Naturally Inspired Highly Stable Salt-Resisting Material for Solar Water Desalination
Abstract
1. Introduction
2. Materials and Methods
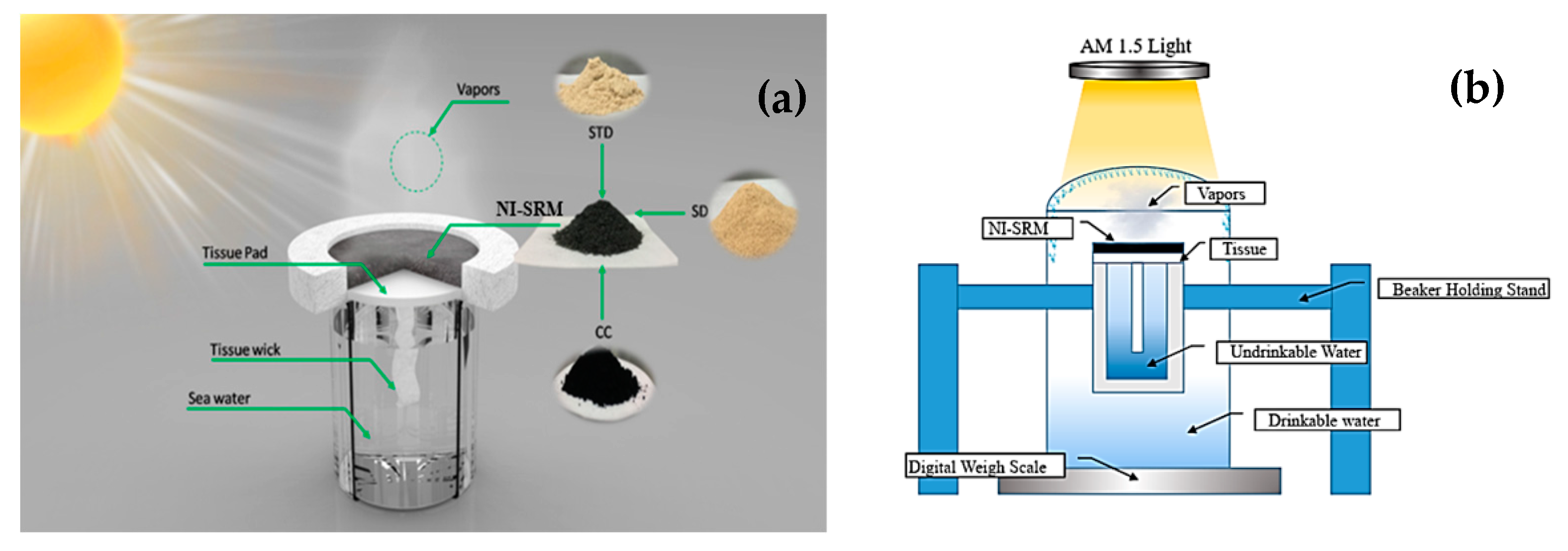
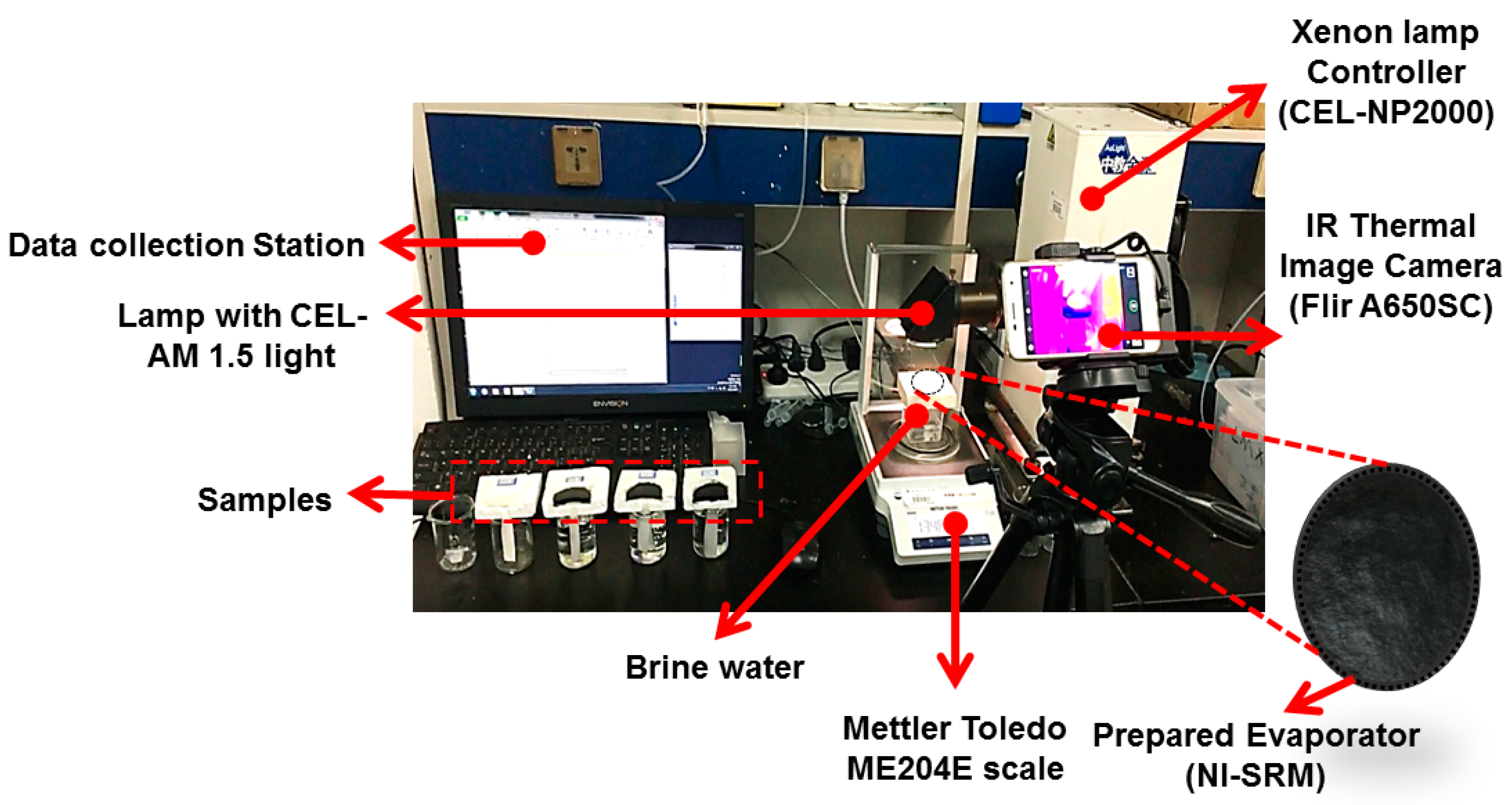
2.1. Experiment Setup
2.2. Characterization
3. Results and Discussion
3.1. Characterization
3.2. Wettablility Control
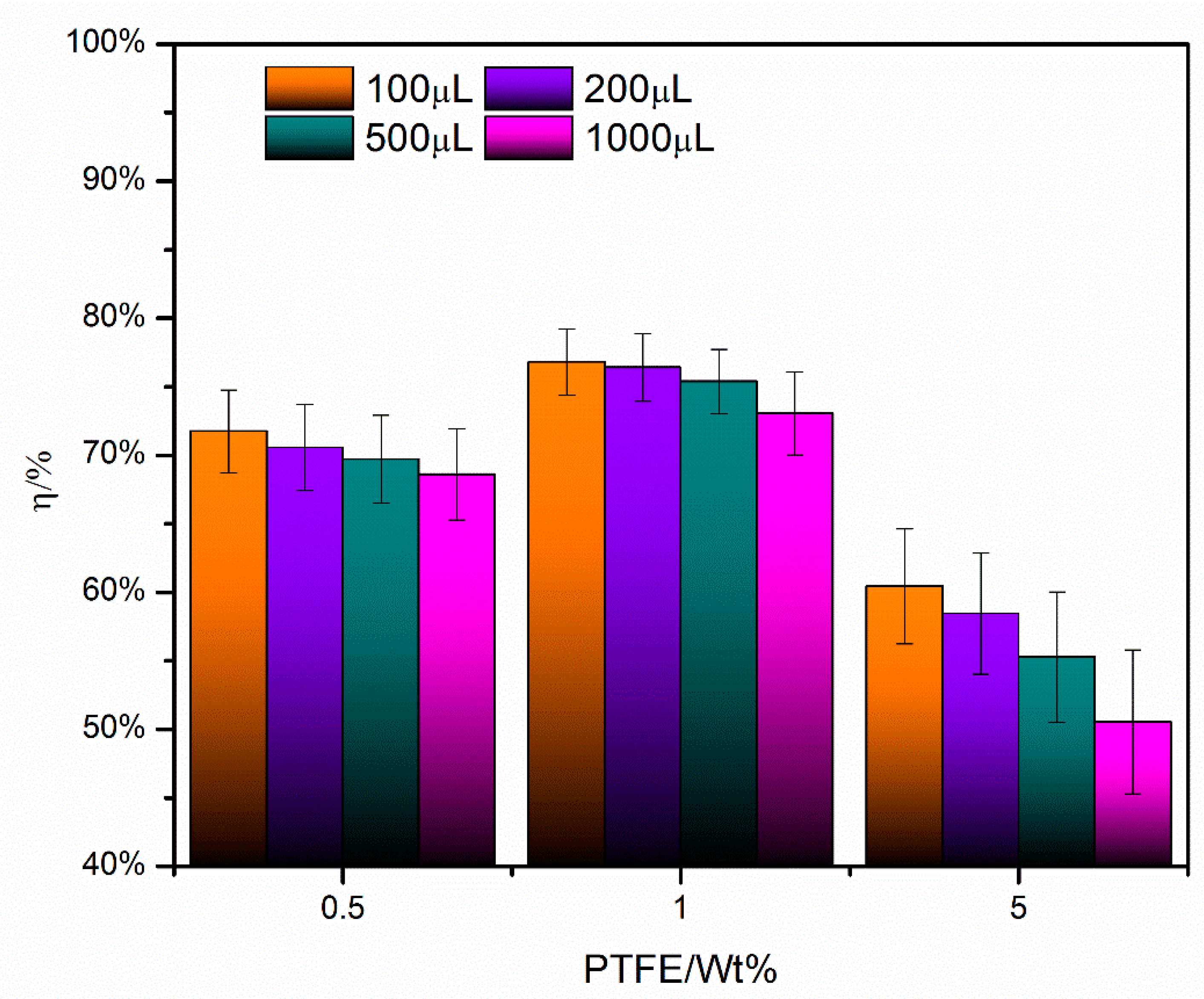
3.3. Effect of Particle Size on Mass Evaporation
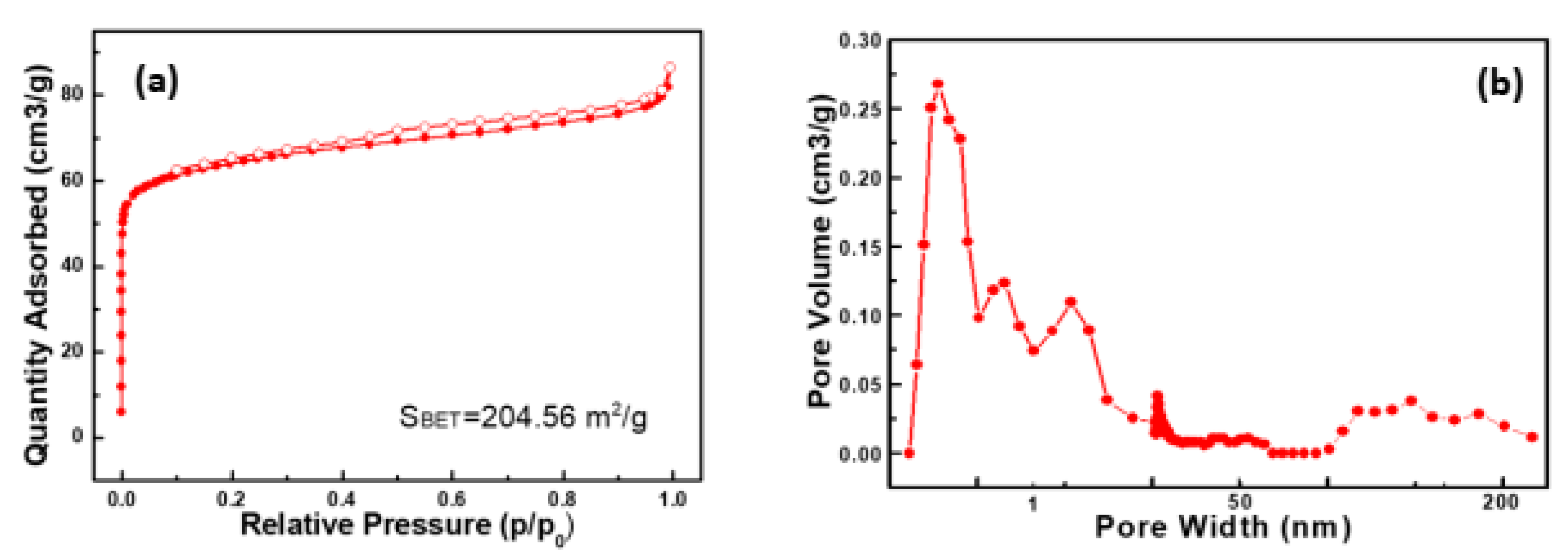
3.4. BET and Surface-Area Analysis
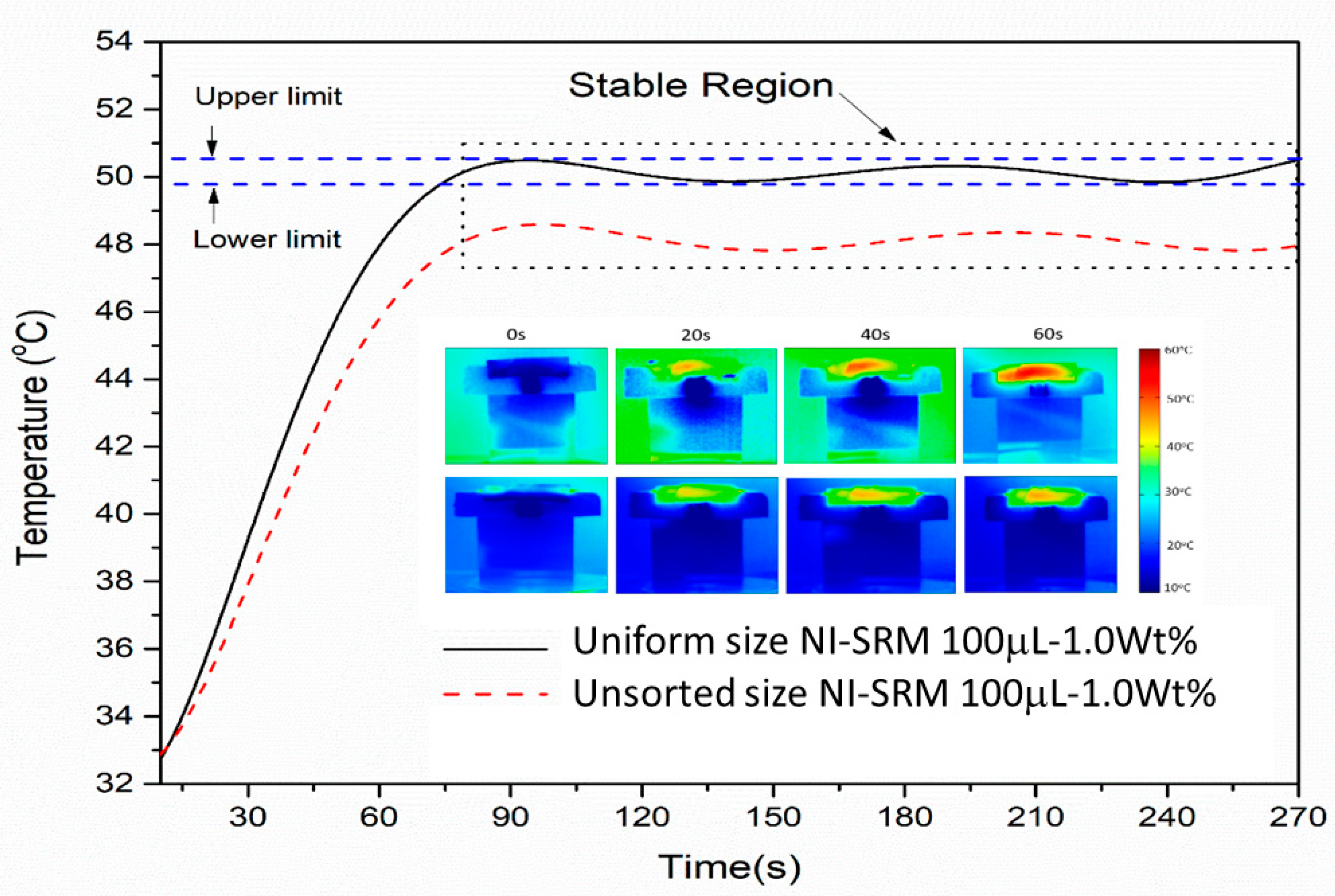
3.5. Temperature Evaluation of NI-SRM
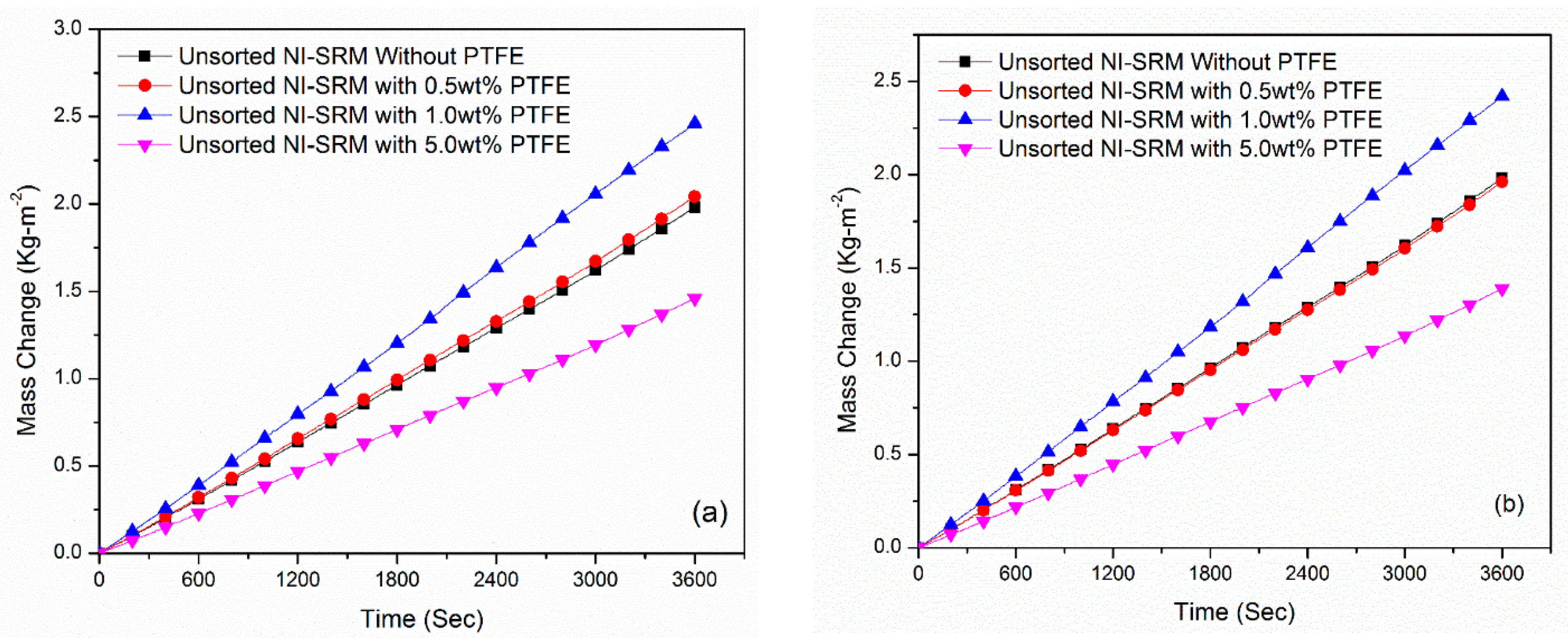
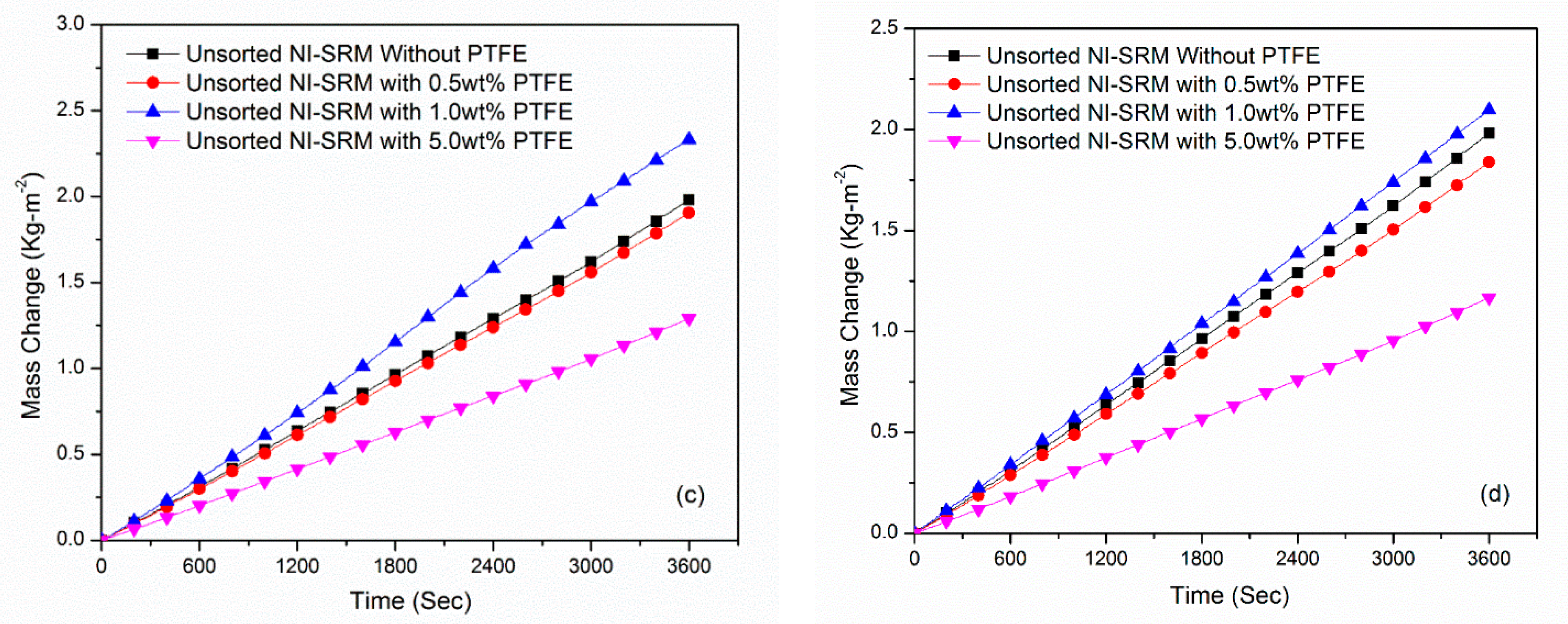
3.6. Stabillity and Evaporation Rate of Sorted-Sized Evaporator
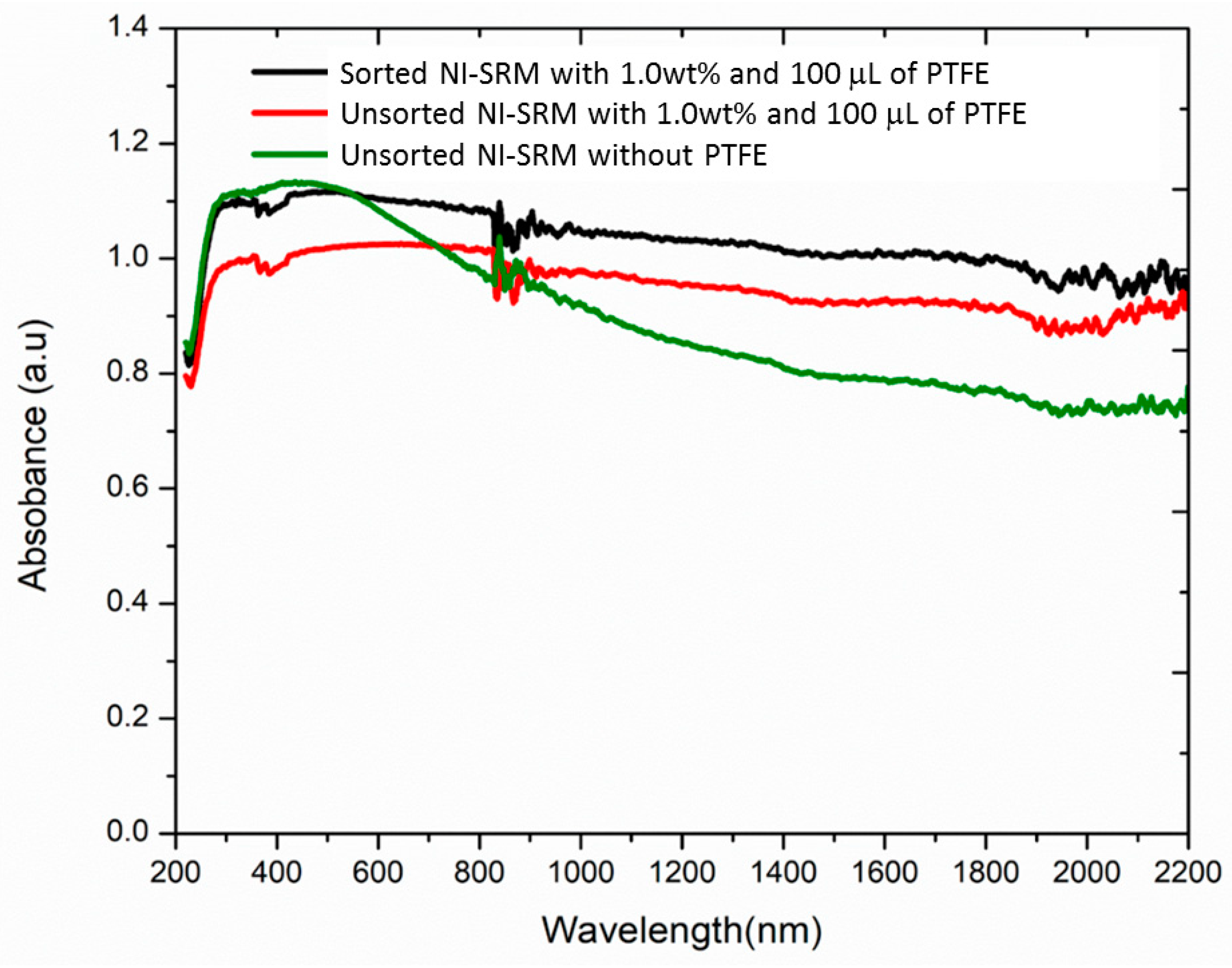
3.7. Evaluation of Surface Wettibilty of NI-SRM
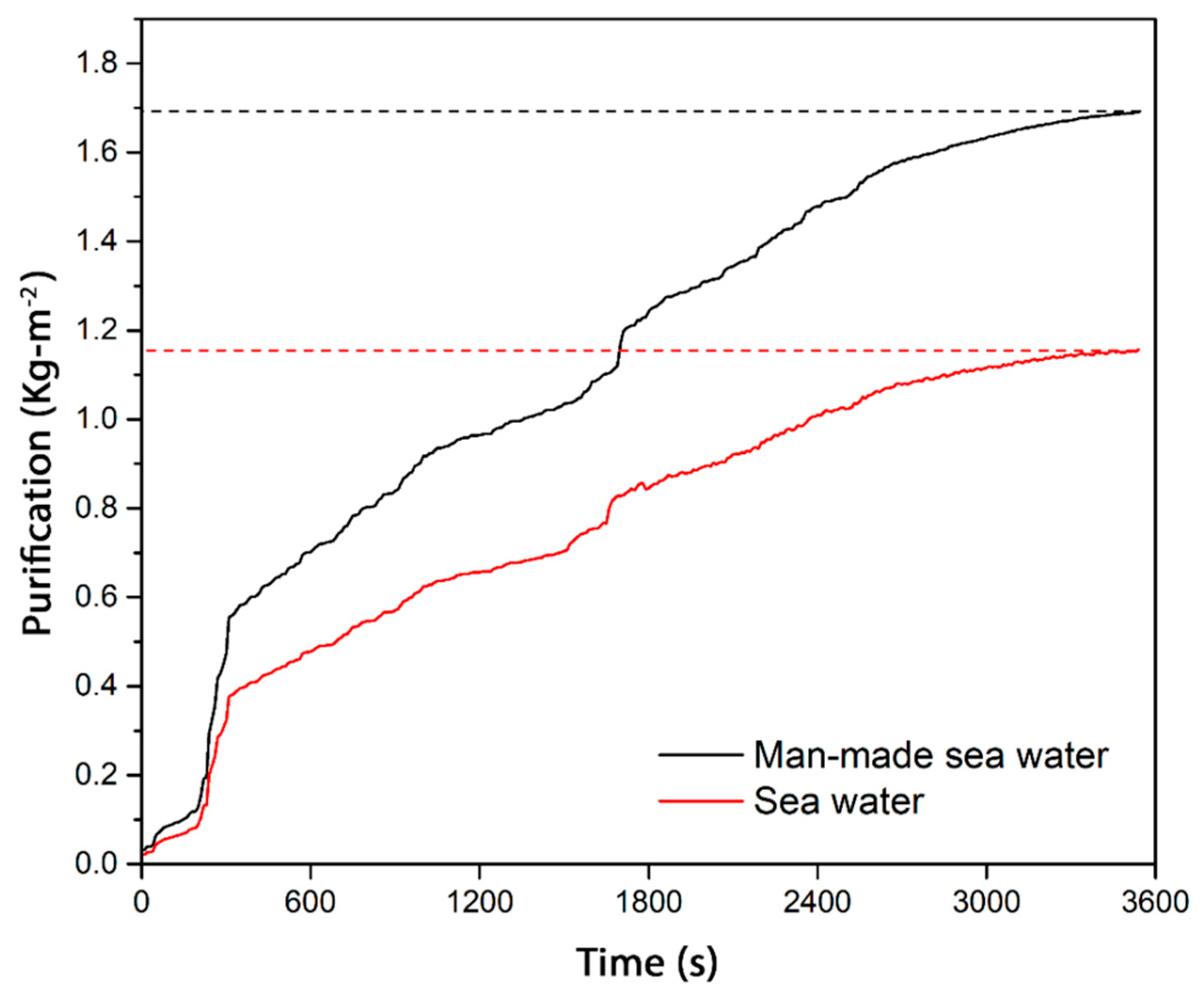
3.8. Water Purification
3.9. Self Salt Replineshment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nag, A. Utilization of charred sawdust as an adsorbent of dyes, toxic salts and oil from water. Process. Saf. Environ. Prot. 1995, 73, 299–304. [Google Scholar]
- Bryant, P.S.; Petersen, J.N.; Lee, J.M.; Brouns, T.M. Sorption of heavy metals by untreated red fir sawdust. Appl. Biochem. Biotechnol. 1992, 34, 777–788. [Google Scholar] [CrossRef]
- Ni, G.; Li, G.; Boriskina, S.V.; Li, H.; Yang, W.; Zhang, T.; Chen, G. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh CK, N.; Ho GW Zhu, L. Recent progress in solar-driven interfacial water evaporation: Advanced designs and applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- He, S.; Chen, C.; Kuang, Y.; Mi, R.; Liu, Y.; Pei, Y.; Kong, W.; Gan, W.; Xie, H.; Hitz, E.; et al. Nature-inspired salt resistant bimodal porous solar evaporator for efficient and stable water desalination. Energy Environ. Sci. 2019, 12, 1558–1567. [Google Scholar] [CrossRef]
- Cho, K.L.; Hill, A.J.; Caruso, F.; Kentish, S.E. Chlorine resistant glutaraldehyde crosslinked polyelectrolyte multilayer membranes for desalination. Adv. Mater. 2015, 27, 2791–2796. [Google Scholar] [CrossRef]
- Hanshik, C.; Jeong, H.; Jeong, K.W.; Choi, S.H. Improved productivity of the MSF (multi-stage flashing) desalination plant by increasing the TBT (top brine temperature). Energy 2016, 107, 683–692. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Guo, W.; Liu, X.; Li, Y. Flexible and robust polyaniline composites for highly efficient and durable solar desalination. ACS Appl. Energy Mater. 2020, 3, 2634–2642. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X.; Liang, H. Application of recoverable carbon nanotube nanofluids in solar desalination system: An experimental investigation. Desalination 2019, 451, 92–101. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Kuravi, S.; Kota, K.; Park, Y.H.; Xu, P. Low-cost and reusable carbon black based solar evaporator for effective water desalination. Desalination 2020, 483, 114412. [Google Scholar] [CrossRef]
- Mckay, G.H.; Blair, H.S.; Gardner, J.R. Adsorption of dyes on chitin. I. Equilibrium studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar]
- Shukla, A.; Zhang, Y.H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.K.; Yoon, S.W.; Park, P.Y.; Park, K.I.; Lee, C.W. Desalination performance of a carbon-based composite electrode. Desalination 2009, 237, 155–161. [Google Scholar] [CrossRef]
- Chao, W.; Sun, X.; Li, Y.; Cao, G.; Wang, R.; Wang, C.; Ho, S.H. Enhanced Directional Seawater Desalination Using a Structure-Guided Wood Aerogel. ACS Appl. Mater. Interfaces 2020, 12, 22387–22397. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Ismail, A.F.; Ng, B.C. Carbon nanotubes for desalination: Performance evaluation and current hurdles. Desalination 2013, 308, 2–14. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles; Nature Publishing Group: London, UK, 2011; pp. 148–159. [Google Scholar]
- Zheng, L.; Wang, J.; Li, J.; Zhang, Y.; Li, K.; Wei, Y. Preparation, evaluation and modification of PVDF-CTFE hydrophobic membrane for MD desalination application. Desalination 2017, 402, 162–172. [Google Scholar] [CrossRef]
- Khairkar, S.R.; Pansare, A.V.; Shedge, A.A.; Chhatre, S.Y.; Suresh, A.K.; Chakrabarti, S.; Patil, V.R.; Nagarkar, A.A. Hydrophobic interpenetrating polyamide-PDMS membranes for desalination, pesticides removal and enhanced chlorine tolerance. Chemosphere 2020, 258, 127179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic light-to-heat conversion membranes with self-healing ability for interfacial solar heating. Adv. Mater. 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Knozowska, K. Hydrophobic ceramic membranes for water desalination. Appl. Sci. 2017, 7, 402. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as efficient solar steam-generation devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Schipper, L.; Vojvodić-Vuković, M. Nitrate removal from groundwater using a denitrification wall amended with sawdust: Field trial. J. Environ. Qual. 1998, 27, 664–668. [Google Scholar] [CrossRef]
- McKay, G.; Poots, V.J. Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. J. Chem. Technol. Biotechnol. 1980, 30, 279–292. [Google Scholar] [CrossRef]
- Liao, R.; Zuo, Z.; Guo, C.; Yuan, Y.; Zhuang, A. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property. Appl. Surf. Sci. 2014, 317, 701–709. [Google Scholar] [CrossRef]
- Obeso, C.G.; Sousa, M.P.; Song, W.; Rodriguez-Pérez, M.A.; Bhushan, B.; Mano, J.F. Modification of paper using polyhydroxybutyrate to obtain biomimetic superhydrophobic substrates. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 51–55. [Google Scholar] [CrossRef]
- Bond, G.R. The role of activated charcoal and gastric emptying in gastrointestinal decontamination: A state-of-the-art review. Ann. Emer. Med. 2002, 39, 273–286. [Google Scholar] [CrossRef]
- Pan, M.; van Staden, J. The use of charcoal in in vitro culture–A review. Plant Growth Regul. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- Sander, M.; Pignatello, J.J. Characterization of charcoal adsorption sites for aromatic compounds: Insights drawn from single-solute and bi-solute competitive experiments. Environ. Sci. Technol. 2005, 39, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kwon, S.; Pignatello, J.J. Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions. Environ. Sci. Technol. 2005, 39, 3990–3998. [Google Scholar] [CrossRef]
- Sands, C.D.; McIntyre, J.L.; Walton, G.S. Use of activated charcoal for the removal of patulin from cider. Appl. Environ. Microbiol. 1976, 32, 388–391. [Google Scholar] [CrossRef]
- Jia, C.; Li, Y.; Yang, Z.; Chen, G.; Yao, Y.; Jiang, F.; Kuang, Y.; Pastel, G.; Xie, H.; Yang, B.; et al. Rich mesostructures derived from natural woods for solar steam generation. Joule 2017, 1, 588–599. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Song, J.; Yang, Z.; Kuang, Y.; Hitz, E.; Jia, C.; Gong, A.; Jiang, F.; Zhu, J.Y.; et al. Highly flexible and efficient solar steam generation device. Adv. Mater. 2017, 29, 1701756. [Google Scholar] [CrossRef]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour. Technol. 2008, 99, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.; Al-Bayati, A.; Sajadi, S.M.; Irajizad, P.; Wang, S.H.; Ghasemi, H. A flexible anti-clogging graphite film for scalable solar desalination by heat localization. J. Mater. Chem. A 2017, 5, 15227–15234. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A hydrophobic surface enabled salt-blocking 2D Ti3C2 MXene membrane for efficient and stable solar desalination. J. Mater. Chem. A 2018, 6, 16196–16204. [Google Scholar] [CrossRef]
- Yi, L.; Ci, S.; Luo, S.; Shao, P.; Hou, Y.; Wen, Z. Scalable and low-cost synthesis of black amorphous Al-Ti-O nanostructure for high-efficient photothermal desalination. Nano Energy 2017, 41, 600–608. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Guo, X.; Zheng, R. Delignified Wood-based Highly Efficient Solar Steam Generation Device via Promoting Both Water Transportation and Evaporation. BioResources 2019, 14, 3758–3767. [Google Scholar]
- Hao, D.; Yang, Y.; Xu, B.; Cai, Z. Efficient solar water vapor generation enabled by water-absorbing polypyrrole coated cotton fabric with enhanced heat localization. Appl. Therm. Eng. 2018, 141, 406–412. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, H.; Jian, M.; Li, Y.; Xia, K.; Zhang, M.; Wang, C.; Wang, Q.; Ma, M.; Zheng, Q.S.; et al. Extremely black vertically aligned carbon nanotube arrays for solar steam generation. ACS Appl. Mater. Interfaces 2017, 9, 28596–28603. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P. Self-floating carbon nanotube membrane on macroporous silica substrate for highly efficient solar-driven interfacial water evaporation. ACS Sustain. Chem. Eng. 2016, 4, 1223–1230. [Google Scholar] [CrossRef]
- Huang, L.; Pei, J.; Jiang, H.; Hu, X. Water desalination under one sun using graphene-based material modified PTFE membrane. Desalination 2018, 442, 1–7. [Google Scholar] [CrossRef]
- Kou, H.; Liu, Z.; Zhu, B.; Macharia, D.K.; Ahmed, S.; Wu, B.; Zhu, M.; Liu, X.; Chen, Z. Recyclable CNT-coupled cotton fabrics for low-cost and efficient desalination of seawater under sunlight. Desalination 2019, 462, 29–38. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Lu, J.; Xu, N.; Chen, C.; Min, X.; Zhu, B.; Li, H.; Zhou, L.; Zhu, S.; et al. Enhancement of interfacial solar vapor generation by environmental energy. Joule 2018, 2, 1331–1338. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Duan, H.; Liu, Y.; Quan, X.; Tao, P.; Shang, W.; Wu, J.; Song, C.; Deng, T. The impact of surface chemistry on the performance of localized solar-driven evaporation system. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Diddens, C.; Tan, H.; Lv, P.; Versluis, M.; Kuerten, J.G.M.; Zhang, X.; Lohse, D. Evaporating pure, binary and ternary droplets: Thermal effects and axial symmetry breaking. arXiv 2017, arXiv:1706.06874. Available online: https://arxiv.org/abs/1706.06874 (accessed on 10 September 2020).
- Wan, R.; Wang, C.; Lei, X.; Zhou, G.; Fang, H. Enhancement of water evaporation on solid surfaces with nanoscale hydrophobic-hydrophilic patterns. Phys. Rev. Lett. 2015, 115, 195901. [Google Scholar] [CrossRef]



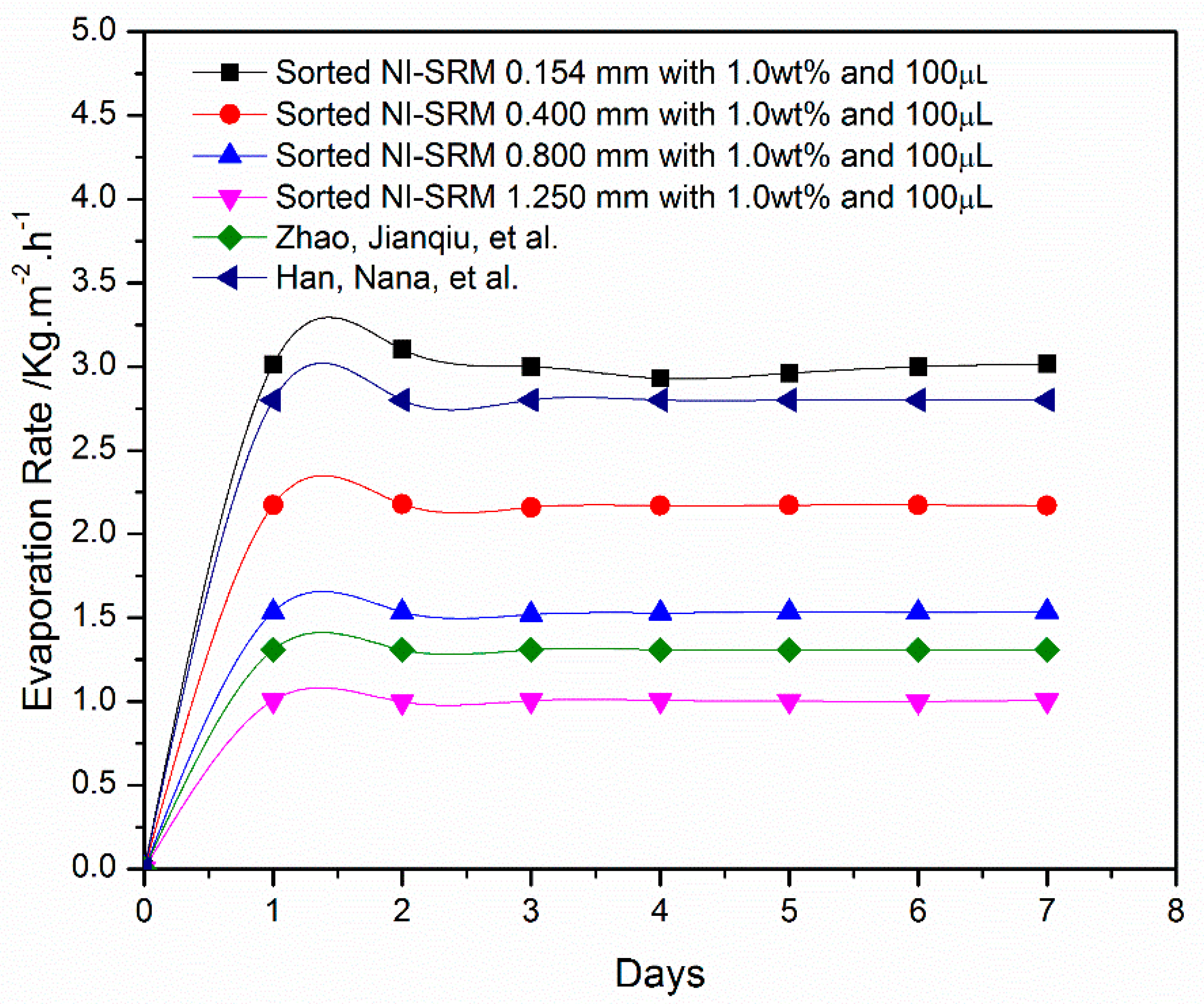


| Sr. | Authors | EV Rate (kg-m−2-h−1) | Solar Condition | Stability | Ref |
|---|---|---|---|---|---|
| 1 | Zhao, Jianqiu et al. | 1.31 | 1 sun | 200 h | [34] |
| 2 | Yi, Luocai et al. | 1.03 | 1 sun | - | [35] |
| 3 | Li, Renyuan et al. | 1.30 | 1 sun | [36] | |
| 4 | He, Yuming et al. | 1.91 min to 1.21 max | 1 sun | 7 cycles | [37] |
| 5 | Hao, Dandan et al. | 1.19 | 1 sun | [38] | |
| 6 | Yin, Zhe et al. | 1.9 | 1 sun | - | [39] |
| 7 | Wang, Yuchao | 0.4 to 1.25 | 1 sun | 2 h | [40] |
| 8 | Huang, Lu et al. | 0.4 to 0.7 | 1 sun | 12 cycles | [41] |
| 9 | Kou, Hui et al. | 1.59 to 0.54 | 1 sun | 0 to 8 h | [42] |
| 10 | Present work | 1.16 to 3.02 | 1 Sun | 7 Days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samo, I.A.; Mughal, W.; Samo, K.A.; Zhong, Y.; Cheng, C.; Zhao, Y.; Siyal, A.A.; Tian, B. Naturally Inspired Highly Stable Salt-Resisting Material for Solar Water Desalination. Processes 2021, 9, 1019. https://doi.org/10.3390/pr9061019
Samo IA, Mughal W, Samo KA, Zhong Y, Cheng C, Zhao Y, Siyal AA, Tian B. Naturally Inspired Highly Stable Salt-Resisting Material for Solar Water Desalination. Processes. 2021; 9(6):1019. https://doi.org/10.3390/pr9061019
Chicago/Turabian StyleSamo, Imran Ahmed, Waqas Mughal, Kamran Ahmed Samo, Yang Zhong, Congtian Cheng, Yajun Zhao, Asif Ali Siyal, and Benqiang Tian. 2021. "Naturally Inspired Highly Stable Salt-Resisting Material for Solar Water Desalination" Processes 9, no. 6: 1019. https://doi.org/10.3390/pr9061019
APA StyleSamo, I. A., Mughal, W., Samo, K. A., Zhong, Y., Cheng, C., Zhao, Y., Siyal, A. A., & Tian, B. (2021). Naturally Inspired Highly Stable Salt-Resisting Material for Solar Water Desalination. Processes, 9(6), 1019. https://doi.org/10.3390/pr9061019






