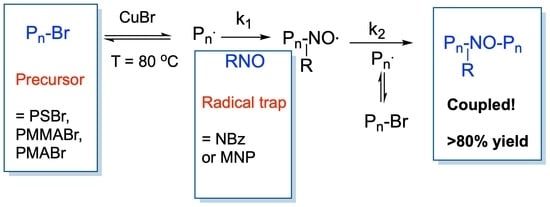
Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates
Abstract
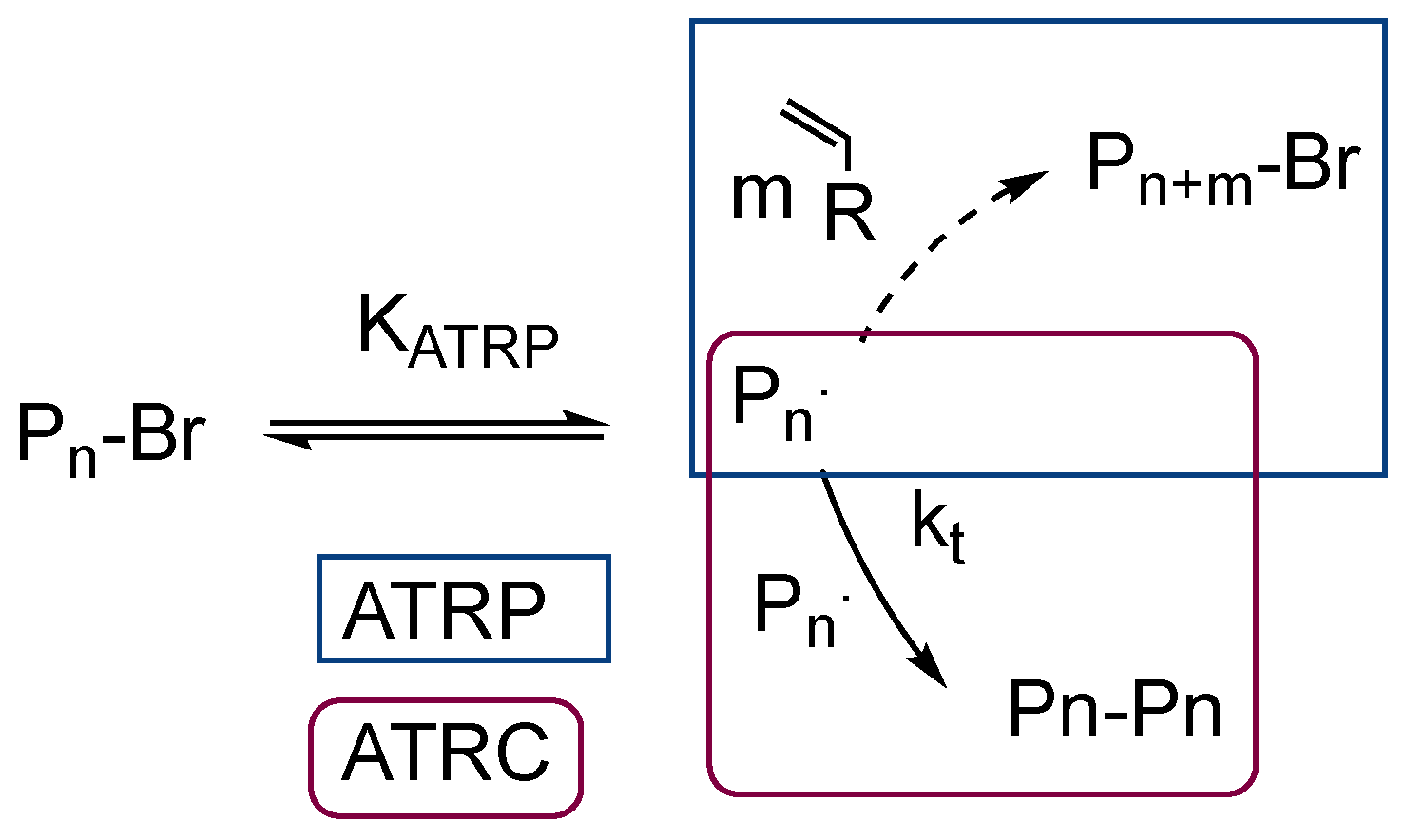
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Typical Procedure for the Synthesis of Monobrominated Polystyrene (PSBr) Using ATRP
2.3. Typical Procedure for RTA-ATRC of PSBr
2.4. Typical Procedure for Thermolysis of PSBr-RTA-ATRC
2.5. Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jenkins, A.D.; Jones, R.G.; Moad, G. Terminology for reversible-deactivation radical polymerization previously called ‘controlled’ radical or ‘living’ radical polymerization (IUPAC Recommendations 2010). Pure. Appl. Chem. 2009, 82, 483–491. [Google Scholar] [CrossRef]
- Shipp, D.A. Reversible-Deactivation Radical Polymerizations. Polym. Rev. 2011, 51, 99–103. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Stalmach, U.; de Boer, B.; Videlot, C.; van Hutten, P.F.; Hadziioannou, G. Semiconducting Diblock Copolymers Synthesized by Means of Controlled Radical Polymerization Techniques. J. Am. Chem. Soc. 2000, 122, 5464–5472. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.; Ell, J.; Siegwart, D.J.; Spanswick, J.; Matyjaszewski, K. Polystyrene with Designed Molecular Weight Distribution by Atom Transfer Radical Coupling. Macromolecules 2004, 37, 3120–3127. [Google Scholar] [CrossRef]
- Thakur, S.; Tillman, E.S. Efficient metal-free coupling of polystyrene chains using silane radical atom abstraction. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3488–3493. [Google Scholar] [CrossRef]
- Nottelet, B.; Lacroix-Desmazes, P.; Boutevin, B. Atom transfer radical coupling of polystyrene and poly(methyl acrylate) synthesized by reverse iodine transfer polymerization. Polymer 2007, 48, 50–57. [Google Scholar] [CrossRef]
- Huang, C.F.; Ohta, Y.; Yokoyama, A.; Yokozawa, T. Efficient Low-Temperature Atom Transfer Radical Coupling and Its Application to Synthesis of Well-Defined Symmetrical Polybenzamides. Macromolecules 2011, 44, 4140–4148. [Google Scholar] [CrossRef]
- Voter, A.F.; Tillman, E.S.; Findeis, P.M.; Radzinski, S.C. Synthesis of Macrocyclic Polymers Formed via Intramolecular Radical Trap-Assisted Atom Transfer Radical Coupling. ACS Macro Lett. 2012, 1, 1066–1070. [Google Scholar] [CrossRef]
- Butcher, W.E.; Radzinski, S.C.; Tillman, E.S. Selective formation of diblock copolymers using radical trap-assisted atom transfer radical coupling. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3619–3626. [Google Scholar] [CrossRef]
- Valente, C.J.; Schellenberger, A.M.; Tillman, E.S. Dimerization of Poly(methyl methacrylate) Chains Using Radical Trap-Assisted Atom Transfer Radical Coupling. Macromolecules 2014, 47, 2226–2232. [Google Scholar] [CrossRef]
- Blackburn, S.C.; Myers, K.D.; Tillman, E.S. Macrocyclic poly(methyl acrylate) and macrocyclic poly(methyl acrylate-block-styrene) synthesized by radical trap-assisted atom transfer radical coupling. Macromol. Chem. Phys. 2015, 68, 284–292. [Google Scholar] [CrossRef]
- Blackburn, S.C. Synthesis of Cyclic Polyacrylate Homopolymers and Diblock Copolymers via RTA-ATRC; Inducing Tacticity by Pi-Pi Stacking; Orthogonality of CuAAC & ATRC. Master’s Thesis, Bucknell University, Lewisburg, PA, USA, 2015. Available online: https://digitalcommons.bucknell.edu/masters_theses/151 (accessed on 14 February 2021).
- Whitfield, R.; Anastasaki, A.; Nikolaou, V.; Jones, G.R.; Engelis, N.G.; Discekici, E.H.; Fleischmann, C.; Willenbacher, J.; Hawker, C.J.; Haddleton, D.M. Universal Conditions for the Controlled Polymerization of Acrylates, Methacrylates, and Styrene via Cu(0)-RDRP. J. Am. Chem. Soc. 2017, 139, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Matyjaszewski, K. Effect of [PMDETA]/[Cu(I)] Ratio, Monomer, Solvent, Counterion, Ligand, and Alkyl Bromide on the Activation Rate Constants in Atom Transfer Radical Polymerization. Macromolecules 2003, 36, 1487–1493. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Braunecker, W.A.; Vacca, A.; Gans, P.; Matyjaszewski, K. Competitive Equilibria in Atom Transfer Radical Polymerization. Macromol. Symp. 2007, 248, 60–70. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.; Spanswick, J.; Gil, R.R.; Siegwart, D.J.; Matyjaszewski, K. Synthesis of Hydroxy-Telechelic Poly(methyl acrylate) and Polystyrene by Atom Transfer Radical Coupling. Macromolecules 2004, 37, 9694–9700. [Google Scholar] [CrossRef]
- Xia, K.; Rubaie, A.; Johnson, B.; Tillman, E.S. ‘Greener’ Coupling of Poly(methyl methacrylate) and Poly(methyl acrylate) Chains using Activators Generated by Electron Transfer and Radical Traps. Macromol. Chem. Phys. 2020, 221, 2000125. [Google Scholar] [CrossRef]
- Arce, M.M.; Pan, C.W.; Thursby, M.M.; Wu, J.P.; Carnicom, E.M.; Tillman, E.S. Influence of Solvent on Radical Trap Assisted Dimerization and Cyclization of Polystyrene Radicals. Macromolecules 2016, 49, 7804–7813. [Google Scholar] [CrossRef]
- Domingues, K.M.; Tillman, E.S. Radical-radical coupling of polystyrene chains using AGET ATRC. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5737–5745. [Google Scholar] [CrossRef]
- Xia, K.; Rubaie, A.J.; Johnson, B.P.; Parker, S.A.; Tillman, E.S. Atom Transfer Coupling Reactions Performed with Benign Reducing Agents and Radical Traps. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2113–2120. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Abruzzese, J.A.; Sidibe, Y.; Myers, K.D.; Tillman, E.S. Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling. Polymers 2014, 6, 2737–2751. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Coyne, W.E.; Myers, K.D.; Tillman, E.S. One pot, two step sequence converting atom transfer radical polymerization directly to radical trap-assisted atom transfer radical coupling. Polymer 2013, 54, 5560–5567. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Tillman, E.S. Polymerization of Styrene and Cyclization to Macrocyclic Polystyrene in a One-pot, Two-Step Sequence. React. Funct. Polym. 2014, 80, 9–14. [Google Scholar] [CrossRef]
- Wu, J.P.; Pan, C.W.; Heiler, K.E.; Ching, M.E.; Tillman, E.S. Altering the Effectiveness of Radical Traps in Atom Transfer Radical Coupling Reactions of Polymer Chains. Polymer 2017, 127, 66–76. [Google Scholar] [CrossRef]
- Jiang, X.; Vamvakaki, M.; Narain, R. Copper-Catalyzed Bimolecular Coupling of α,ω-Dibromide-Functionalized Poly(γ-caprolactone). Macromolecules 2010, 43, 3228–3232. [Google Scholar] [CrossRef]
- Sasaki, D.; Suzuki, Y.; Hagiwara, T.; Yano, S.; Sawaguchi, T. Synthesis and applications of triblock and multiblock copolymers using telechelic oligopropylene. Polymer 2008, 49, 4094–4100. [Google Scholar] [CrossRef]





| Trial | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) d | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| 1 | PBN a | 40 | 5:5:10:0.6 | 1570 | 1.08 | 1580 | <0.1 |
| 2 | NBz b | 40 | 5:5:10:0.6 | 1570 | 1.08 | 1680 | 0.13 |
| 3 | NBz | 40 | 2.5:4:8:0.6 | 2060 | 1.08 | 2700 | 0.47 |
| 4 | NBz | 40 | 2.5:4:8:1 | 2900 | 1.1 | 4000 | 0.55 |
| 5 | NBz | 80 | 2.5:4:8:1 | 3520 | 1.06 | 4860 | 0.55 |
| 6 | NBz | 80 | 2.5:4:8:0.6 | 2900 | 1.1 | 4140 | 0.60 |
| 7 | NBz | 80 | 2.5:4:8:1 | 7740 | 1.07 | 11,400 | 0.64 |
| 8 | MNP c | 40 | 2.5:4:8:0.6 | 2050 | 1.17 | 2550 | 0.39 |
| 9 | MNP | 40 | 5:5:10:0.6 | 7670 | 1.19 | 9590 | 0.40 |
| 10 | MNP | 80 | 2.5:4:8:0.6 | 4710 | 1.07 | 7990 | 0.82 |
| 11 | MNP | 80 | 2.5:4:8:1 | 1420 | 1.06 | 2710 | 0.96 |
| Trial | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) d | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| 12 | PBN a | 40 | 5:5:10:0.6 | 4370 | 1.18 | 5260 | 0.34 |
| 13 | NBz b | 40 | 2.5:4:8:0.6 | 4370 | 1.18 | 6920 | 0.74 |
| 14 | NBz | 40 | 5:5:10:0.6 | 4370 | 1.18 | 7030 | 0.76 |
| 15 | NBz | 80 | 2.5:4:8:0.6 | 2870 | 1.16 | 5360 | 0.93 |
| 16 | NBz | 80 | 2.5:4:8:1 | 2870 | 1.16 | 5070 | 0.87 |
| 17 | MNP c | 80 | 2.5:4:8:1 | 2870 | 1.16 | 3170 | 0.19 |
| Trial | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) d | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| 18 | PBN a | 40 | 5:5:10:0.6 | 2750 | 1.11 | 3000 | 0.17 |
| 19 | NBz b | 40 | 2.5:4:8:0.6 | 2710 | 1.09 | 4310 | 0.74 |
| 20 | NBz | 40 | 5:5:10:0.6 | 2750 | 1.11 | 4500 | 0.78 |
| 21 | NBz | 80 | 2.5:4:8:0.6 | 3000 | 1.01 | 4460 | 0.65 |
| 22 | NBz | 80 | 2.5:4:8:1 | 2170 | 1.08 | 3400 | 0.72 |
| 23 | MNP c | 40 | 2.5:4:8:0.6 | 2780 | 1.11 | 4730 | 0.82 |
| 24 | MNP | 40 | 5:5:10:0.6 | 2260 | 1.08 | 4080 | 0.90 |
| 25 | MNP | 80 | 2.5:4:8:1 | 3450 | 1.08 | 5950 | 0.84 |
| Polymer | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) e | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
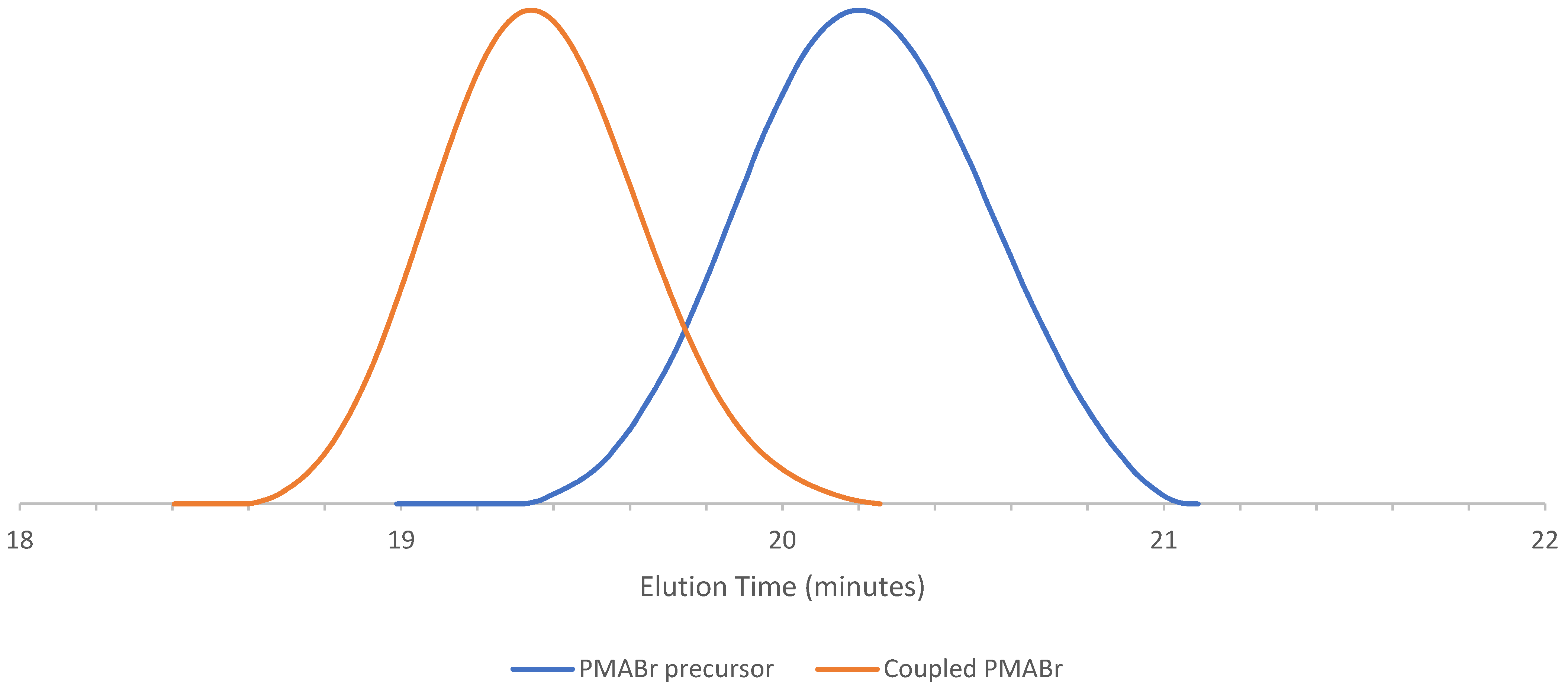
| PMABr c | MNP a | 80 | 2.5:4:8:1 | 1420 | 1.06 | 2710 | 0.96 |
| PMMABr c | NBz b | 80 | 2.5:4:8:1 | 2870 | 1.16 | 5070 | 0.87 |
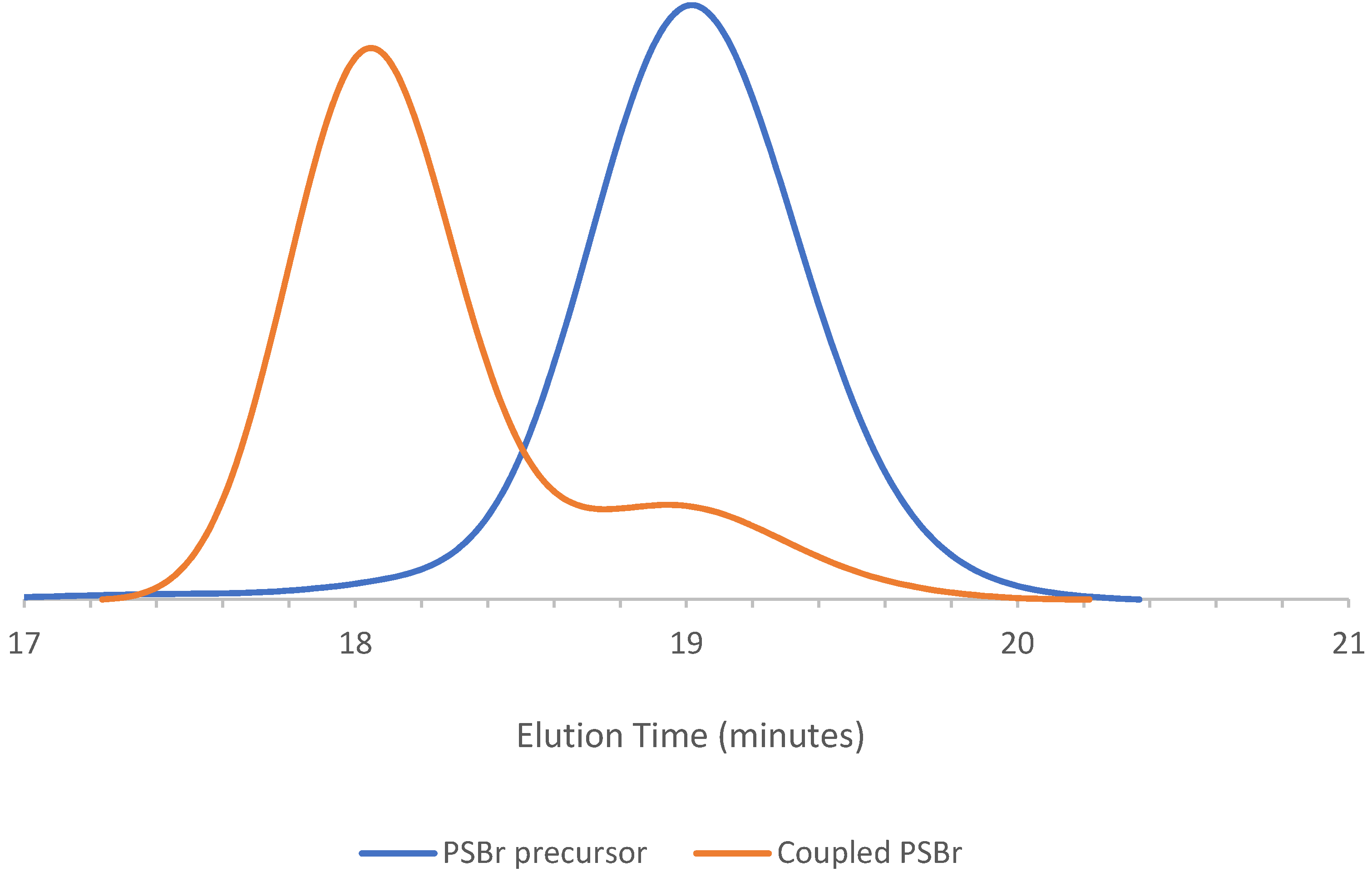
| PSBr d | MNP | 80 | 2.5:4:8:1 | 3450 | 1.08 | 5950 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andry, J.J.; Lee, J.J.; Wu, J.; Xia, K.; Tillman, E.S. Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates. Processes 2021, 9, 1001. https://doi.org/10.3390/pr9061001
Andry JJ, Lee JJ, Wu J, Xia K, Tillman ES. Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates. Processes. 2021; 9(6):1001. https://doi.org/10.3390/pr9061001
Chicago/Turabian StyleAndry, Joseph J., Jaenic J. Lee, Jessica Wu, Katherine Xia, and Eric S. Tillman. 2021. "Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates" Processes 9, no. 6: 1001. https://doi.org/10.3390/pr9061001
APA StyleAndry, J. J., Lee, J. J., Wu, J., Xia, K., & Tillman, E. S. (2021). Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates. Processes, 9(6), 1001. https://doi.org/10.3390/pr9061001