Pool Boiling Performance of Water and Self-Rewetting Fluids on Hybrid Functionalized Aluminum Surfaces
Abstract
1. Introduction
1.1. Surface Roughness and Topography
1.2. Surface Wettability
1.3. Self-Rewetting Fluids
1.4. Boiling of SRFs on Modified Surfaces
1.5. Motivation of This Work
2. Materials and Methods
2.1. Surface Functionalization and Evaluation
2.2. Boiling Performance Evaluation
2.3. Data Reduction and Measurement Uncertainty
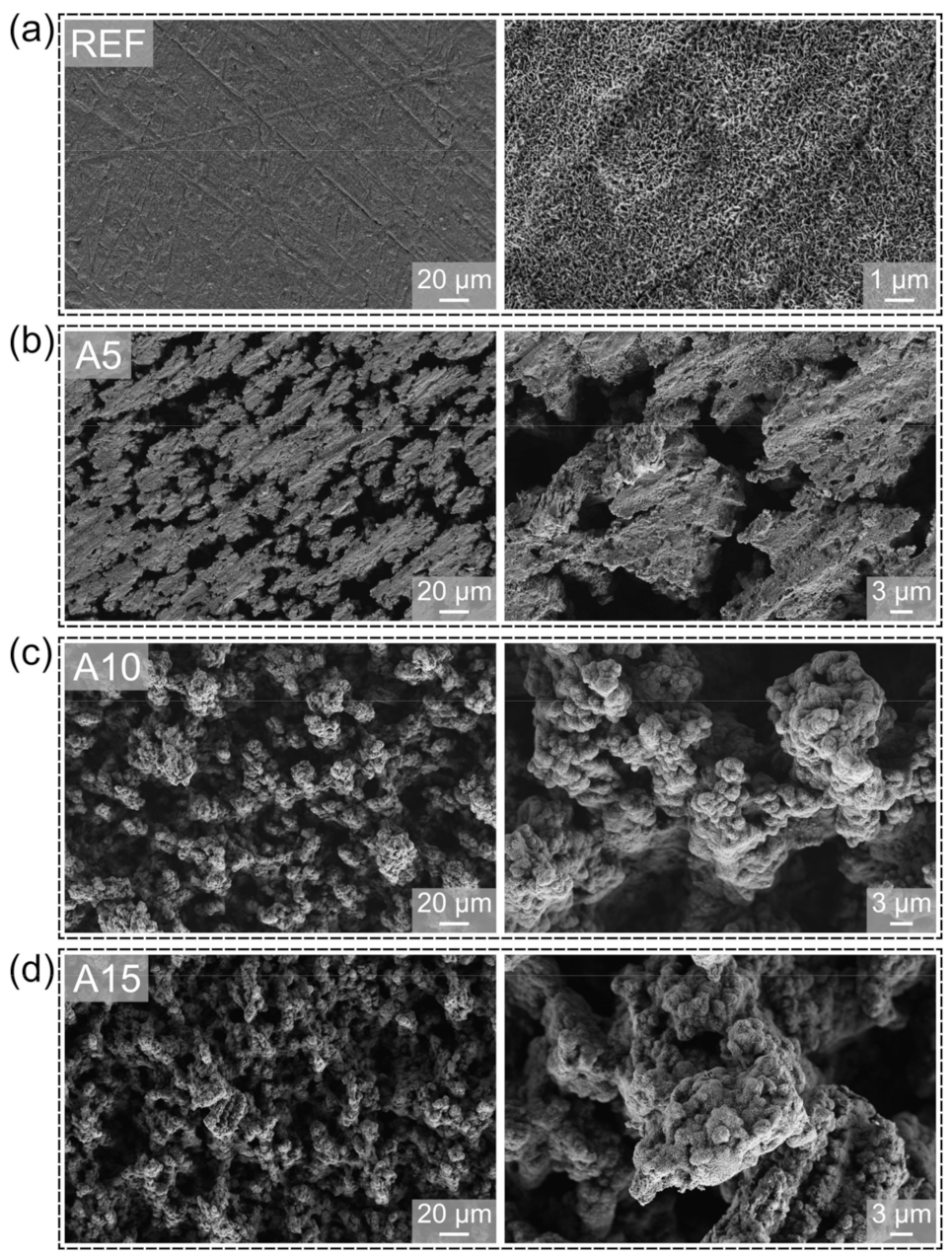
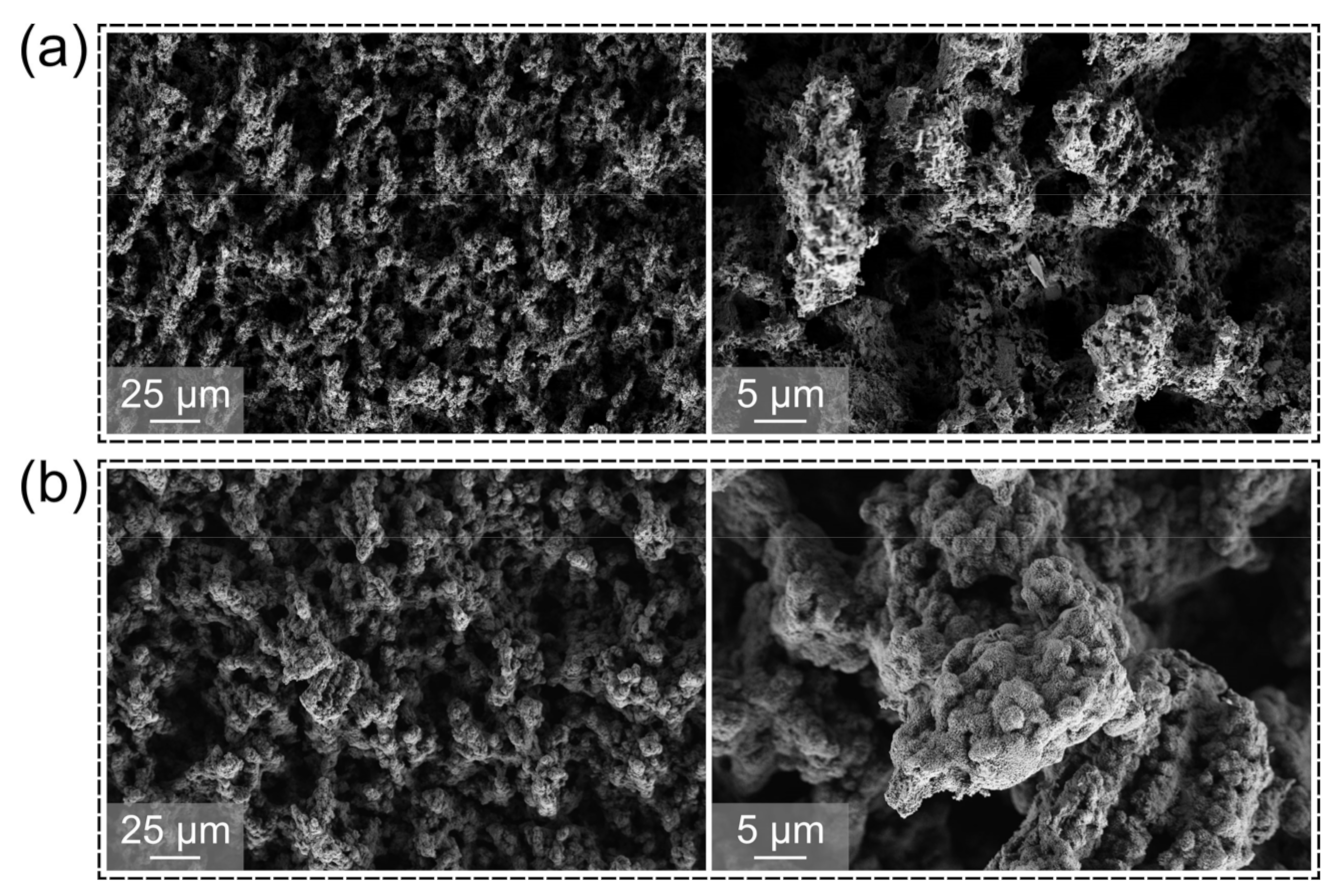
3. Surface Characteristics
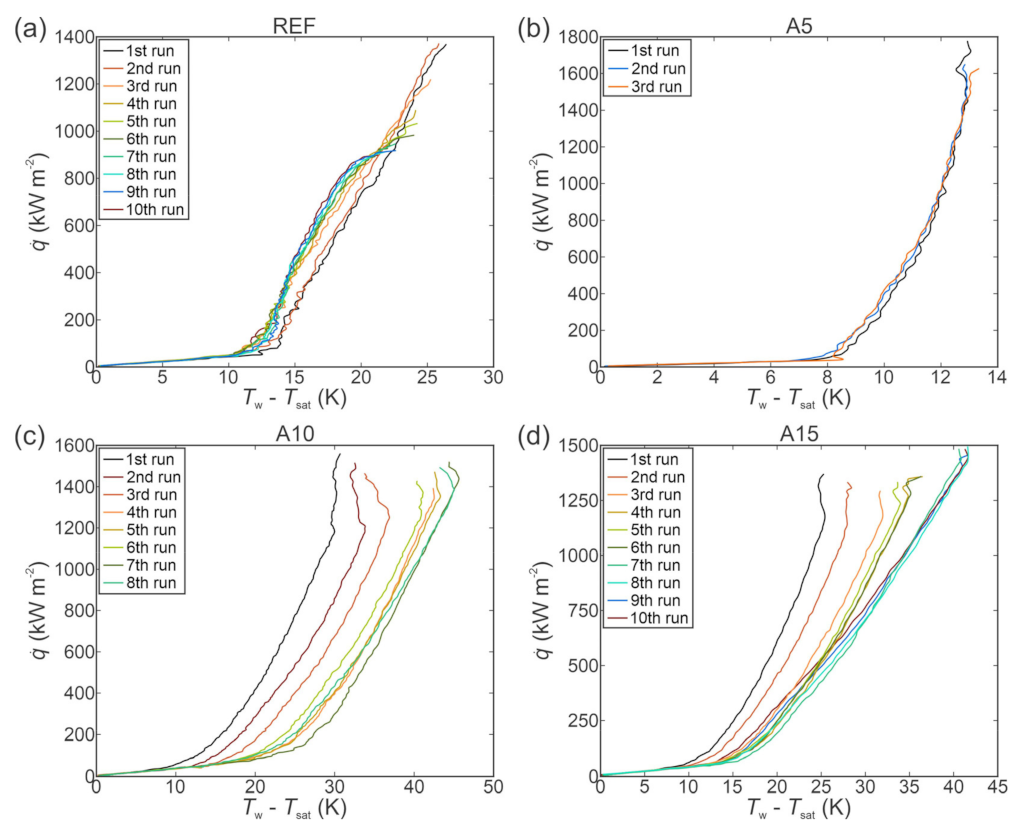
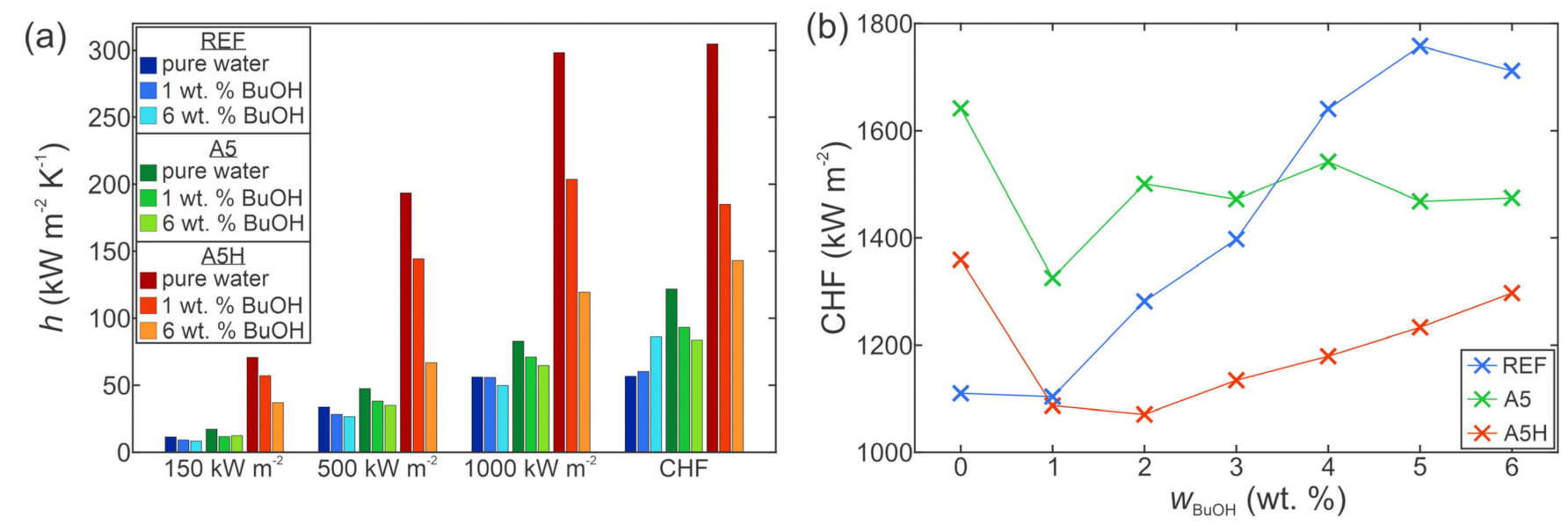
4. Boiling Performance Using Water
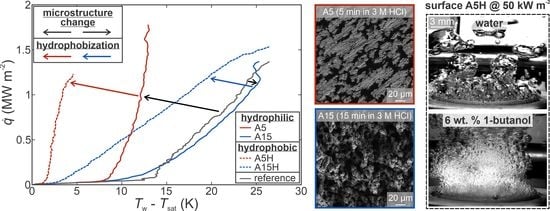
4.1. Effect of Surface Microstructure
4.2. Effect of Hydrophobization
5. Boiling Performance Using Self-Rewetting Fluids
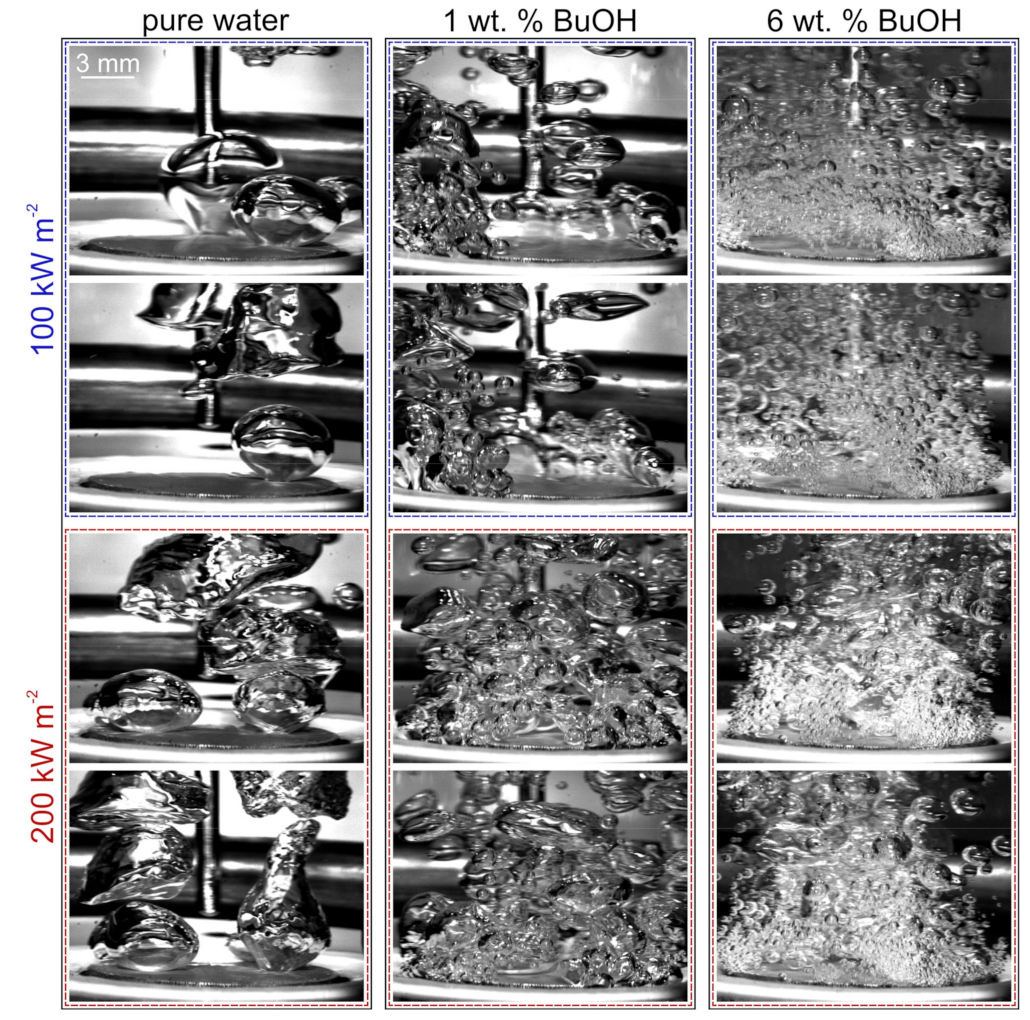
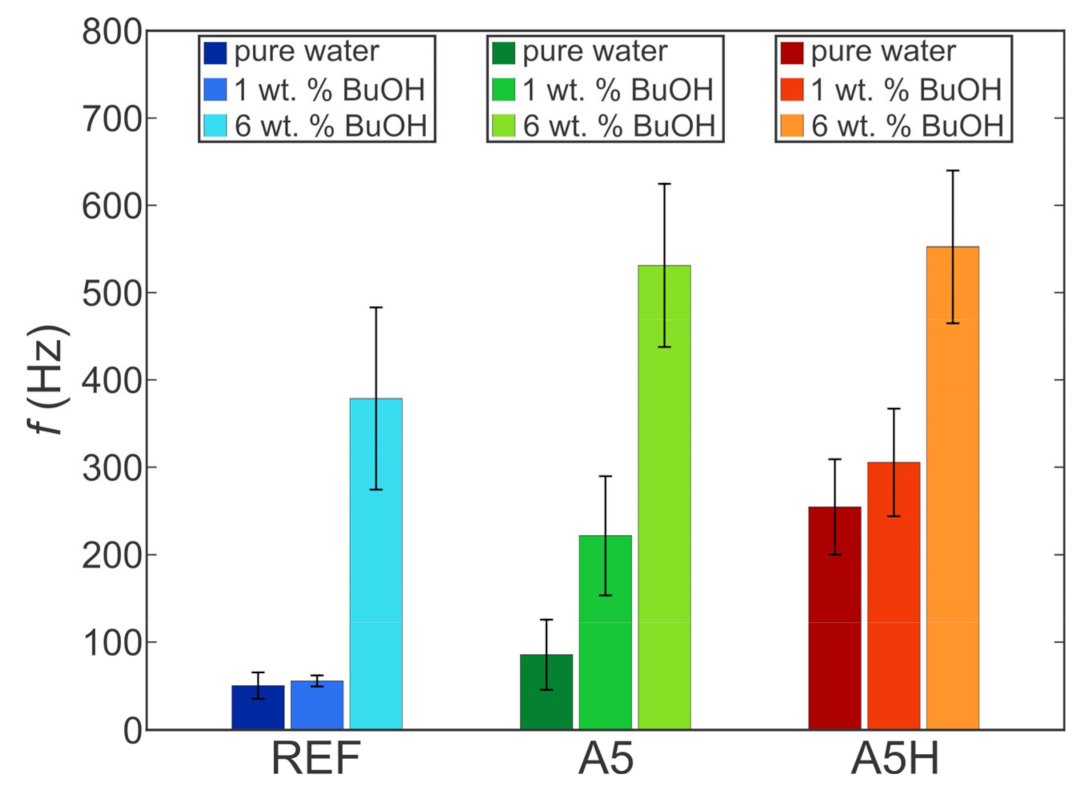
5.1. Effect of Self-Rewetting Fluid Concentration
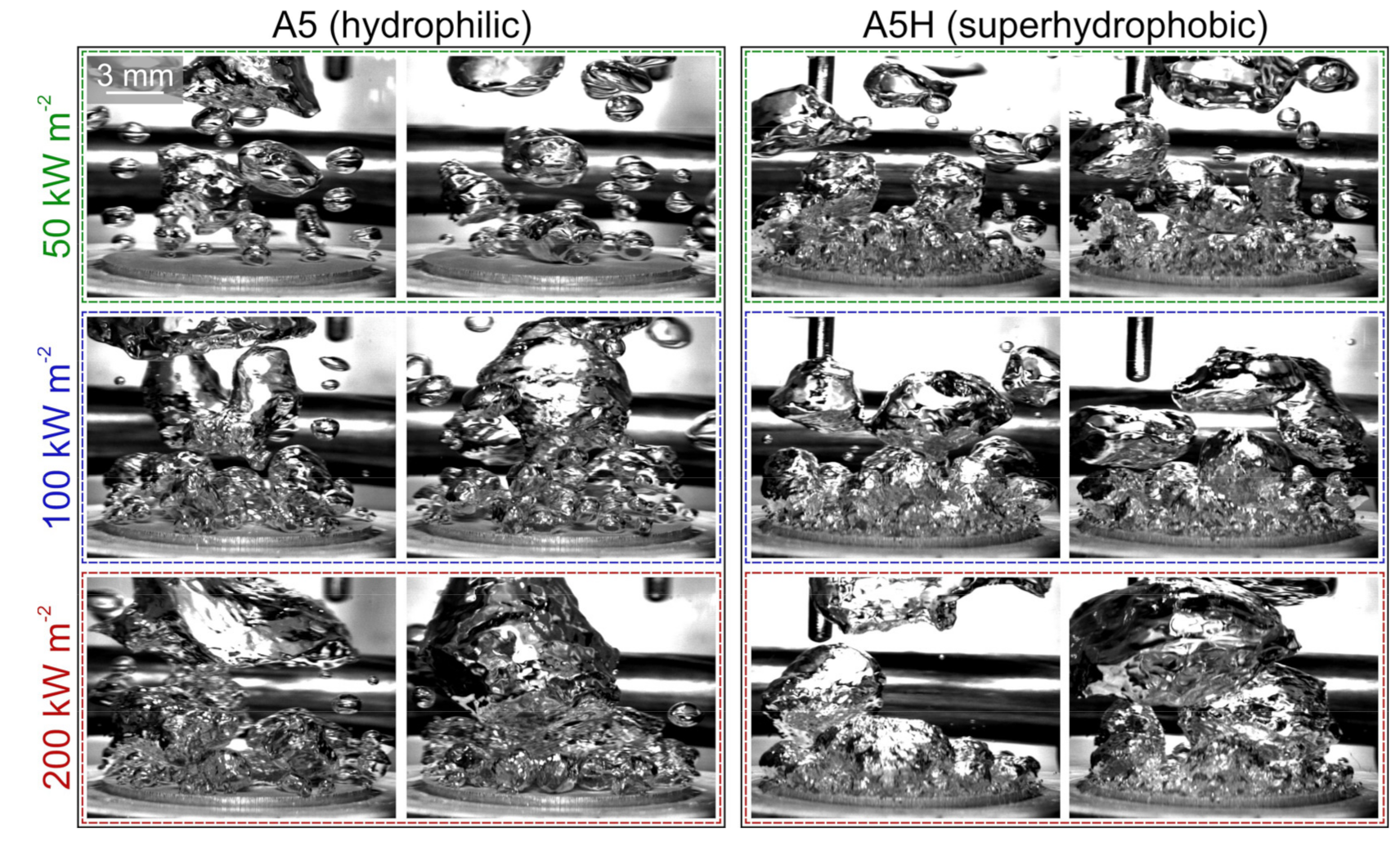
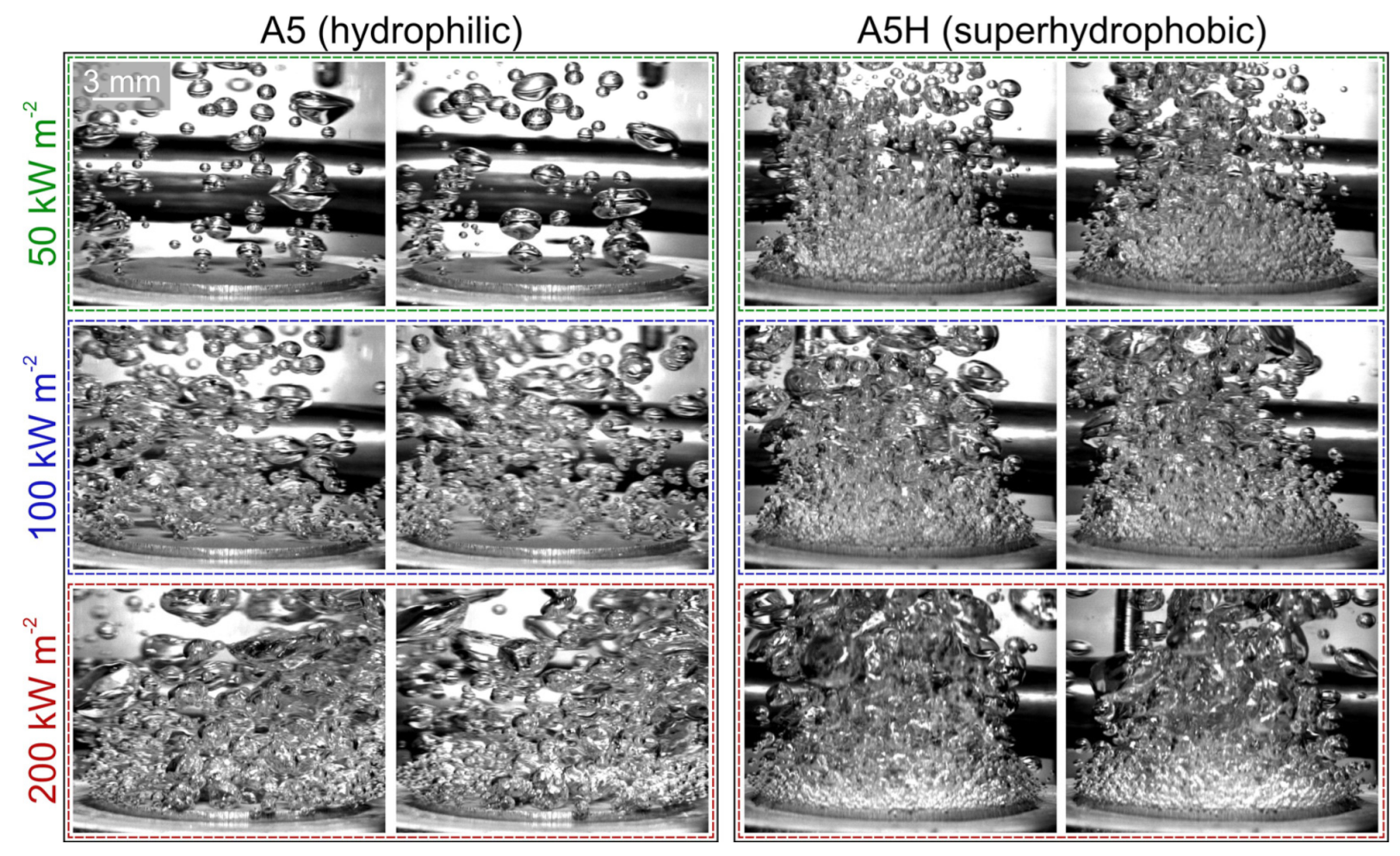
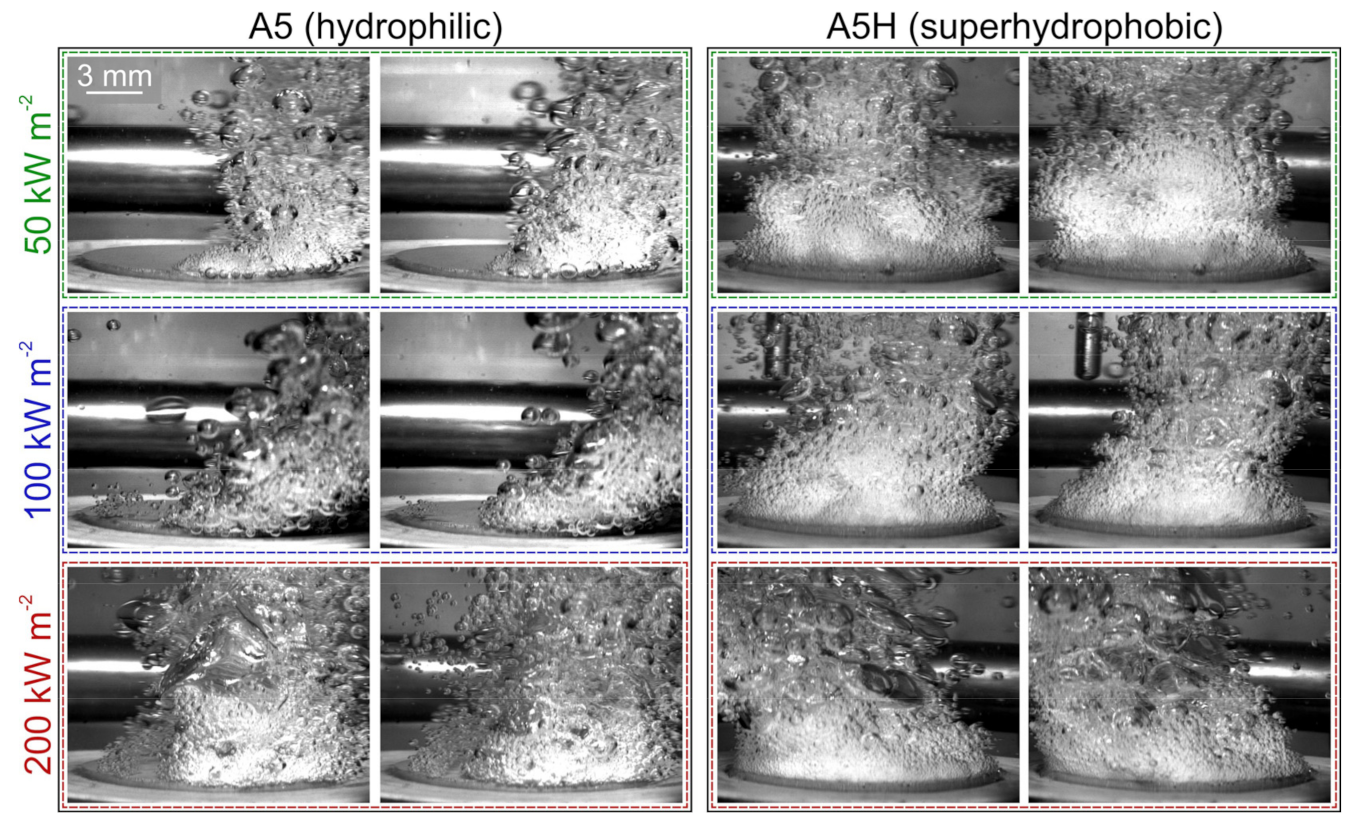
5.2. Comparison of Bubble Dynamics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Latin symbols | |
| f | bubble nucleation frequency (Hz) |
| h | heat transfer coefficient (W m−2 K−1) |
| k | thermal conductivity (W m−1 K−1) |
| heat flux (W m−2) | |
| T | temperature (°C) |
| u | uncertainty |
| x | distance (m) |
| Greek symbols | |
| Δ | difference |
| Subscripts | |
| f | fluid |
| s | sample |
| sat | saturated |
| w | wall |
| Abbreviations | |
| Ax | aluminum surface etched for x minutes |
| AxH | aluminum surface etched for x minutes with subsequent hydrophobization |
| CHF | critical heat flux |
| CVD | chemical vapor deposition |
| HTC | heat transfer coefficient |
| ONB | onset of nucleate boiling |
| PEEK | polyether ether ketone |
| REF | untreated reference sample |
| SEM | scanning electron microscope |
| SRF | self-rewetting fluid |
References
- Honda, H.; Wei, J.J. Enhanced boiling heat transfer from electronic components by use of surface microstructures. Exp. Therm. Fluid Sci. 2004, 28, 159–169. [Google Scholar] [CrossRef]
- Fujita, Y.; Tsutsui, M. Heat transfer in nucleate pool boiling of binary mixtures. Int. J. Heat Mass Transf. 1994, 37, 291–302. [Google Scholar] [CrossRef]
- Allred, T.P.; Weibel, J.A.; Garimella, S.V. Enabling Highly Effective Boiling from Superhydrophobic Surfaces. Phys. Rev. Lett. 2018, 120. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Bai, Q. Critical heat flux of binary mixtures in pool boiling and its correlation in terms of Marangoni number. Int. J. Refrig. 1997, 20, 616–622. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Kandlikar, S.G. Nanoscale surface modification techniques for pool boiling enhancementa critical review and future directions. Heat Transf. Eng. 2011, 32, 827–842. [Google Scholar] [CrossRef]
- Rioux, R.P.; Nolan, E.C.; Li, C.H. A systematic study of pool boiling heat transfer on structured porous surfaces: From nanoscale through microscale to macroscale. AIP Adv. 2014, 4. [Google Scholar] [CrossRef]
- You, S.M.; Kim, J.; Jun, S.; Godinez, J.; Lee, J. Effect of surface roughness on pool boiling heat transfer of water on a superhydrophilic aluminum surface. J. Heat Transf. 2017, 139, 101501. [Google Scholar] [CrossRef]
- Peng, G.S.; Chen, K.H.; Fang, H.C.; Chao, H.; Chen, S.Y. EIS study on pitting corrosion of 7150 aluminum alloy in sodium chloride and hydrochloric acid solution. Mater. Corros. 2010, 61, 783–789. [Google Scholar] [CrossRef]
- Lee, C.Y.; Zhang, B.J.; Kim, K.J. Morphological change of plain and nano-porous surfaces during boiling and its effect on nucleate pool boiling heat transfer. Exp. Therm. Fluid Sci. 2012, 40, 150–158. [Google Scholar] [CrossRef]
- Godinez, J.C.; Fadda, D.; Lee, J.; You, S.M. Development of a stable Boehmite layer on aluminum surfaces for improved pool boiling heat transfer in water. Appl. Therm. Eng. 2019, 541–549. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement by surface modification. Int. J. Heat Mass Transf. 2019, 128, 892–933. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, S.; Li, X.; Wang, S. Heat transfer enhancement of subcooled pool boiling with self-rewetting fluid. Int. J. Heat Mass Transf. 2015, 83, 64–68. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement with additives and nanofluids. Int. J. Heat Mass Transf. 2018, 124, 423–453. [Google Scholar] [CrossRef]
- Hsu, Y.Y. On the size range of active nucleation cavities on a heating surface. J. Heat Transf. 1962, 84, 207–213. [Google Scholar] [CrossRef]
- Zupančič, M.; Može, M.; Gregorčič, P.; Golobič, I. Nanosecond laser texturing of uniformly and non-uniformly wettable micro structured metal surfaces for enhanced boiling heat transfer. Appl. Surf. Sci. 2017, 399, 480–490. [Google Scholar] [CrossRef]
- Može, M.; Senegačnik, M.; Gregorčič, P.; Hočevar, M.; Zupančič, M.; Golobič, I. Laser-engineered microcavity surfaces with a nanoscale superhydrophobic coating for extreme boiling performance. ACS Appl. Mater. Interfaces 2020, 12, 24419–24431. [Google Scholar] [CrossRef]
- Voglar, J.; Gregorčič, P.; Zupančič, M.; Golobič, I. Boiling performance on surfaces with capillary-length-spaced one- and two-dimensional laser-textured patterns. Int. J. Heat Mass Transf. 2018, 127, 1188–1196. [Google Scholar] [CrossRef]
- Gregorčič, P.; Zupančič, M.; Golobič, I. Scalable surface microstructuring by a fiber laser for controlled nucleate boiling performance of high- and low-surface-tension fluids. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Zakšek, P.; Zupančič, M.; Gregorčič, P.; Golobič, I. Investigation of nucleate pool boiling of saturated pure liquids and ethanol-water mixtures on smooth and laser-textured surfaces. Nanoscale Microscale Thermophys. Eng. 2020, 24, 29–42. [Google Scholar] [CrossRef]
- Luo, C.; Xiang, M. Angle inequality for judging the transition from Cassie-Baxter to Wenzel states when a water drop contacts bottoms of grooves between micropillars. Langmuir 2012, 28, 13636–13642. [Google Scholar] [CrossRef]
- Zhang, F.; Jacobi, A.M. Aluminum surface wettability changes by pool boiling of nanofluids. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 438–444. [Google Scholar] [CrossRef]
- Webb, R.L. The evolution of enhanced surface geometries for nucleate boiling. Heat Transf. Eng. 1981, 2, 46–69. [Google Scholar] [CrossRef]
- Može, M. Effect of boiling-induced aging on pool boiling heat transfer performance of untreated and laser-textured copper surfaces. Appl. Therm. Eng. 2020, 181, 116025. [Google Scholar] [CrossRef]
- Gregorčič, P. Comment on “Bioinspired reversible switch between underwater superoleophobicity/superaerophobicity and oleophilicity/aerophilicity and improved antireflective property on the nanosecond laser-ablated superhydrophobic titanium surfaces”. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, A.; Zhang, W.; Yarin, A.L. Pool boiling in deep and shallow vessels and the effect of surface nano-texture and self-rewetting. Int. J. Heat Mass Transf. 2018, 127, 857–866. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, J.; Kong, D.; Lee, H.; Kim, S.-M. Analysis of the enhancing mechanism in pool boiling heat transfer through wetting speed for rough aluminum surfaces with FC-72. Int. J. Heat Mass Transf. 2020, 150, 119325. [Google Scholar] [CrossRef]
- Hui, L.; Liu, M.; Cai, Y.; Lv, Y. Fouling resistance on chemically etched hydrophobic surfaces in nucleate pool boiling. Chem. Eng. Technol. 2015, 38, 416–422. [Google Scholar] [CrossRef]
- Li, L.; Breedveld, V.; Hess, D.W. Creation of superhydrophobic stainless steel surfaces by acid treatments and hydrophobic film deposition. ACS Appl. Mater. Interfaces 2012, 4, 4549–4556. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhang, J.Y.; Mou, L.W.; Zhang, Y.H.; Fan, L.W. Enhanced transitional heat flux by wicking during transition boiling on microporous hydrophilic and superhydrophilic surfaces. Int. J. Heat Mass Transf. 2019, 141, 835–844. [Google Scholar] [CrossRef]
- Ferjančič, K.; Golobič, I. Surface effects on pool boiling CHF. Exp. Therm. Fluid Sci. 2002, 25, 565–571. [Google Scholar] [CrossRef]
- Vachon, R.I.; Tanger, G.E.; Davis, D.L.; Nix, G.H. Pool boiling on polished and chemically etched stainless-steel surfaces. ASME J. heat Transf. 1968, 80, 231–238. [Google Scholar] [CrossRef]
- Allred, T.P.; Weibel, J.A.; Garimella, S.V. The petal effect of parahydrophobic surfaces offers low receding contact angles that promote effective boiling. Int. J. Heat Mass Transf. 2019, 135, 403–412. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Li, L.; Shek, Y.N.; Xu, D. Design of Cassie-wetting nucleation sites in pool boiling. Int. J. Heat Mass Transf. 2019, 132, 25–33. [Google Scholar] [CrossRef]
- Jo, H.; Ahn, H.S.; Kang, S.; Kim, M.H. A study of nucleate boiling heat transfer on hydrophilic, hydrophobic and heterogeneous wetting surfaces. Int. J. Heat Mass Transf. 2011, 54, 5643–5652. [Google Scholar] [CrossRef]
- Teodori, E.; Valente, T.; Malavasi, I.; Moita, A.S.; Marengo, M.; Moreira, A.L.N. Effect of extreme wetting scenarios on pool boiling conditions. Appl. Therm. Eng. 2017, 115, 1424–1437. [Google Scholar] [CrossRef]
- Kim, J.S.; Girard, A.; Jun, S.; Lee, J.; You, S.M. Effect of surface roughness on pool boiling heat transfer of water on hydrophobic surfaces. Int. J. Heat Mass Transf. 2018, 118, 802–811. [Google Scholar] [CrossRef]
- Bourdon, B.; Di Marco, P.; Rioboo, R.; Marengo, M.; De Coninck, J. Enhancing the onset of pool boiling by wettability modification on nanometrically smooth surfaces. Int. Commun. Heat Mass Transf. 2013, 45, 11–15. [Google Scholar] [CrossRef]
- Abe, Y.; Iwaski, A.; Tanaka, K. Thermal management with self-rewetting fluids. Microgravity Sci. Technol. 2005, 15, 148–152. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Shoji, M. Boiling heat transfer of butanol aqueous solution-augmentation of critical heat flux. J. ASTM Int. 2011, 8. [Google Scholar] [CrossRef]
- Abe, Y.; Savino, R. Self-rewetting fluids. In Boiling: Research and Advances; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ohta, H. Nucleate boiling of mixtures. In Boiling: Research and Advances; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hu, B.; Zhang, S.; Wang, D.; Chen, X.; Si, X.; Zhang, D. Experimental study of nucleate pool boiling heat transfer of self-rewetting solution by surface functionalization with TiO2 nanostructure. Can. J. Chem. Eng. 2019, 97, 1399–1406. [Google Scholar] [CrossRef]
- Sahu, R.P.; Sinha-Ray, S.; Sinha-Ray, S.; Yarin, A.L. Pool boiling of Novec 7300 and self-rewetting fluids on electrically-assisted supersonically solution-blown, copper-plated nanofibers. Int. J. Heat Mass Transf. 2016, 95, 83–93. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Du, X.; Yang, Y. Boiling characteristics of water and self-rewetting fluids in packed bed of spherical glass beads. Exp. Therm. Fluid Sci. 2015, 68, 537–544. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, S.; Huang, J.; Song, M. Marangoni effect on pool boiling heat transfer enhancement of self-rewetting fluid. Int. J. Heat Mass Transf. 2018, 127, 1263–1270. [Google Scholar] [CrossRef]
- Butzhammer, L.; Köhler, W. Thermocapillary and thermosolutal Marangoni convection of ethanol and ethanol–water mixtures in a microfluidic device. Microfluid. Nanofluid. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Shoji, M.; Nishiguchi, S. Boiling heat transfer of butanol aqueous solution. In Proceedings of the 9th International Conference on Nanochannels, Microchannels, and Minichannels, ICNMM 2011; ASME: Edmonton, Kanada, 2011; Volume 2, pp. 649–654. [Google Scholar]
- Namura, K.; Nakajima, K.; Suzuki, M. Investigation of transition from thermal- to solutal-Marangoni flow in dilute alcohol/water mixtures using nano-plasmonic heaters. Nanotechnology 2018, 29. [Google Scholar] [CrossRef]
- Sitar, A.; Golobič, I. Heat transfer enhancement of self-rewetting aqueous n-butanol solutions boiling in microchannels. Int. J. Heat Mass Transf. 2015, 81, 198–206. [Google Scholar] [CrossRef]
- Sitar, A.; Zupančič, M.; Crivellari, M.; Golobič, I. The onset of nucleate boiling of self-rewetting fluids in microchannels. IOP Conf. Ser. Earth Environ. Sci. 2017, 93. [Google Scholar] [CrossRef]
- Zhou, L.; Li, W.; Ma, T.; Du, X. Experimental study on boiling heat transfer of a self-rewetting fluid on copper foams with pore-density gradient structures. Int. J. Heat Mass Transf. 2018, 124, 210–219. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Golobič, I. Investigation of the scatter in reported pool boiling CHF measurements including analysis of heat flux and measurement uncertainty evaluation methodology. Appl. Therm. Eng. 2020, 169. [Google Scholar] [CrossRef]
- Alwitt, R.S. The Growth of Hydrous Oxide Films on Aluminum. J. Electrochem. Soc. 1974, 121, 1322–1328. [Google Scholar] [CrossRef]
- Din, R.U.; Yuksel, S.; Jellesen, M.S.; Møller, P.; Ambat, R. Steam Assisted Accelerated Growth of Oxide Layer on Aluminium Alloys. In Proceedings of the Eurocorr 2013, Estoril, Portugal, 1–5 September 2013; Volume 1090, pp. 1–7. [Google Scholar]
- Min, J.; Webb, R.L. Long-term wetting and corrosion characteristics of hot water treated aluminum and copper fin stocks. Int. J. Refrig. 2002, 25, 1054–1061. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Golobič, I. Pattern geometry optimization on superbiphilic aluminum surfaces for enhanced pool boiling heat transfer. Int. J. Heat Mass Transf. 2020, 161, 120265. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium; Elsevier Science: london, UK, 2004; ISBN 9780080444956. [Google Scholar]
- Katarkar, A.S.; Majumder, B.; Pingale, A.D.; Belgamwar, S.U.; Bhaumik, S. A review on the effects of porous coating surfaces on boiling heat transfer. Mater. Today Proc. 2021, 44, 362–367. [Google Scholar] [CrossRef]
- Polyaev, V.M.; Kichatov, B.V. Boiling of solutions on porous surfaces. Theor. Found. Chem. Eng. 2000, 34, 22–26. [Google Scholar] [CrossRef]
- Chang, J.Y.; You, S.M. Boiling heat transfer phenomena from micro-porous and porous surfaces in saturated FC-72. Int. J. Heat Mass Transf. 1997, 40, 4437–4447. [Google Scholar] [CrossRef]
- Patil, C.M.; Kandlikar, S.G. Review of the manufacturing techniques for porous surfaces used in enhanced pool boiling. Heat Transf. Eng. 2014, 35, 887–902. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Hočevar, M.; Golobič, I.; Gregorčič, P. Surface chemistry and morphology transition induced by critical heat flux incipience on laser-textured copper surfaces. Appl. Surf. Sci. 2019, 490, 220–230. [Google Scholar] [CrossRef]
- Nikaido, N.; Shirai, S.; Iihashi, M.; Umemoto, S. Process for Treating the Surface of Aluminium or Aluminium Alloy. U.S. Patent US3945899A, 23 March 1978. [Google Scholar]
- Jones, B.J.; Garimella, S.V. Effects of Surface Roughness on the Pool Boiling of Water. In Proceedings of the ASME/JSME 2007 Thermal Engineering Heat Transfer Summer Conference; ASME: New York, NY, USA, 2007; Volume 2, pp. 219–227. [Google Scholar]
- Zupančič, M.; Steinbücher, M.; Gregorčič, P.; Golobič, I. Enhanced pool-boiling heat transfer on laser-made hydrophobic/superhydrophilic polydimethylsiloxane-silica patterned surfaces. Appl. Therm. Eng. 2015, 91, 288–297. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Wang, H.; Pan, T. Bubble formation on superhydrophobic-micropatterned copper surfaces. Appl. Therm. Eng. 2012, 35, 112–119. [Google Scholar] [CrossRef]
- Raza, M.Q.; Kumar, N.; Raj, R. Effect of foamability on pool boiling critical heat flux with nanofluids. Soft Matter 2019, 15, 5308–5318. [Google Scholar] [CrossRef]




| Sample Name | Treatment | Contact Angle before Boiling (°) |
|---|---|---|
| REF | none | 58° ± 11° |
| A5 | 5 min etching | 68° ± 8° |
| A10 | 10 min etching | <1° |
| A15 | 15 min etching | <1° |
| A5H | 5 min etching + hydrophobization | 157° ± 3° |
| A15H | 15 min etching + hydrophobization | 160° ± 3° |
| Heat Flux (kW m−2) | A15 vs. REF | A15H vs. REF | A15H vs. A15 | A5 vs. REF | A5H vs. REF | A5H vs. A5 |
|---|---|---|---|---|---|---|
| 150 | +6% | +179% | +163% | +54% | +559% | +327% |
| 500 | −5% | +78% | +88% | +64% | +582% | +316% |
| 1000 | −5% | +34% | +41% | +90% | +583% | +260% |
| CHF | +5% | +12% | +7% | +165% | +488% | +122% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Može, M.; Vajc, V.; Zupančič, M.; Šulc, R.; Golobič, I. Pool Boiling Performance of Water and Self-Rewetting Fluids on Hybrid Functionalized Aluminum Surfaces. Processes 2021, 9, 1058. https://doi.org/10.3390/pr9061058
Može M, Vajc V, Zupančič M, Šulc R, Golobič I. Pool Boiling Performance of Water and Self-Rewetting Fluids on Hybrid Functionalized Aluminum Surfaces. Processes. 2021; 9(6):1058. https://doi.org/10.3390/pr9061058
Chicago/Turabian StyleMože, Matic, Viktor Vajc, Matevž Zupančič, Radek Šulc, and Iztok Golobič. 2021. "Pool Boiling Performance of Water and Self-Rewetting Fluids on Hybrid Functionalized Aluminum Surfaces" Processes 9, no. 6: 1058. https://doi.org/10.3390/pr9061058
APA StyleMože, M., Vajc, V., Zupančič, M., Šulc, R., & Golobič, I. (2021). Pool Boiling Performance of Water and Self-Rewetting Fluids on Hybrid Functionalized Aluminum Surfaces. Processes, 9(6), 1058. https://doi.org/10.3390/pr9061058