A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose
Abstract
1. Introduction
2. Materials and Methods
- Fitting of single time-derivative thermogravimetric curves obtained at different temperatures versus time. The curves are fitted to time derivative logistic functions, (Equation (1)). The fittings were performed with the Gnumeric software.
- Fitting and analysis of the rate parameter values obtained at different temperatures versus temperature. For this task, the R software was used, and Equations (2) and (5) were tested and compared.
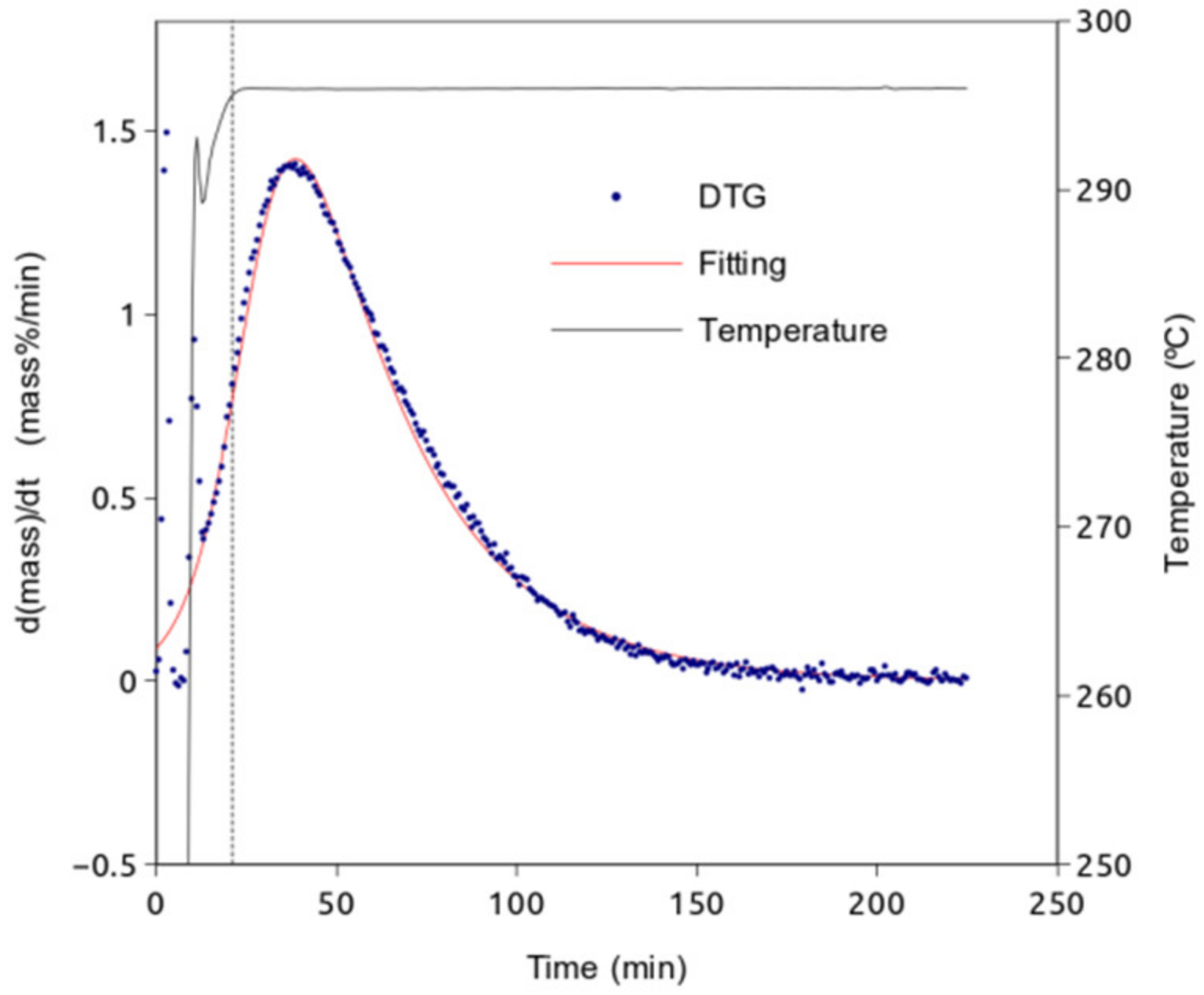
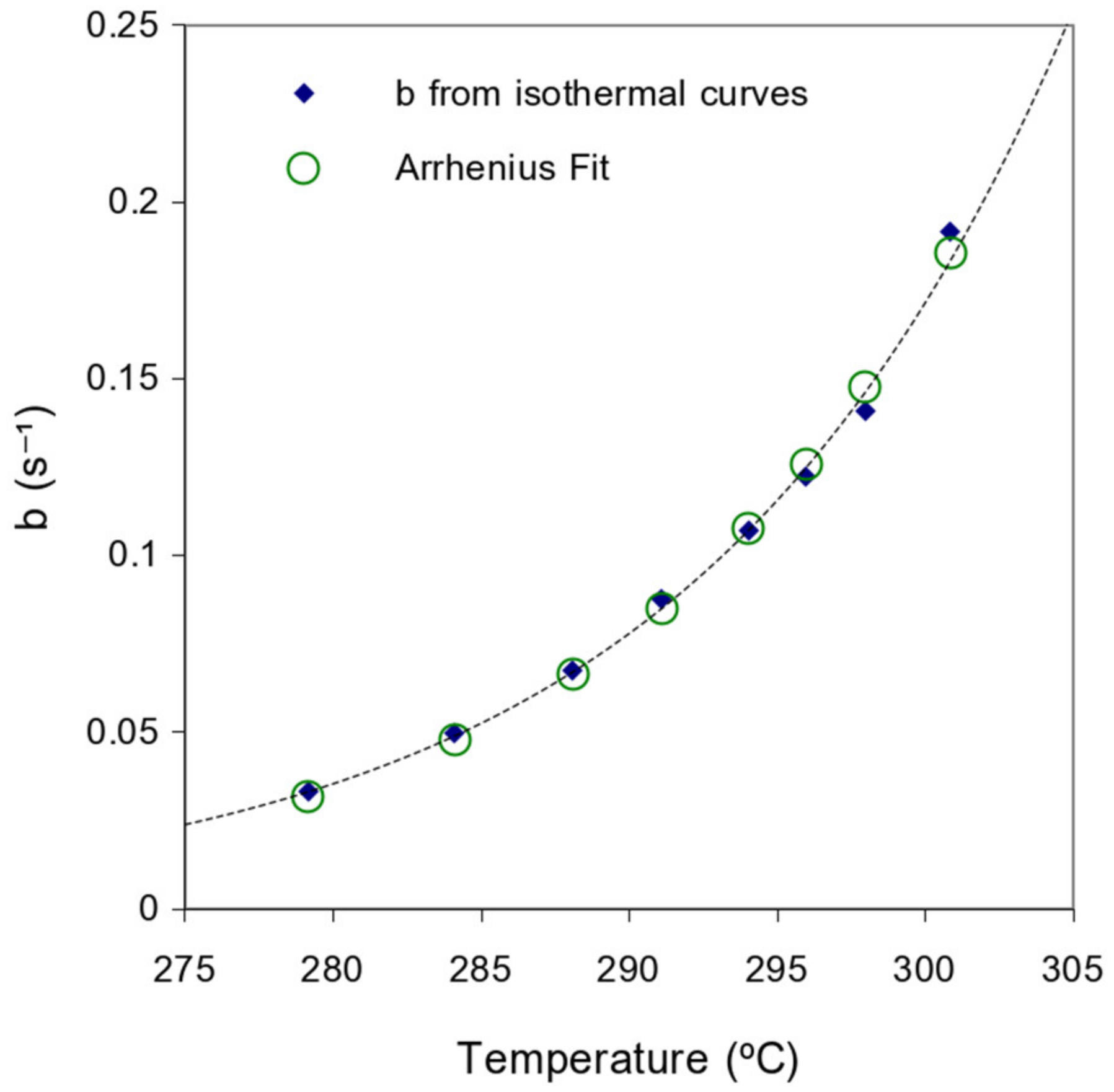
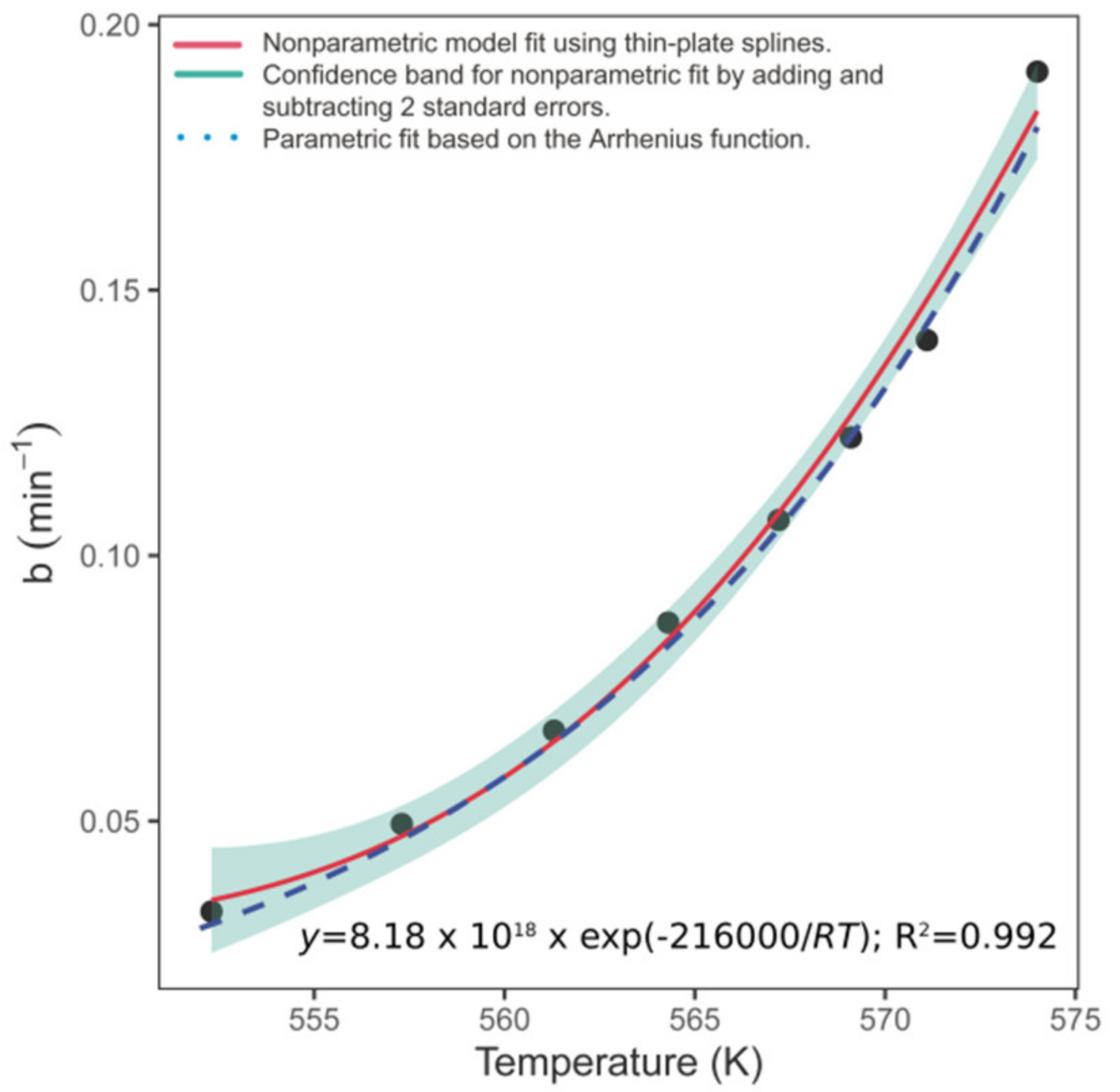
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Fukaya, Y.; Ohno, H. Chapter 8—Energy-Saving Biomass Processing with Polar Ionic Liquids. In Research Approaches to Sustainable Biomass Systems; Tojo, S., Hirasawa, T., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 205–223. ISBN 978-0-12-404609-2. [Google Scholar]
- Cellulose Products—Epis. Available online: https://epis.org/cellulose-products (accessed on 30 January 2021).
- Roy Choudhury, A.K. Antistatic and Soil-Release Finishes. In Principles of Textile Finishing; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–318. ISBN 978-0-08-100646-7. [Google Scholar]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose Nanocomposites: Fabrication and Biomedical Applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A Kinetic Model for Pyrolysis of Cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Bradbury, A.G.W. Thermal Degradation of Cellulose in Air and Nitrogen at Low Temperatures. J. Appl. Polym. Sci. 1979, 23, 1431–1442. [Google Scholar] [CrossRef]
- Antal, M.J.; Varhegyi, G.; Jakab, E. Cellulose Pyrolysis Kinetics: Revisited. Ind. Eng. Chem. Res. 1998, 37, 1267–1275. [Google Scholar] [CrossRef]
- Varhegyi, G.; Grønli, M.G.; Blasi, C.D. Effects of Sample Origin, Extraction, and Hot-Water Washing on the Devolatilization Kinetics of Chestnut Wood. Ind. Eng. Chem. Res. 2004, 43, 2356–2367. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Chaos, M.; Chen, R.; Lu, S. Estimation of Beech Pyrolysis Kinetic Parameters by Shuffled Complex Evolution. Bioresour. Technol. 2016, 200, 658–665. [Google Scholar] [CrossRef]
- Sfakiotakis, S.; Vamvuka, D. Development of a Modified Independent Parallel Reactions Kinetic Model and Comparison with the Distributed Activation Energy Model for the Pyrolysis of a Wide Variety of Biomass Fuels. Bioresour. Technol. 2015, 197, 434–442. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Álvarez-García, A.; Sebio-Puñal, T.; Zaragoza-Fernández, S.; Álvarez-García, B.; Díaz-Díaz, A.; Janeiro, J.; Artiaga, R. Kinetics of Thermal Degradation of Cellulose: Analysis Based on Isothermal and Linear Heating Data. BioResources 2016, 11, 5870–5888. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. Influence of Mineral Matter on Pyrolysis of Palm Oil Wastes. Combust. Flame 2006, 146, 605–611. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Leng, E.; Ferreiro, A.I.; Liu, T.; Gong, X.; Costa, M.; Li, X.; Xu, M. Experimental and Kinetic Modelling Investigation on the Effects of Crystallinity on Cellulose Pyrolysis. J. Anal. Appl. Pyrolysis 2020, 152, 104863. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal Degradation and Stability of Starch under Different Processing Conditions. Starch-Stärke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Mironova, M.; Makarov, I.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Levin, I. Improvement in Carbonization Efficiency of Cellulosic Fibres Using Silylated Acetylene and Alkoxysilanes. Fibers 2019, 7, 84. [Google Scholar] [CrossRef]
- Romagnoli, M.; Galotta, G.; Antonelli, F.; Sidoti, G.; Humar, M.; Kržišnik, D.; Čufar, K.; Davidde Petriaggi, B. Micro-Morphological, Physical and Thermogravimetric Analyses of Waterlogged Archaeological Wood from the Prehistoric Village of Gran Carro (Lake Bolsena-Italy). J. Cult. Herit. 2018, 33, 30–38. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C. Kinetics of Primary Product Formation from Wood Pyrolysis. Ind. Eng. Chem. Res. 2001, 40, 5547–5556. [Google Scholar] [CrossRef]
- Lanzetta, M.; Di Blasi, C.; Buonanno, F. An Experimental Investigation of Heat-Transfer Limitations in the Flash Pyrolysis of Cellulose. Ind. Eng. Chem. Res. 1997, 36, 542–552. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C 2009, 113, 20097–20107. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R.; Huber, G.W. A Distributed Activation Energy Model for the Pyrolysis of Lignocellulosic Biomass. Green Chem. 2013, 15, 1331. [Google Scholar] [CrossRef]
- Şerbănescu, C. Kinetic Analysis of Cellulose Pyrolysis: A Short Review. Chem. Pap. 2014, 68, 847–860. [Google Scholar] [CrossRef]
- Gracia-Fernández, C.; Álvarez-García, B.; Gómez-Barreiro, S.; López-Beceiro, J.; Artiaga, R. Significant Hidden Temperature Gradients in Thermogravimetric Tests. Polym. Test. 2018, 68, 388–394. [Google Scholar] [CrossRef]
- Díaz-Díaz, A.M.; López-Beceiro, J.; Li, Y.; Cheng, Y.; Artiaga, R. Crystallization Kinetics of a Commercial Poly(Lactic Acid) Based on Characteristic Crystallization Time and Optimal Crystallization Temperature. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Gracia-Fernández, C.; Artiaga, R. A Kinetic Model That Fits Nicely Isothermal and Non-Isothermal Bulk Crystallizations of Polymers from the Melt. Eur. Polym. J. 2013, 49, 2233–2246. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Fontenot, S.A.; Gracia-Fernández, C.; Artiaga, R.; Chartoff, R. A Logistic Kinetic Model for Isothermal and Nonisothermal Cure Reactions of Thermosetting Polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wackerly, D.D.; Mendenhall, W.; Scheaffer, R.L. Mathematical Statistics with Applications, 6th ed.; Duxbury: Pacific Grove, CA, USA, 2002; ISBN 978-0-534-37741-0. [Google Scholar]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Francisco-Fernández, M.; Artiaga, R. Simulation Study for Generalized Logistic Function in Thermal Data Modeling. J. Therm. Anal. Calorim. 2014, 118, 1253–1268. [Google Scholar] [CrossRef]
- Nash, J.C. Spreadsheets in Statistical Practice—Another Look. Am. Stat. 2006, 60, 287–289. [Google Scholar] [CrossRef]
- Price, K.V.; Storn, R.M.; Lampinen, J.A. Differential Evolution: A Practical Approach to Global Optimization; Natural Computing Series; Springer: Berlin, Germany; New York, NY, USA, 2005; ISBN 978-3-540-20950-8. [Google Scholar]
- Robles-Bykbaev, Y.; Tarrío-Saavedra, J.; Quintana-Pita, S.; Díaz-Prado, S.; García Sabán, F.J.; Naya, S. Statistical Degradation Modelling of Poly(D,L-Lactide-Co-Glycolide) Copolymers for Bioscaffold Applications. PLoS ONE 2018, 13, e0204004. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall: London, UK; CRC: London, UK, 2017; ISBN 978-1-315-37027-9. [Google Scholar]



| Temperature (°C) | C (Mass %) | τ | b (min−1) | ASE |
|---|---|---|---|---|
| 279.2 | 80.18 | 3.28 | 0.03 | 2.77 × 10−4 |
| 284.1 | 82.55 | 3.52 | 0.05 | 3.99 × 10−4 |
| 288.1 | 83.17 | 3.63 | 0.07 | 4.31 × 10−4 |
| 291.1 | 81.5 | 3.78 | 0.09 | 1.08 × 10−3 |
| 294.1 | 83.22 | 3.78 | 0.11 | 1.01 × 10−3 |
| 296.0 | 85.62 | 3.89 | 0.12 | 6.08 × 10−4 |
| 298.0 | 84.68 | 4.41 | 0.14 | 5.84 × 10−4 |
| 300.9 | 85.5 | 4.43 | 0.19 | 6.13 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose. Processes 2021, 9, 551. https://doi.org/10.3390/pr9030551
López-Beceiro J, Díaz-Díaz AM, Álvarez-García A, Tarrío-Saavedra J, Naya S, Artiaga R. A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose. Processes. 2021; 9(3):551. https://doi.org/10.3390/pr9030551
Chicago/Turabian StyleLópez-Beceiro, Jorge, Ana María Díaz-Díaz, Ana Álvarez-García, Javier Tarrío-Saavedra, Salvador Naya, and Ramón Artiaga. 2021. "A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose" Processes 9, no. 3: 551. https://doi.org/10.3390/pr9030551
APA StyleLópez-Beceiro, J., Díaz-Díaz, A. M., Álvarez-García, A., Tarrío-Saavedra, J., Naya, S., & Artiaga, R. (2021). A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose. Processes, 9(3), 551. https://doi.org/10.3390/pr9030551







