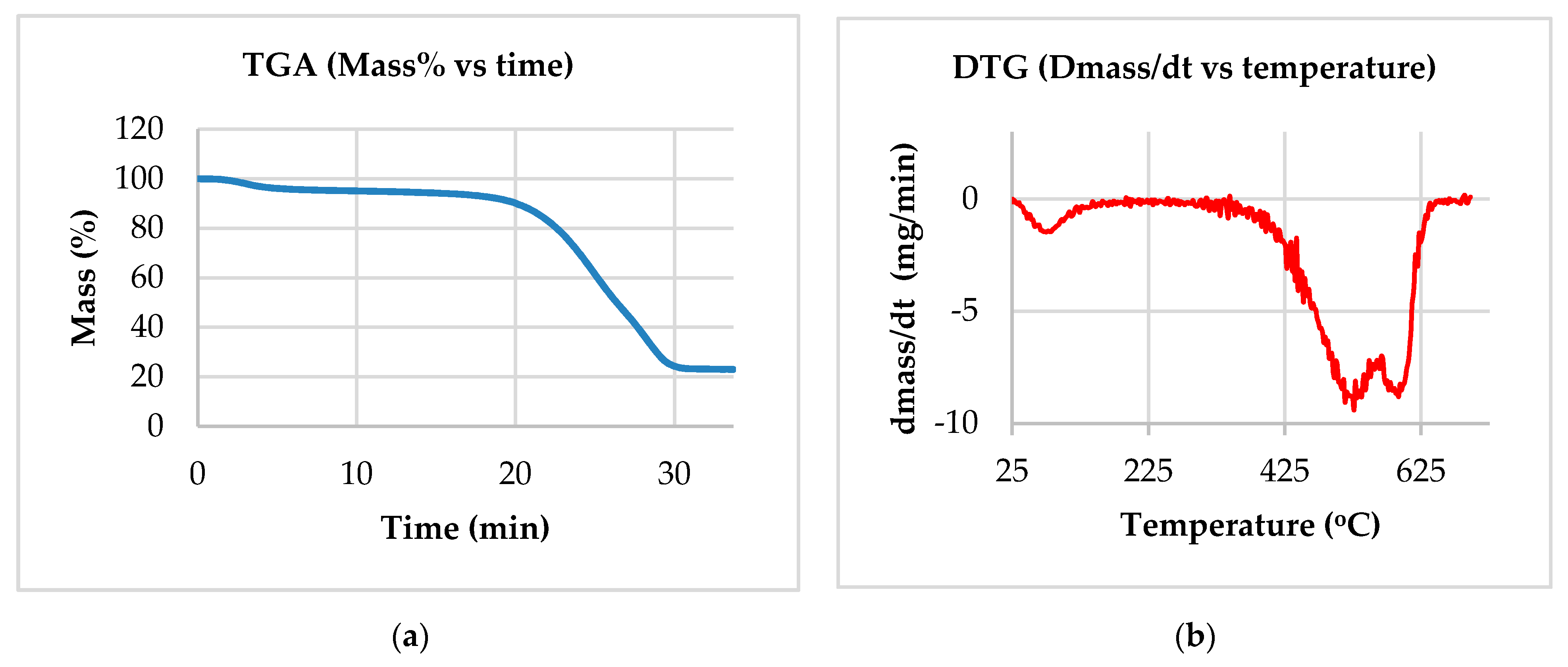
2.4. Thermogravimetric Analysis (TGA/DTGA)
2.5. Apparent Pyrolysis Kinetics
2.6. Fuel Quality Indexes
3. Results
3.1. Experimental Results Used in the Kinetic Study
3.2. Estimation of the Pyrolysis Reaction Order
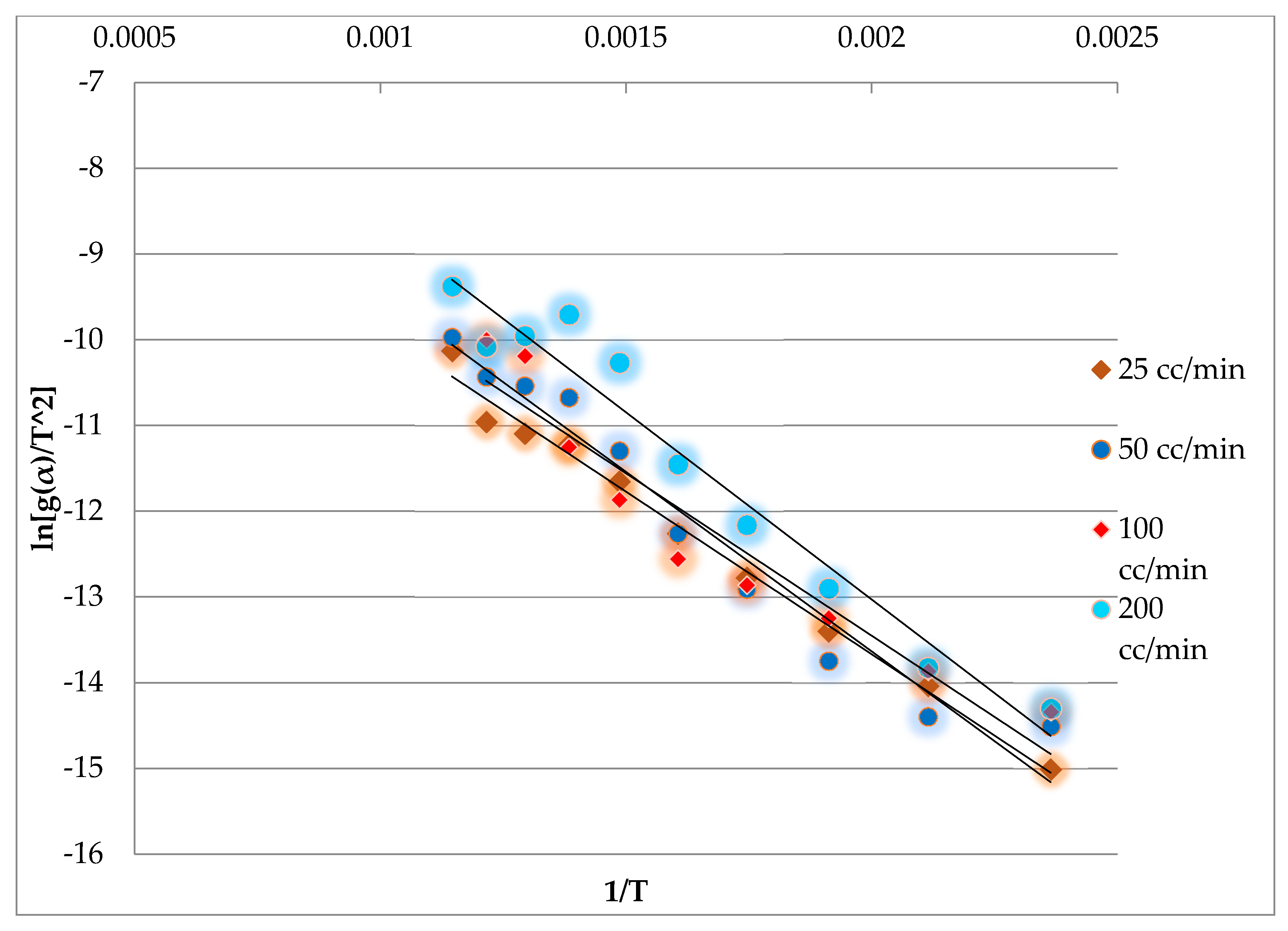
3.3. Effect of the Pyrolysis Temperature on the Kinetic Parameters
3.4. Effect of the Carrier Gas Flow Rate (FN2) on the Kinetic Parameters
3.5. Effect of the Heating Rate (q) on the Kinetic Parameters
3.6. Comparison of Peach Seeds Pyrolysis Kinetic Parameters with Bibliographic Data
3.7. Estimation of the Combustion Index C
Estimation of the Pyrolysis Index D
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zabaniotou, A.; Stamou, K. Balancing Waste and Nutrient Flows between Urban Agglomerations and Rural Ecosystems: Biochar for Improving Crop Growth and Urban Air Quality in the Mediterranean Region. Atmosphere 2020, 11, 539. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Circular economy synergistic opportunities of decentralized thermochemical systems for bioenergy and biochar production fueled with agro-industrial wastes with environmental sustainability and social acceptance: A review. Curr. Sustain. Renew. Energy Rep. 2018, 5, 150–155. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A review of the process development and challenges from initial researches up to the commercialization stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- European Environmental Agency. The Circular Economy and the Bioeconomy—Partners in Sustainability; European Environment Agency, Ed.; Office of the European Union: Luxembourg, 2018; ISBN 978-92-9213-974-2. [Google Scholar]
- Ubando, A.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiorea, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of food waste biorefinery: A review on valorisation pathways, tech-no-economic constraints, and environmental assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Huisingh, D. Food Waste Reduction and Valorisation: Sustainability Assessment and Policy Analysis. J. Clean. Prod. 2019, 207, 788. [Google Scholar] [CrossRef]
- EU; European Commission; Directorate-General for Agriculture and Rural Development. Agriculture in the European Union: Statistical and Economic Information; Agriculture and Rural Development European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Valverde, C. European Union: Stone Fruit Production Annually; USDA: Washington, DC, USA, 2019.
- Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The Potential of Tree Fruit Stone and Seed Wastes in Greece as Sources of Bioactive Ingredients. Recycling 2018, 3, 9. [Google Scholar] [CrossRef]
- Babu, B.V.; Chaurasia, A.S. Modeling, simulation and estimation of optimum parameters in pyrolysis of biomass. Energy Convers. Manag. 2003, 44, 2135–2158. [Google Scholar] [CrossRef]
- Hashimoto, K.; Hasegawa, I.; Hayashi, J.; Mae, K. Correlations of kinetic parameters in biomass pyrolysis with solid residue yield and lignin content. Fuel 2011, 90, 104–112. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Suzana, Y.; Khan, M.I.; Khan, J. Kinetic modeling and optimization of biomass pyrolysis for bio-oil production. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2065–2071. [Google Scholar] [CrossRef]
- Yiin, C.L.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M.; Kida, T. Thermogravimetric analysis and kinetic modeling of low-transition-temperature mixtures pretreated oil palm empty fruit bunch for possible maximum yield of pyrolysis oil. Bioresour. Technol. 2018, 255, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ezekoye, O.A.; Zhang, J.; Wang, C.; Lu, S. The effect of chemical reaction kinetic parameters on the bench-scale pyrolysis of lignocellulosic biomass. Fuel 2018, 232, 147–153. [Google Scholar] [CrossRef]
- Santos, V.O.; Queiroz, L.S.; Araujo, R.O.; Ribeiro, F.C.P.; Guimarães, M.N.; da Costa, C.E.F.; Chaar, J.S.; de Souza, L.K.C. Pyrolysis of acai seed biomass: Kinetics and thermodynamic parameters using thermogravimetric analysis. Bioresour. Technol. Rep. 2020, 12, 100553. [Google Scholar] [CrossRef]
- Burnham, A.K.; Dinh, L. A comparison of iso conversional and model-fitting approaches to kinetic parameter estimation and application predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Burra, K.R.G.; Gupta, A.K. Modeling of biomass pyrolysis kinetics using sequential multi-step reaction model. Fuel 2019, 237, 1057–1067. [Google Scholar] [CrossRef]
- Antal, M.J.J.; Varhegyi, G. Cellulose pyrolysis kinetics: The current state of knowledge. Ind. Eng. Chem. Res. 1995, 34, 703–717. [Google Scholar] [CrossRef]
- Vtirhegyi, G.; Antal, M.J.; Jakab, E.; Szabo, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Lumping kinetic model for hydrotreating of bio-oil from the fast pyrolysis of biomass. Energy Sources Part A 2009, 31, 639–645. [Google Scholar] [CrossRef]
- Ojha, D.K.; Viju, D.; Vinu, R. Fast pyrolysis kinetics of alkali lignin: Evaluation of apparent rate parameters and product time evolution. Bioresour. Technol. 2017, 241, 142–151. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose. Processes 2021, 9, 551. [Google Scholar] [CrossRef]
- Gagliano, F.A.; Nocera, F.; Patania, F.; Bruno, M.; Scirè, S. Kinetic of the Pyrolysis Process of Peach and Apricot Pits by TGA and DTGA Analysis. Int. J. Heat Technol. 2016, 34, S553–S560. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Lovera, R.G.; Escalona, N.; Sepúlveda, C.; Sotelo, J.L.; García, J. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem. Eng. J. 2015, 279, 788–798. [Google Scholar] [CrossRef]
- Fernandez, A.; Saffe, A.; Mazza, G.; Rodriguez, R. Nonisothermal drying kinetics of biomass fuels by thermogravimetric analysis under oxidative and inert atmosphere. Dry. Technol. 2017, 35, 163–172. [Google Scholar] [CrossRef]
- Janković1, B.Z.; Radojević, M.B.; Balać, M.M.; Stojiljković, D.D.; Manić, N.G. Thermogravimetric study on the pyrolysis kinetic mechanism of waste biomass from fruit processing industry. Therm. Sci. 2020, 24, 191. [Google Scholar] [CrossRef]
- Radojević, M.; Janković, B.; Jovanović, V.; Stojiljković, D.; Manić, N. Comparative pyrolysis kinetics of various biomasses based on model-free and DAEM approaches improved with numerical optimization procedure. PLoS ONE 2018, 13, e0206657. [Google Scholar] [CrossRef]
- Ingrao, C.; Bacenetti, J.; Bezama, A.; Blok, V.; Goglio, P.; Koukios, E.G.; Lindner, M.; Nemecek, T.; Siracusa, V.; Zabaniotou, A.; et al. The potential roles of bio-economy in the transition to equitable, sustainable, post fossil-carbon societies: Findings from this virtual special issue. J. Clean. Prod. 2018, 204, 471–488. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.-L.C.; Bezerra, F.W.F.; Pinto, R.-H.H.; de Carvalho Junior, P.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Altynbaeva, D.; Astafev, A.; Tabakaev, R. Kinetics of biomass low-temperature pyrolysis by coats-redfern method. MATEC Web Conf. 2018, 194. [Google Scholar] [CrossRef]
- Greek Canners Association. 2020. Available online: https://ekeenglish.weebly.com/ (accessed on 12 October 2020).
- NETZSCH, (n.d.). TG 209 F3 Tarsus® [Apparatus and Software]. Available online: https://www.netzsch-thermal-analysis.com/en/products-solutions/thermogravimetric-analysis/tg-209-f3-tarsus/ (accessed on 30 November 2020).
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Galwey, A.K.; Brown, M.E. Chapter 3: Kinetic Background to Thermal Analysis and Calorimetry. In Handbook of Thermal Analysis and Calorimetry, 1; Brown, M.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 147–224. [Google Scholar]
- Kissinger, H.E.; Blaine, R.L. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar]
- Coats, A.; Redfern, J. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Vlaev, L.; Markovska, I. Non-Isothermal Kinetics of Pyrolysis of Rice Husk. Thermochim. Acta 2003, 406, 1–7. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating Parameters for Bio-oil Production in Biomass Pyrolysis: A Review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Punia, R.; Kumar, S. Chapter 1: Thermogravimetric characterization of wood stalks as gasification and pyrolysis feedstock. In Recent Advances in Bioenergy Research, 3; Kumar, S., Sarma, A.K., Tyagi, S.K., Yadav, Y.K., Eds.; SSS-NIRE: Kapurthala, India, 2014; pp. 29–30. [Google Scholar]
- Chen, G.-B.; Li, J.-W.; Lin, H.-T.; Wu, F.-H.; Chao, Y.-C. A Study of the Production and Combustion Characteristics of Pyrolytic Oil from Sewage Sludge Using the Taguchi Method. Energies 2018, 11, 2260. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, B.; Chen, X.; Yuan, Y.; Zhong, L.; Qiao, X.; Zhang, Y.; Yuan, M.; Shu, J.; Wang, P. Thermogravimetric Analysis of Combustion Characteristics of Palm Oil and Rapeseed Oil Biodiesel. Biotechnology 2014, 14, 9–15. [Google Scholar] [CrossRef][Green Version]
- Song, C.-Z.; Wen, J.-H.; Li, Y.-Y.; Dan, H.; Shi, X.-Y.; Xin, S. Thermogravimetric Assessment of Combustion Characteristics of Blends of Lignite Coals with Coal Gangue. In Proceedings of the 3rd Annual International Conference on Mechanics and Mechanical Engineering, Hohhot, China, 16–18 December 2016; Atlantis Press: Paris, France, 2017; pp. 490–494. [Google Scholar]
- Yao, X.; Xu, K.; Liang, Y. Assessing the effects of different process parameters on the pyrolysis behaviors and thermal dynamics of corncob fractions. BioResources 2017, 12, 2748–2767. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xu, M.; Sun, R.F.; Sun, L. Study on Biomass Pyrolysis Kinetics, ASME. J. Eng. Gas Turbines Power 2006, 128, 493–496. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, X.; Fang, P.; Ye, L.; Huang, J.; Wu, H.; Tang, Z.; Chen, D. Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions. Sustainability 2020, 12, 464. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.; Liu, H.; Jia, C.; Xu, X. Reactivity and Kinetic Analysis of Biomass during Combustion, 17/A; Elsevier: Amsterdam, The Netherlands, 2012; pp. 869–875. [Google Scholar]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
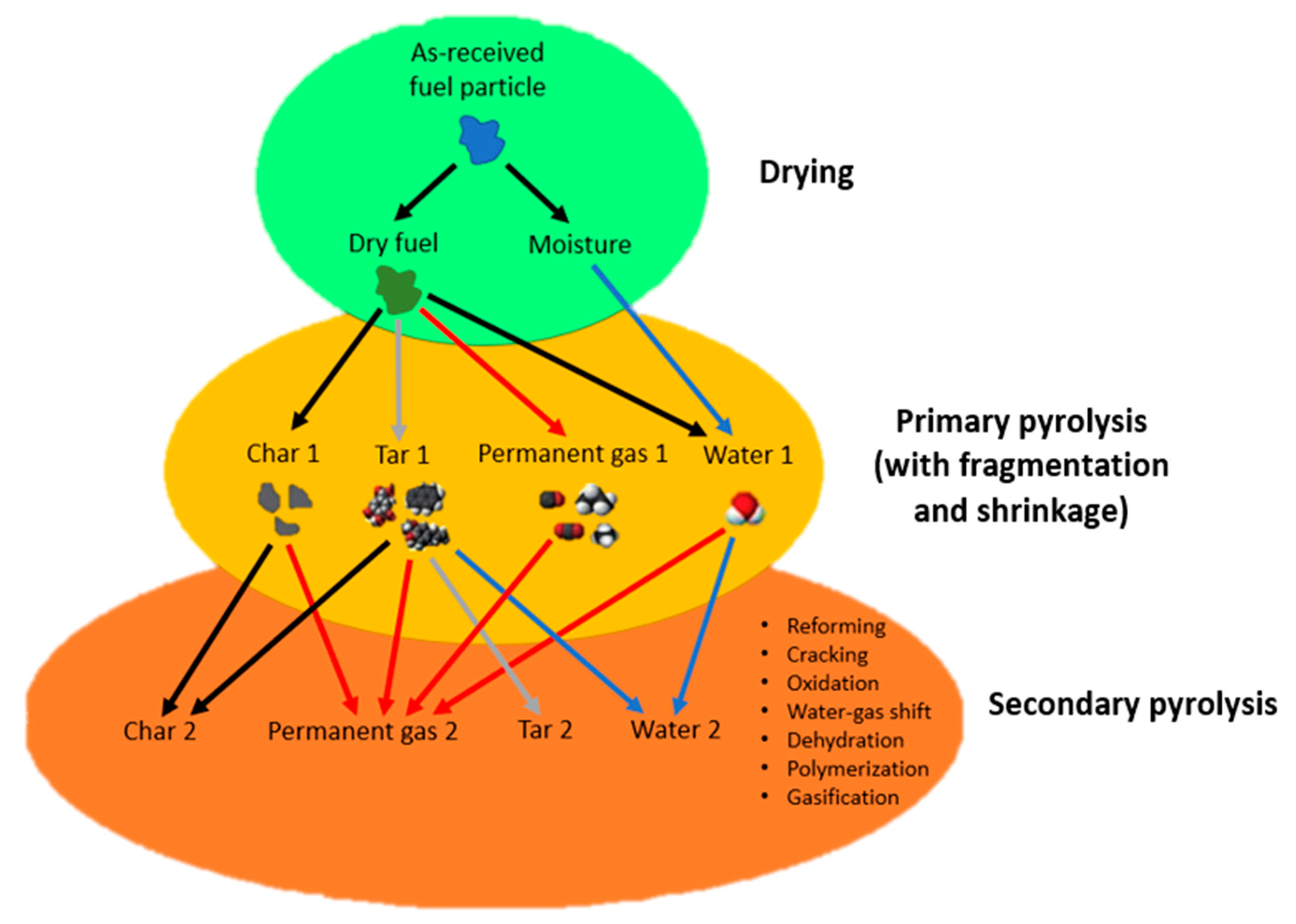




| Rate-Determining Mechanism | Reaction Type | f(a) | g(a) |
|---|---|---|---|
| Chemical reaction | First-order | 1 − a | −ln(1 − a) |
| Second-order | (1 − a)2 | (1 − a)−1 − 1 | |
| Third-order | (1 − a)3 | [(1 − a)−2 − 1]/2 | |
| nth-order | (1 − a)n | [(1 − a)1−n − 1]/(n − 1) − 1 | |
| Random nucleation and nuclei growth | Bi-dimensional | 2(1 − a)[−ln(1 − a)]1/2 | [−ln(1 − a)]1/2 |
| Three-dimensional | 3(1 − a)[−ln(1 − a)]2/3 | [−ln(1 − a)]1/3 | |
| Diffusion | One-way transport | 1/(2a) | a2 |
| Two-way transport | [−ln(1 − a)]−1 | a + (1 − a)ln(1 − a) | |
| Ginstling–Brounshtein equation | (2/3)(1 − a)2/3 − (1 − a)1/3 | 1 − 2a/3 − (1 − a)2/3 |
| Composition % wt./wt. | Pretreated Peach Seeds |
|---|---|
| C | 52.4 |
| H | 6.46 |
| N | 6.18 |
| S | 0.00 |
| O | 18.94 |
| Ash | 16.02 |
| Experimental Set 1 | FN2 = 100 cm3/min and q = 170 C/min. | ||||
| Tmax (°C) | 474 | 674 | 783 | ||
| Biochar (gr) | 0.2644 | 0.2455 | 0.2313 | ||
| Vapors (gr) | 0.7210 | 0.8076 | 0.7831 | ||
| Experimental Set 2 | Tmax= 700 C, q = 170 C/min. | ||||
| Flow rate of N2 (cc/min) | 25 | 50 | 100 | 200 | |
| Biochar (gr) | 0.2376 | 0.2353 | 0.2455 | 0.1556 | |
| Vapors (gr) | 0.7796 | 0.7793 | 0.8076 | 0.8923 | |
| Experimental Set 3 | Tmax = 700 K, FN2 = 100 cm3/min | ||||
| Heating rate, q (C/min) | 102 | 171 | 254 | ||
| Biochar (gr) | 0.2586 | 0.2455 | 0.227 | ||
| Vapors (gr) | 0.7540 | 0.8076 | 0.7905 | ||
| Tmax (°C) | 474 | 674 | 783 |
|---|---|---|---|
| R2 | 0.9888 | 0.9215 | 0.9520 |
| E (kJ/mol) | 56.7 | 31.5 | 23.3 |
| A (min−1) | 1.34 × 106 | 1.82 × 103 | 0.13 × 103 |
| Flow Rate of N2 (FN2) (cc/min) | 25 | 50 | 100 | 200 |
|---|---|---|---|---|
| R2 | 0.9902 | 0.9576 | 0.9215 | 0.9577 |
| E (kJ/mol) | 31.5 | 34.8 | 31.5 | 36.3 |
| A (min−1) | 1.48 × 103 | 3.46 × 103 | 1.82 × 103 | 10.0 × 103 |
| Heating Rate, (q) (K/min) | 102 | 171 | 254 |
|---|---|---|---|
| R2 | 0.9694 | 0.9215 | 0.9136 |
| E (kJ/mol) | 33.8 | 31.5 | 50.4 |
| A (min−1) | 5.4 × 103 | 1.82 × 103 | 716 × 103 |
| Βiomass Residue | Process | Activation Energy (Ε, in kJ/mol) | Pre-Exponential Factor (A, in min−1) | Ref. |
|---|---|---|---|---|
| Peach seeds | Slow pyrolysis | 30–60 | 103–104 | Present study |
| Corn stalk | TG-DTG | 66.518 | 189.6 × 103 | [47] |
| Wheat straw | TG-DTG | 70.516 | 117.6 × 106 | [47] |
| Tree skin | TG-DGT | 77.316 | 157.2 × 105 | [47] |
| Peanut shell | TG-DGT | 84.47 | 88.2 × 106 | [47] |
| Cotton | TG-DGT | 200.9 | 175.8 × 1014 | [47] |
| Peach Seeds | ||||||
|---|---|---|---|---|---|---|
| Sample | Peach Seeds | Pyrolytic Biochar | ||||
| TGA/ DTG | 1st Peak, °C | 1st Peak, °K | 2nd Peak, °C | 2nd Peak, K | Peak, °C | Peak, K |
| , %/min | 7.8 | 7.8 | 5.2 | 5.2 | 9 | 9 |
| , %/min | 2.8 | 2.8 | 2.8 | 2.8 | 4.5 | 4.5 |
| Ti, °C/K | 195 | 468 | 475 | 748 | 400 | 673 |
| Te, °C/K | 445 | 718 | 635 | 908 | 625 | 898 |
| Combustion index C | 1.9 × 10−6 | 1.39 × 10−7 | 1.02 × 10−7 | 2.87 × 10−8 | 4.05 × 10−7 | 9.96 × 10−8 |
| Sample | Peach Seeds, 1st Peak, | Peach Seeds Biochar | Peanut Shells [48] | Corn Stalk [49] | Rice Straw [49] | Rice Shells [49] | SAWDUST [49] |
|---|---|---|---|---|---|---|---|
| , %/min | 7.8 | 9 | 4.21 | 13.49 | 11.56 | 9.49 | 14.86 |
| , %/min | 2.8 | 4.5 | 0.55 | 4.57 | 3.65 | 2.89 | 3.73 |
| Ti, °C/K | 195 | 400 | 236 °C | 261 | 257 | 276 | 293 |
| Te, °C/K | 445 | 625 | 346 °C | 535 | 564 | 568 | 578 |
| Combustion index, C | 1.29 × 10−6 | 4.05 × 10−7 | 5.6 × 10−8 | 1.69 × 10−6 | 1.39 × 10−7 | 6.34 × 10−7 | 1.12 × 10−6 |
| Pretreated Peach Seeds, °C | Pretreated Peach Seeds, °K | Corn Stalk, [49] | MSW (×10−7) [50] | |
|---|---|---|---|---|
| , %/min | 8.8 | 8.8 | 0.881 | 0.61 |
| , %/min | 2.19 | 2.19 | 0.148 | - |
| Starting temperature (Ts), °C, T | 195 | 468 | 183.3 | - |
| Peak temperature (Tmax), °C, T | 420 | 693 | 334.4 | - |
| ΔT1/2 | 50 | 50 | 69.2 | 50–68 (*) |
| D | 4.7 × 10−6 | 1.18 × 10−6 | 3.0 × 10−8 | 1.1–6.6 (*) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altantzis, A.-I.; Kallistridis, N.-C.; Stavropoulos, G.; Zabaniotou, A. Apparent Pyrolysis Kinetics and Index-Based Assessment of Pretreated Peach Seeds. Processes 2021, 9, 905. https://doi.org/10.3390/pr9060905
Altantzis A-I, Kallistridis N-C, Stavropoulos G, Zabaniotou A. Apparent Pyrolysis Kinetics and Index-Based Assessment of Pretreated Peach Seeds. Processes. 2021; 9(6):905. https://doi.org/10.3390/pr9060905
Chicago/Turabian StyleAltantzis, Angelos-Ikaros, Nikolaos-Christos Kallistridis, George Stavropoulos, and Anastasia Zabaniotou. 2021. "Apparent Pyrolysis Kinetics and Index-Based Assessment of Pretreated Peach Seeds" Processes 9, no. 6: 905. https://doi.org/10.3390/pr9060905
APA StyleAltantzis, A.-I., Kallistridis, N.-C., Stavropoulos, G., & Zabaniotou, A. (2021). Apparent Pyrolysis Kinetics and Index-Based Assessment of Pretreated Peach Seeds. Processes, 9(6), 905. https://doi.org/10.3390/pr9060905







