A Dual Reactor for Isothermal Thermochemical Cycles of H2O/CO2 Co-Splitting Using La0.3Sr0.7Co0.7Fe0.3O3 as an Oxygen Carrier
Abstract
1. Introduction
2. Methodology
2.1. Catalyst Preparation and Characterization
2.2. Experimental Procedure
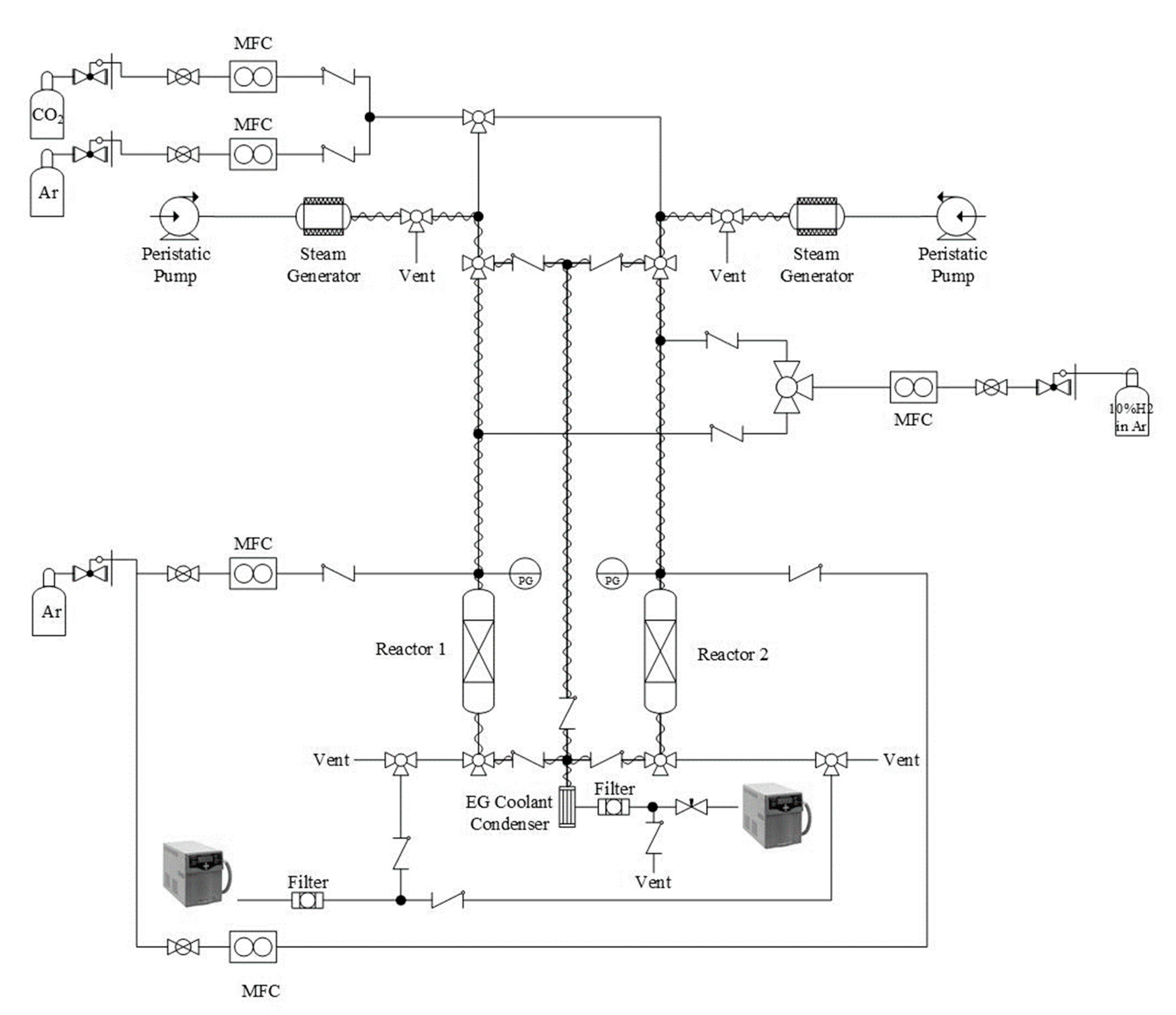
2.2.1. Reactor Set-Up
2.2.2. Single Reactor Experiments
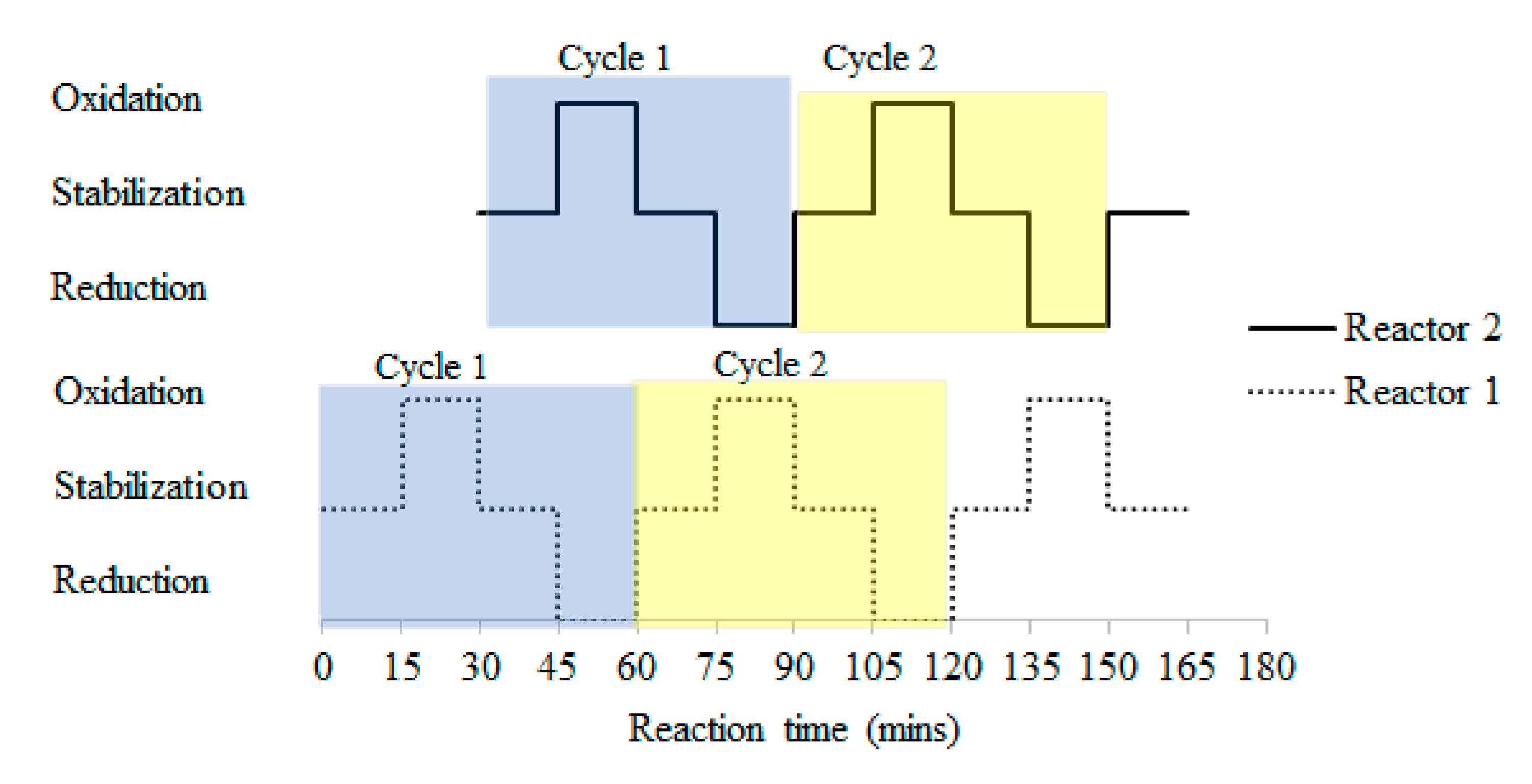
2.2.3. Dual-Reactor System Experiments
2.2.4. Solid Conversion and Reactive Gases Conversion
2.2.5. Gas-Solid Kinetics Analysis
3. Result and Discussion
3.1. Reactivity Study
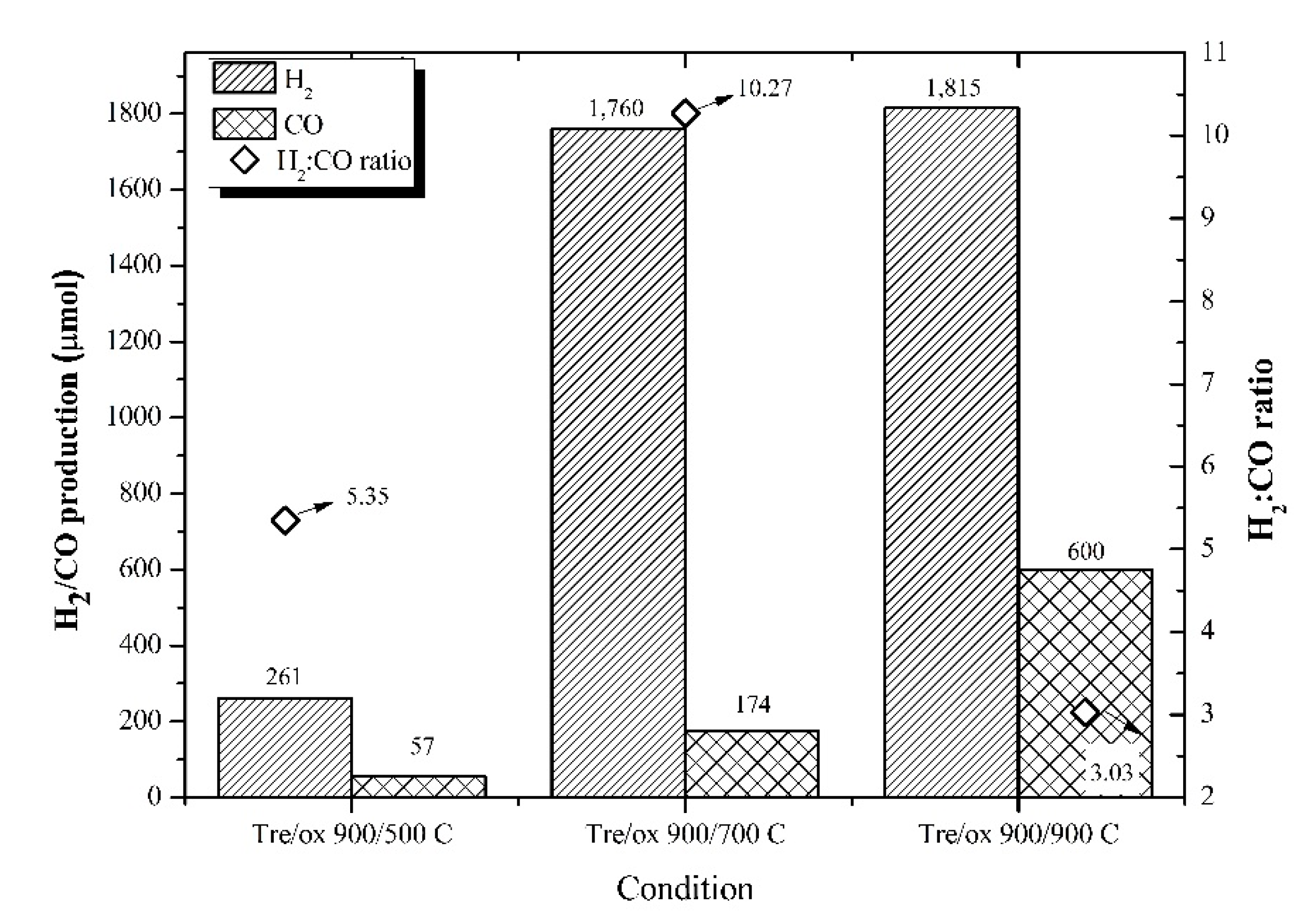
3.2. Reduction Temperature Effect
3.3. Oxidation Temperature Effect
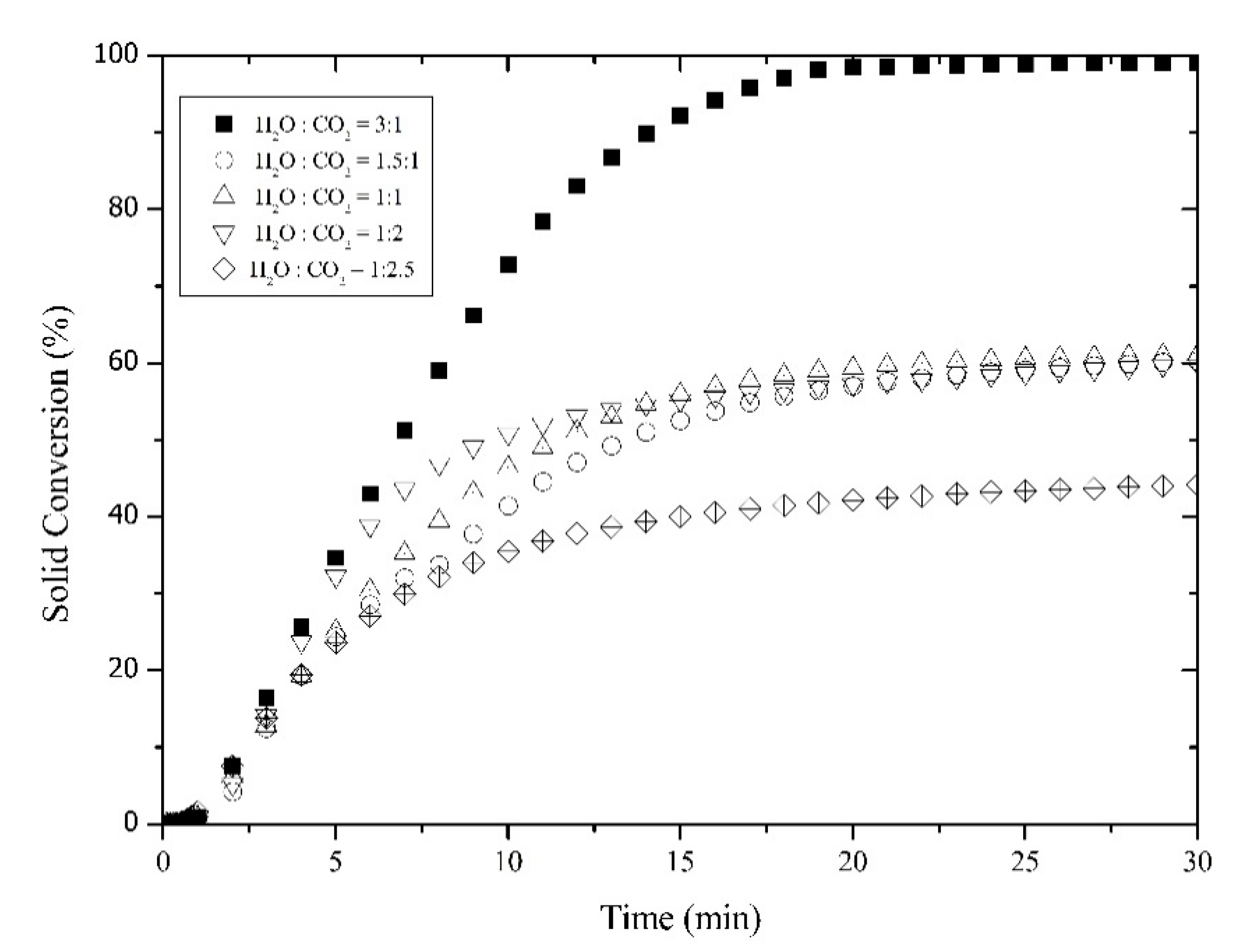
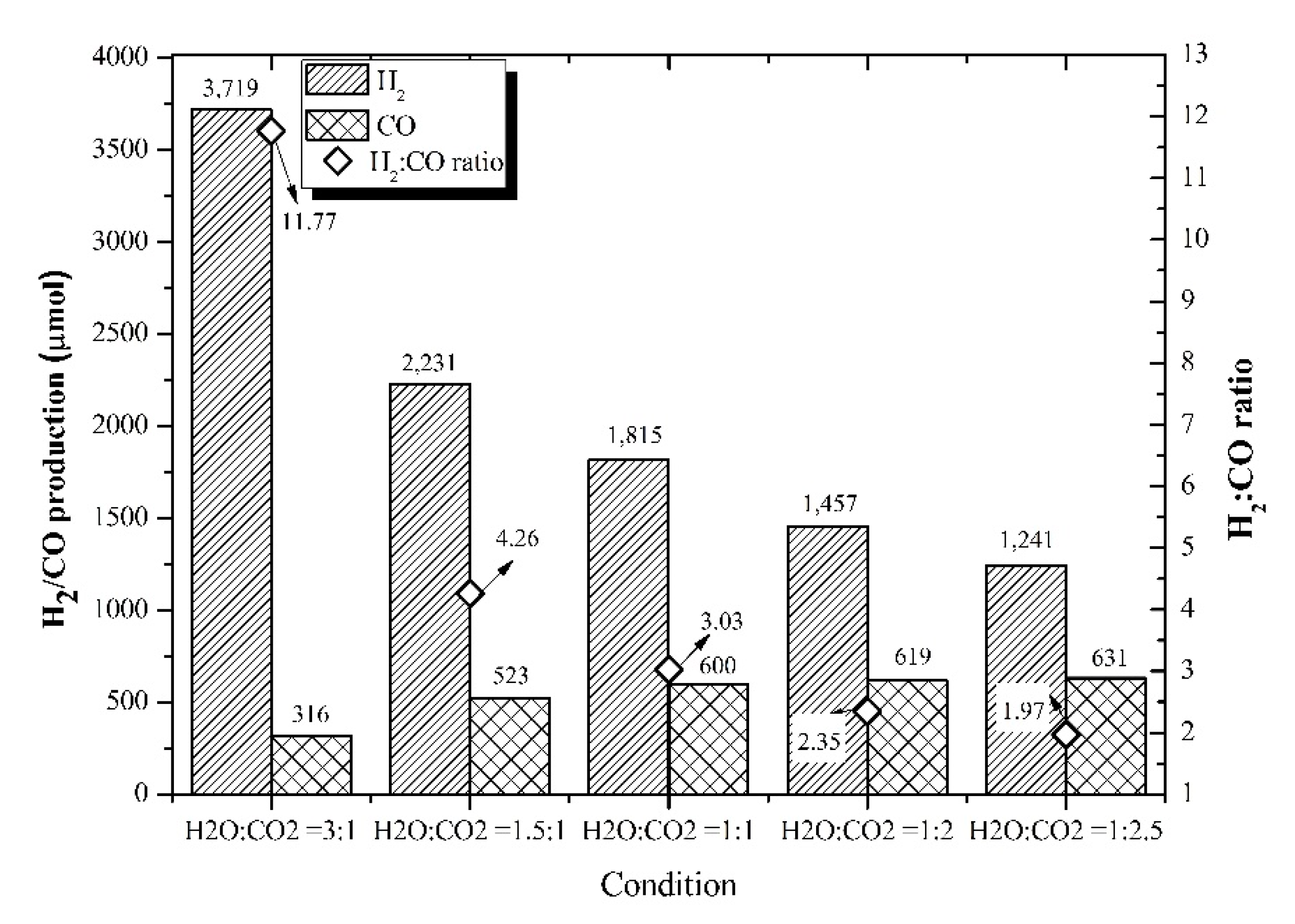
3.4. Feed Molar Ratio Effect
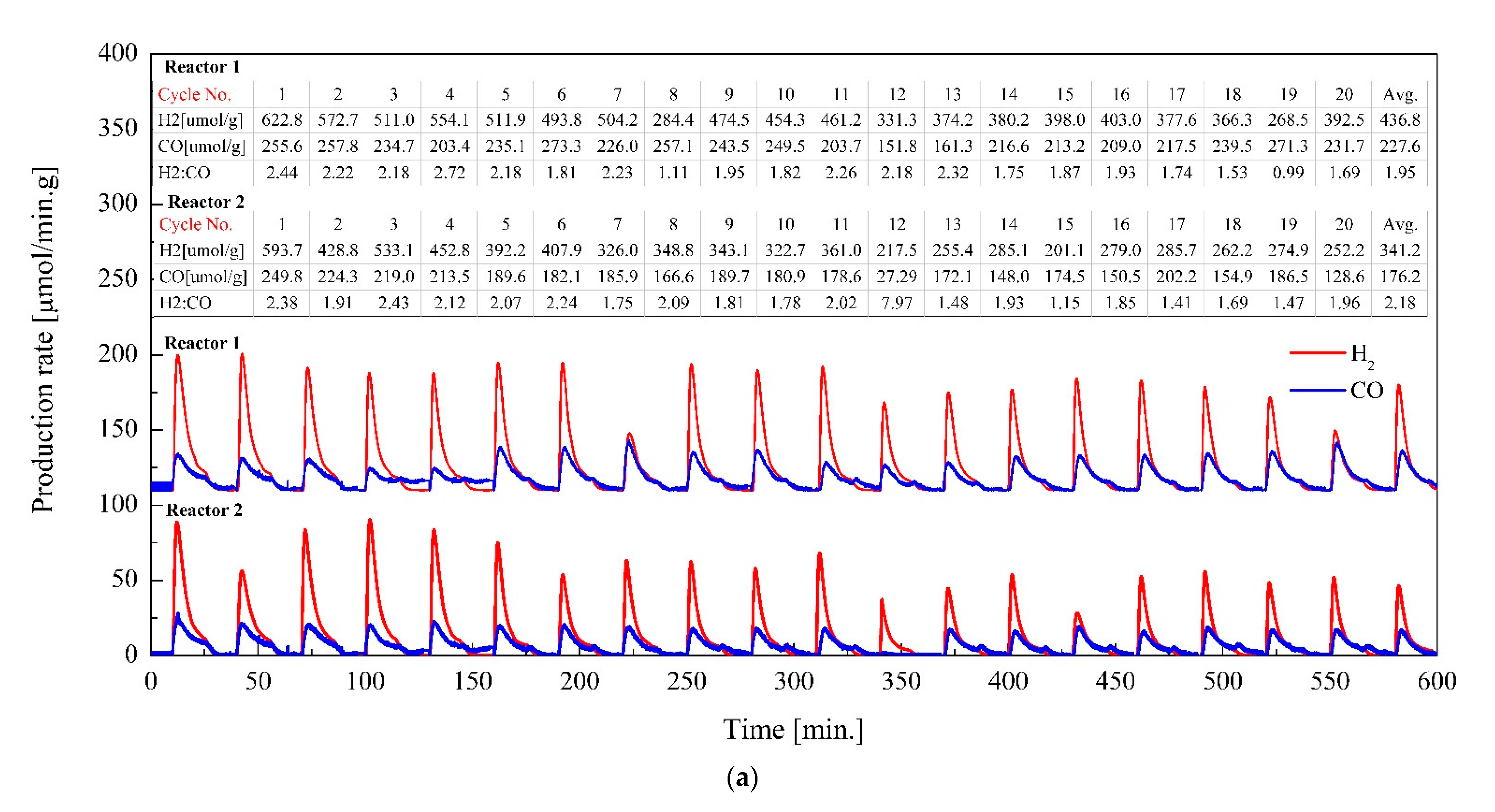
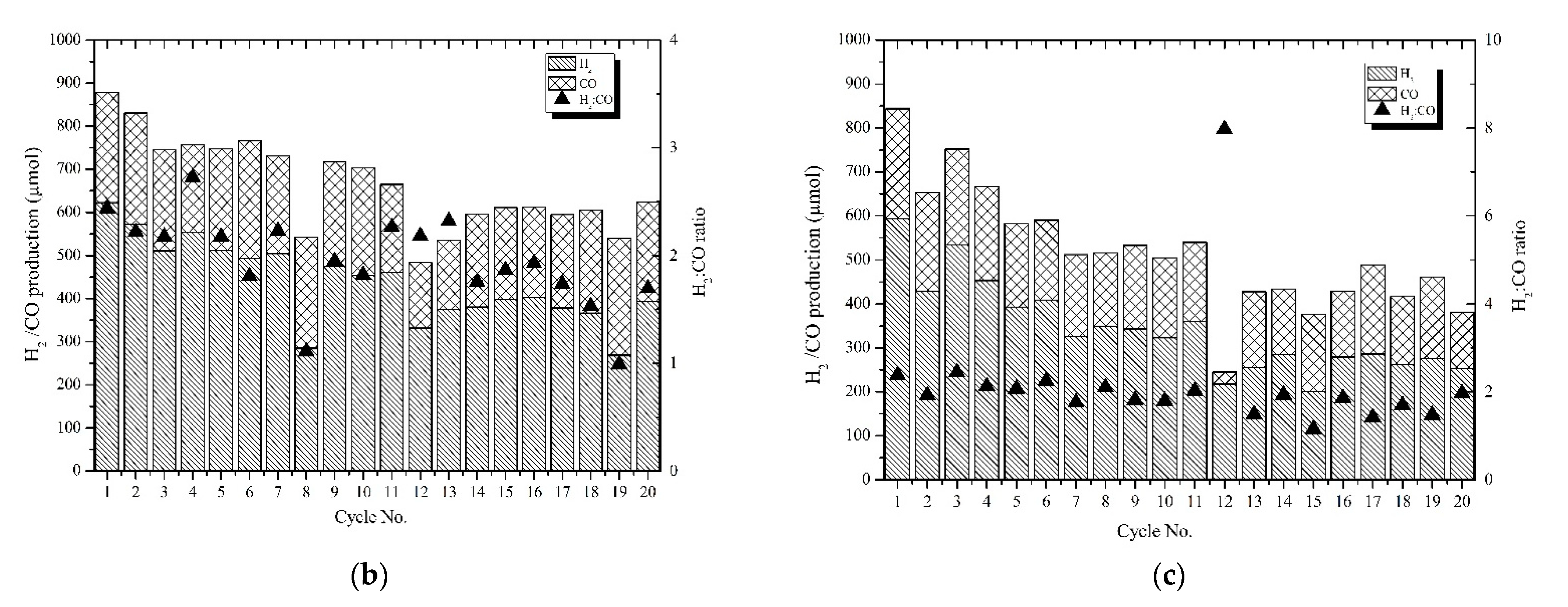
3.5. Efficiency of the Dual Reactor System under 10 h Operation
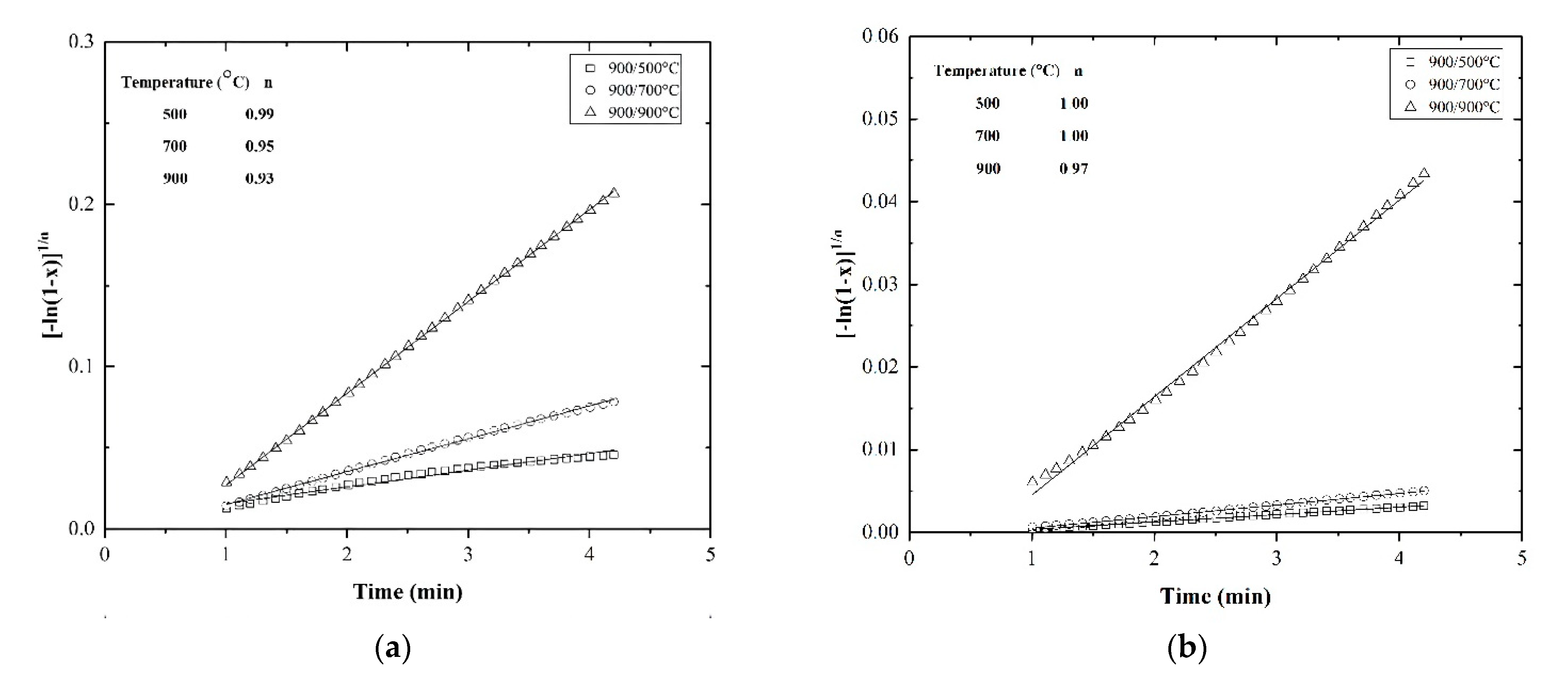
3.6. Kinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, C.S. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Olah, G.A.; Prakash Surya, G.K.; Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef]
- Fletcher, E.A. Solarthermal processing: A review. J. Sol. Energ. Eng. 2001, 123, 63–74. [Google Scholar] [CrossRef]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef]
- Le, G.A.; Abanades, S.; Flamant, G. CO2 and H2O splitting for thermochemical production of solar fuels using nonstoichiometric ceria and ceria/zirconia solid solutions. Energy Fuels 2011, 25, 4836–4845. [Google Scholar]
- Chueh, W.C.; Haile, S.M. A thermochemical study of ceria: Exploiting an old material for new modes of energy conversion and CO2 mitigation. Philos. Trans. R Soc. Lond. Ser. A 2010, 368, 3269–3294. [Google Scholar] [CrossRef] [PubMed]
- Abanades, S.; Chambon, M. CO2 dissociation and upgrading from two-step solar thermochemical processes based on ZnO/Zn and SnO2/SnO redox pairs. Energy Fuels 2010, 24, 6667–6674. [Google Scholar] [CrossRef]
- GáLvez, M.E.; Loutzenhiser, P.G.; Hischier, I.; Steinfeld, A. CO2 splitting via two-step solar thermochemical cycles with Zn/ZnO and FeO/Fe3O4 redox reactions: Thermodynamic analysis. Energy Fuels 2008, 22, 3544–3550. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; GáLvez, M.E.; Hischier, I.; Stamatiou, A.; Frei, A.; Steinfeld, A. CO2 splitting via two-step solar thermochemical cycles with Zn/ZnO and FeO/Fe3O4 redox reactions II: Kinetic analysis. Energy Fuels 2009, 23, 2832–2839. [Google Scholar] [CrossRef]
- Venstrom, L.J.; Davidson, J.H. Splitting water and carbon dioxide via the heterogeneous oxidation of zinc vapor: Thermodynamic considerations. ASME Conf. Proc. 2010, 2010, 79–88. [Google Scholar]
- Stamatiou, A.; Loutzenhiser, P.G.; Steinfeld, A. Syngas production from H2O and CO2 over Zn particles in a packed-bed reactor. AIChE J. 2012, 58, 625–631. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Elena, G.M.; Hischier, I.; Graf, A.; Steinfeld, A. CO2 splitting in an aerosol flow reactor via the two-step Zn/ZnO solar thermochemical cycle. Chem. Eng. Sci. 2010, 65, 1855–1864. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Meier, A.; Steinfeld, A. Review of the two-step H2O/CO2-splitting solar thermochemical cycle based on Zn/ZnO redox reactions. Materials 2010, 3, 4922–4938. [Google Scholar] [CrossRef]
- Stamatiou, A.; Loutzenhiser, P.G.; Steinfeld, A. Solar syngas production via H2O/CO2-splitting thermochemical cycles with Zn/ZnO and FeO/Fe3O4 redox reactions. Chem. Mater. 2009, 22, 851–859. [Google Scholar] [CrossRef]
- Khamhangdatepon, T.; Tongnan, V.; Hartley, M.; Sornchamni, T.; Siri-Nguan, N.; Laosiripojana, N.; Li, K.; Hartley, U.W. Mechanisms of synthesis gas production via thermochemical cycles over La0.3Sr0.7Co0.7Fe0.3O3. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Experimental screening of perovskite oxides as efficient redox materials for solar thermochemical CO2 conversion. Sustain. Energy Fuels 2018, 2, 843–854. [Google Scholar] [CrossRef]
- Wang, L.; Al-Mamun, M.; Liu, P.; Wang, Y.; Yang, H.G.; Zhao, H. Notable hydrogen production on LaxCa1−xCoO3 perovskites via two-step thermochemical water splitting. J. Mater. Sci. 2018, 53, 6796–6806. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Botas, J.Á.; Sanz, R.; Marugán, J. Perovskite materials for hydrogen production by thermochemical water splitting. Int. J. Hydrog. Energy 2016, 41, 19329–19338. [Google Scholar] [CrossRef]
- Wang, L.; Al-Mamun, M.; Zhong, Y.L.; Liu, P.; Wang, Y.; Yang, H.G.; Zhao, H. Enhanced thermochemical water splitting through formation of oxygen vacancy in La0.6Sr0.4BO3−δ (B = Cr, Mn, Fe, Co, and Ni) Perovskites. ChemPlusChem 2018, 83, 924–928. [Google Scholar] [CrossRef]
- Bork, A.H.; Kubicek, M.; Struzik, M.; Rupp, J.L.M. Perovskite La0.6Sr0.4Cr1−xCoxO3−δ solid solutions for solar-thermochemical fuel production: Strategies to lower the operation temperature. J. Mater. Chem. A 2015, 3, 15546–15557. [Google Scholar] [CrossRef]
- Demont, A.; Abanades, S.; Beche, E. Investigation of perovskite structures as oxygen-exchange redox materials for hydrogen production from thermochemical two-step water-splitting cycles. J. Phys. Chem. C 2014, 118, 12682–12692. [Google Scholar] [CrossRef]
- Sibieude, F.; Ducarroir, M.; Tofighi, A.; Ambriz, J. High temperature experiments with a solar furnace: The decomposition of Fe3O4, Mn3O4, CdO. Int. J. Hydrogen Energy 1982, 7, 79–88. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Flamant, G.; Lemort, F. Two-step water splitting thermochemical cycle based on iron oxide redox pair for solar hydrogen production. Energy 2007, 32, 1124–1133. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Gao, L.; Guo, L. Synthesis and characterization of doped LaCrO3 perovskite prepared by EDTA–citrate complexing method. J. Alloy. Compd. 2008, 458, 346–350. [Google Scholar] [CrossRef]



| Condition No. | H2O/CO2 Molar Ratio | H2O Molar Flow Rate (mmol/min) | CO2 Molar Flow Rate (mmol/min) | Total Flow Rate (mL/min) | Reduction Temperature (°C) | Oxidation Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | 1 to 1 | 3.89 | 3.89 | 200 | 500 | 500 |
| 2 | 1 to 1 | 3.89 | 3.89 | 200 | 700 | 700 |
| 3 | 1 to 1 | 3.89 | 3.89 | 200 | 900 | 900 |
| 4 | 1 to 1 | 3.89 | 3.89 | 200 | 500 | 900 |
| 5 | 1 to 1 | 3.89 | 3.89 | 200 | 700 | 900 |
| 6 | 1 to 1 | 3.89 | 3.89 | 200 | 900 | 500 |
| 7 | 1 to 1 | 3.89 | 3.89 | 200 | 900 | 700 |
| 8 | 3 to 1 | 3.89 | 1.30 | 200 | 900 | 900 |
| 9 | 1.5 to 1 | 3.89 | 2.59 | 200 | 900 | 900 |
| 10 | 1 to 2 | 3.89 | 7.79 | 200 | 900 | 900 |
| 11 | 1 to 2.5 | 3.89 | 9.72 | 250 | 900 | 900 |
| Condition No. | ∆δ |
|---|---|
| 1 | 0.04 |
| 2 | 0.20 |
| 3 | 0.25 |
| 4 | 0.13 |
| 5 | 0.17 |
| 6 | 0.03 |
| 7 | 0.20 |
| 8 | 0.43 |
| 9 | 0.29 |
| 10 | 0.22 |
| 11 | 0.20 |
| Condition No. | Reduction Time (min) | Oxidation Time (min) | Number of Cycles in 10 h | Productivities (μmol/g) |
|---|---|---|---|---|
| 1 | 15 | 15 | 20 | 23,640 |
| 2 | 30 | 60 | 7 | 25,200 |
| 3 | 30 | 30 | 10 | 28,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamhangdatepon, T.; Sornchamni, T.; Siri-Nguan, N.; Laosiripojana, N.; Hartley, U.W. A Dual Reactor for Isothermal Thermochemical Cycles of H2O/CO2 Co-Splitting Using La0.3Sr0.7Co0.7Fe0.3O3 as an Oxygen Carrier. Processes 2021, 9, 1018. https://doi.org/10.3390/pr9061018
Khamhangdatepon T, Sornchamni T, Siri-Nguan N, Laosiripojana N, Hartley UW. A Dual Reactor for Isothermal Thermochemical Cycles of H2O/CO2 Co-Splitting Using La0.3Sr0.7Co0.7Fe0.3O3 as an Oxygen Carrier. Processes. 2021; 9(6):1018. https://doi.org/10.3390/pr9061018
Chicago/Turabian StyleKhamhangdatepon, Tatiya, Thana Sornchamni, Nuchanart Siri-Nguan, Navadol Laosiripojana, and Unalome Wetwatana Hartley. 2021. "A Dual Reactor for Isothermal Thermochemical Cycles of H2O/CO2 Co-Splitting Using La0.3Sr0.7Co0.7Fe0.3O3 as an Oxygen Carrier" Processes 9, no. 6: 1018. https://doi.org/10.3390/pr9061018
APA StyleKhamhangdatepon, T., Sornchamni, T., Siri-Nguan, N., Laosiripojana, N., & Hartley, U. W. (2021). A Dual Reactor for Isothermal Thermochemical Cycles of H2O/CO2 Co-Splitting Using La0.3Sr0.7Co0.7Fe0.3O3 as an Oxygen Carrier. Processes, 9(6), 1018. https://doi.org/10.3390/pr9061018







