A Critical Review on the Development of Ionic Liquids-Based Nanofluids as Heat Transfer Fluids for Solar Thermal Energy
Abstract
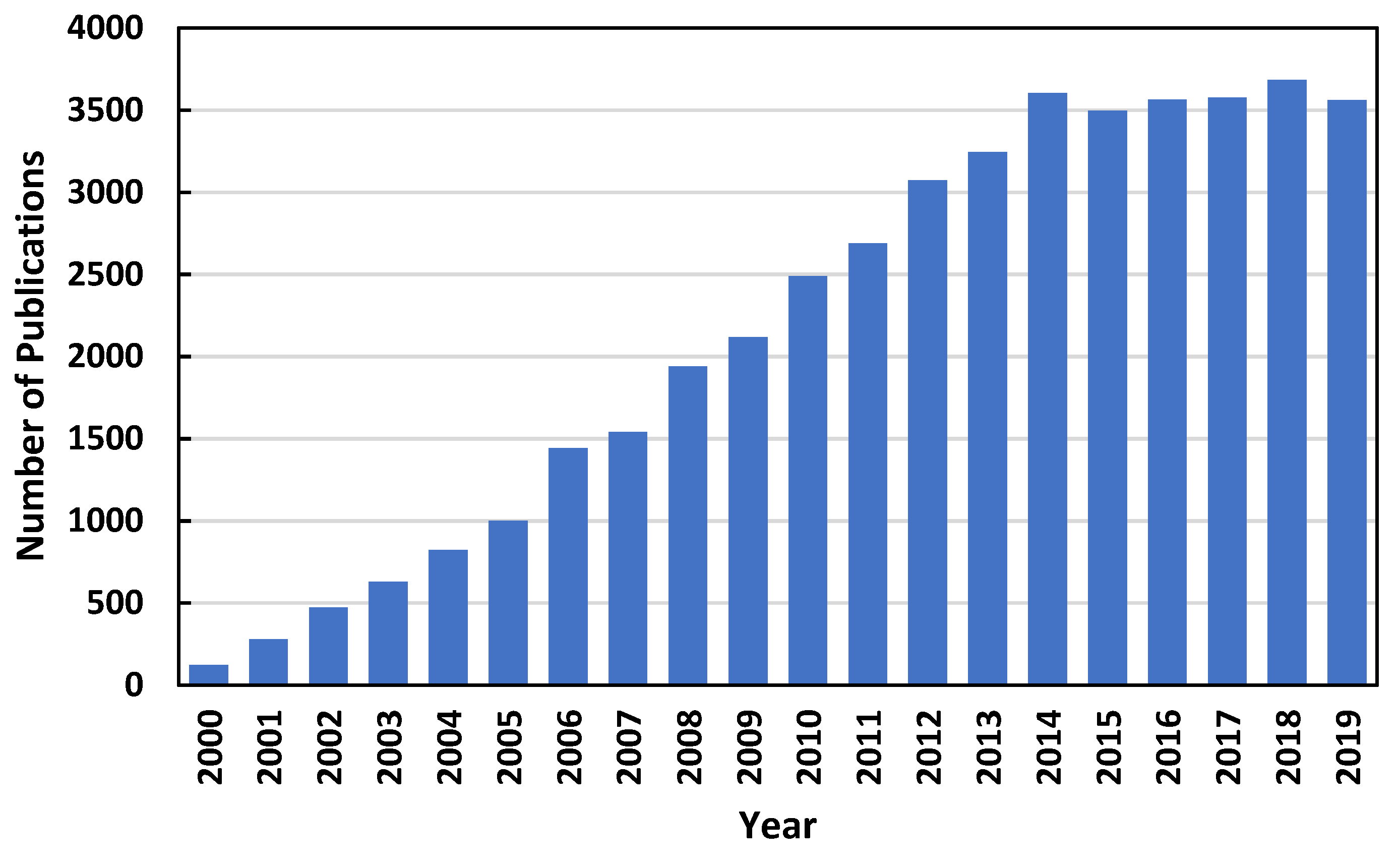
1. Introduction
2. Synthesis and Thermophysical Property Characterizations of ILs-Based Nanofluids
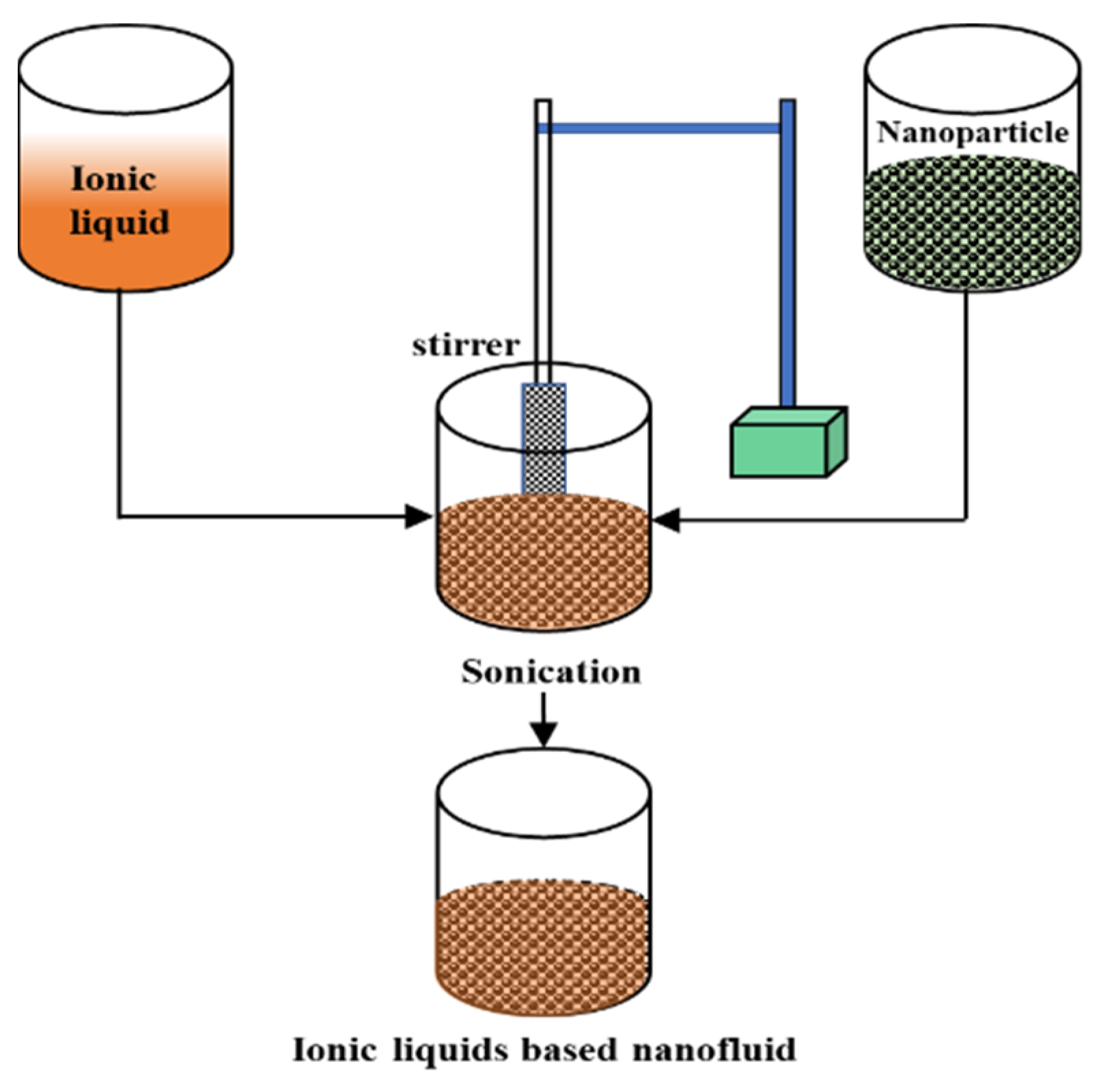
2.1. State-of-the-Art Synthesis Technique
2.2. Experimental Measurements, and Theoretical and Empirical Correlation
3. Literature Survey of Thermophysical Properties of ILs-Based Nanofluids
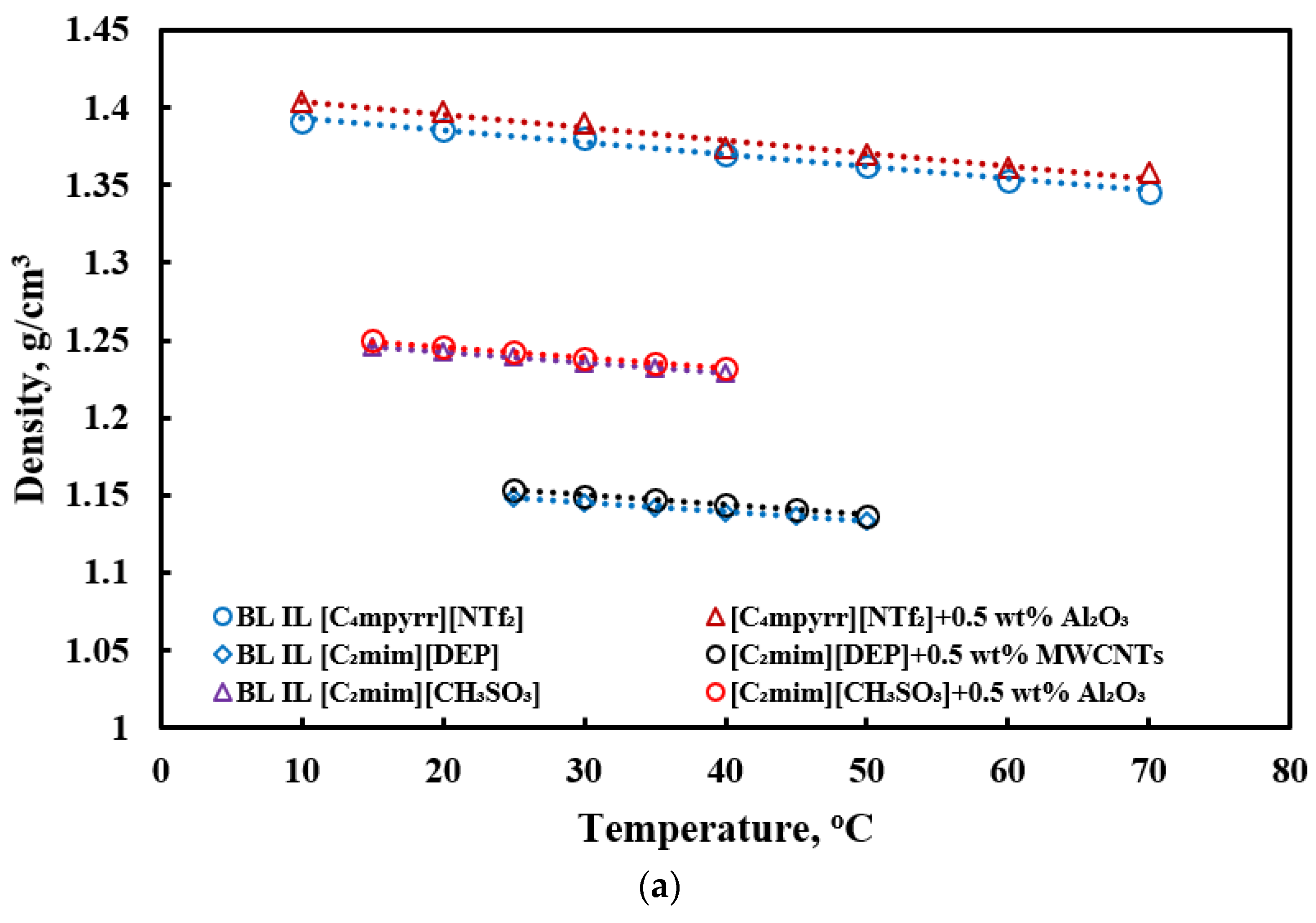
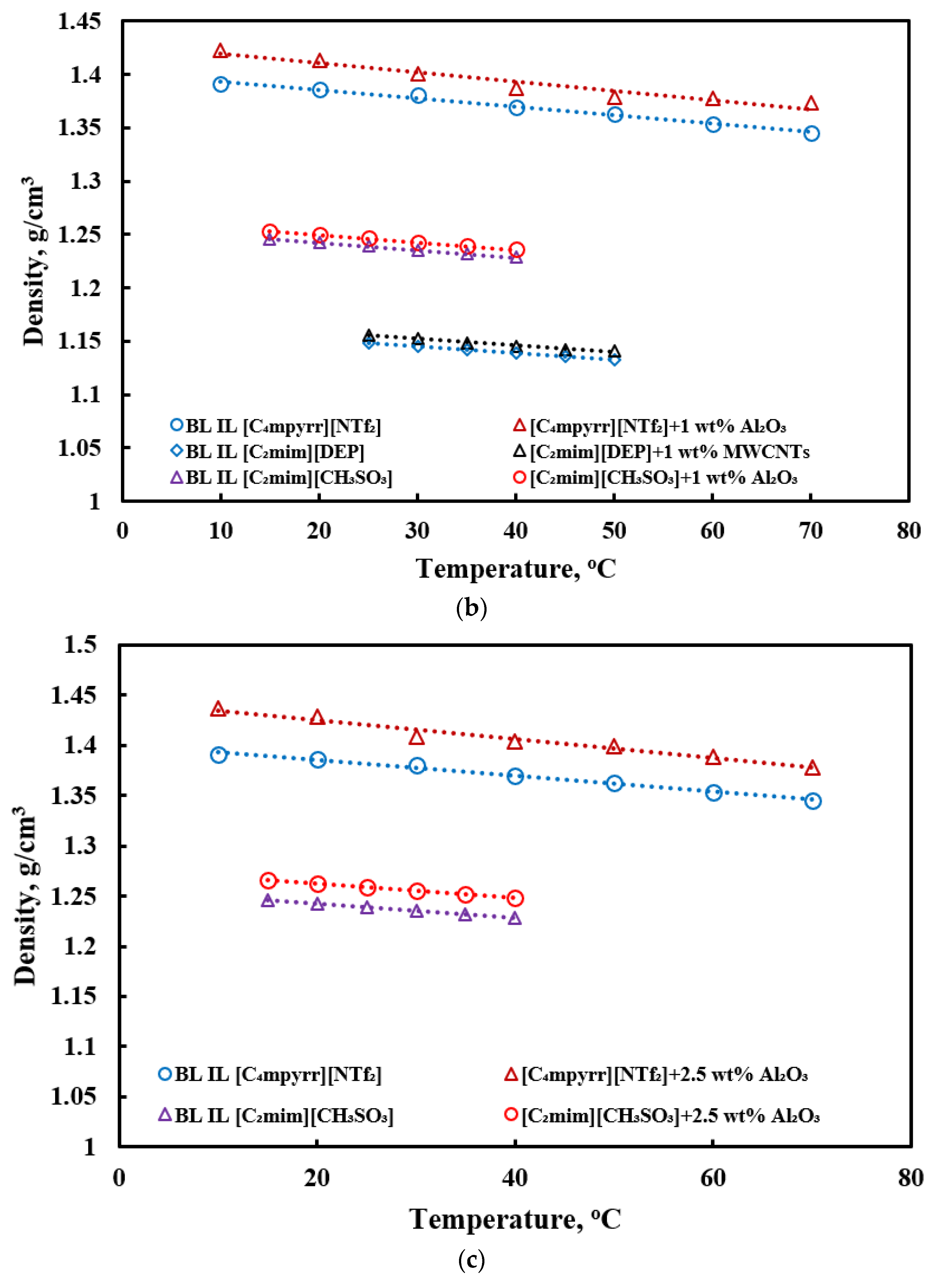
3.1. Density
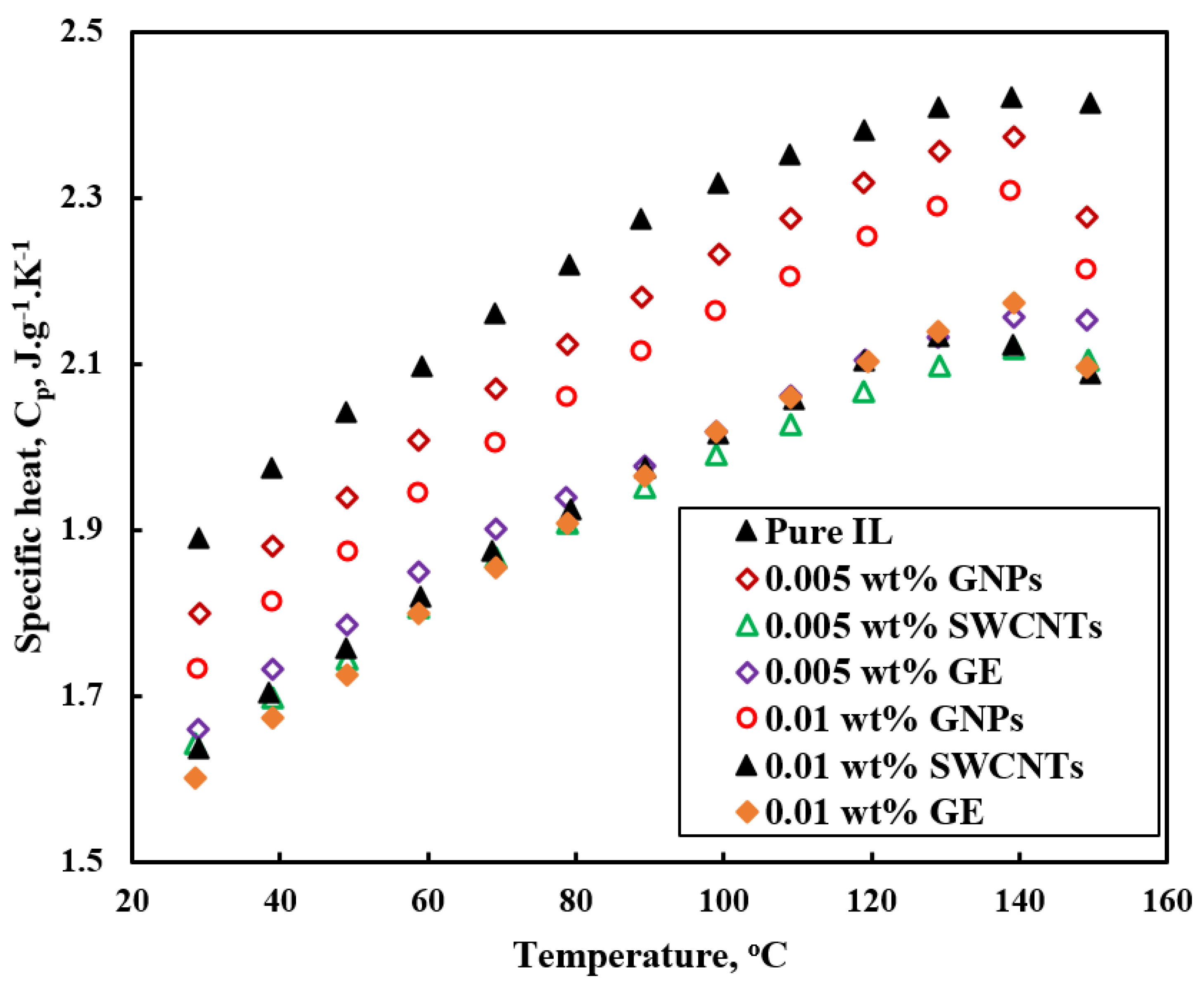
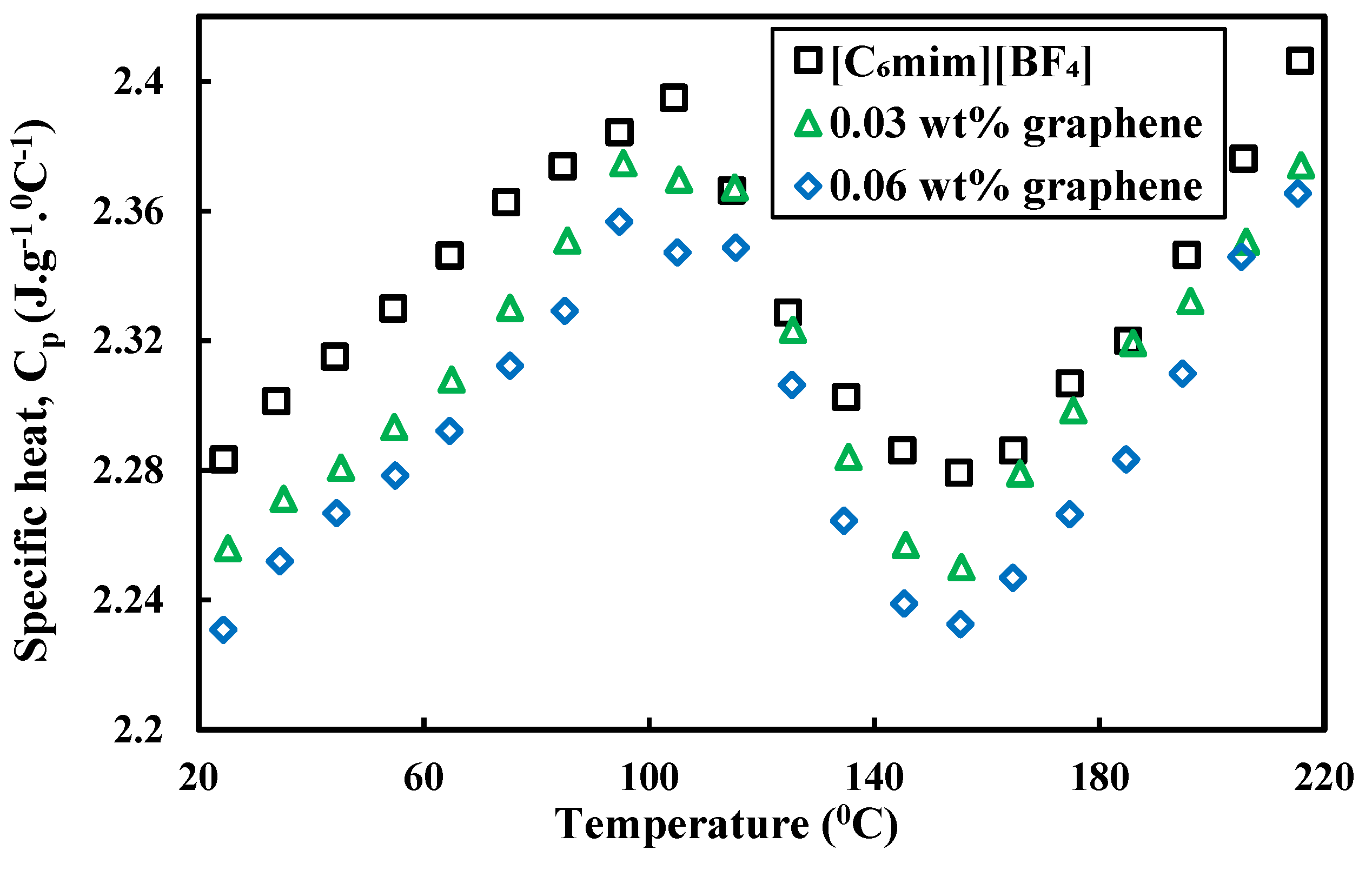
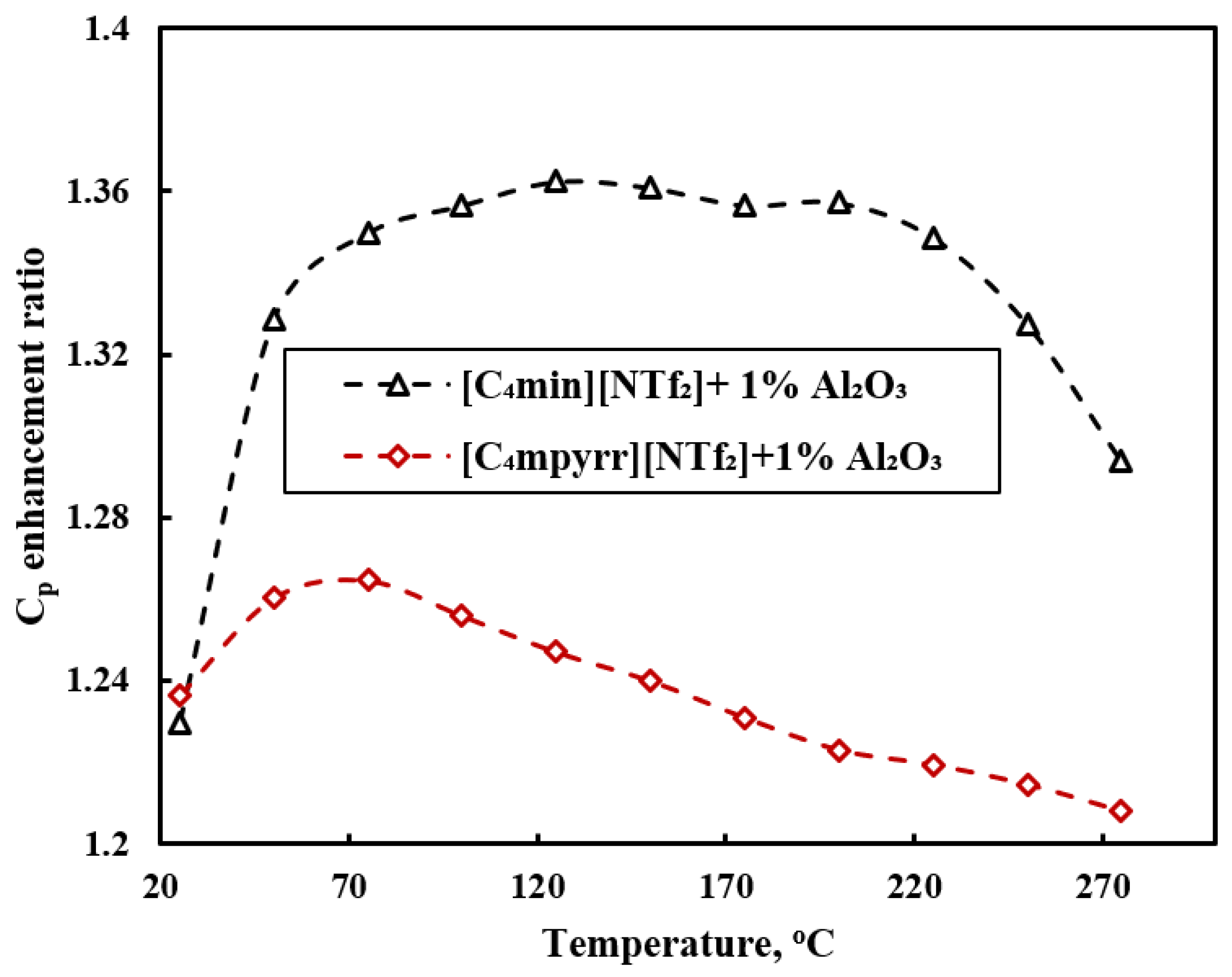
3.2. Specific Heat
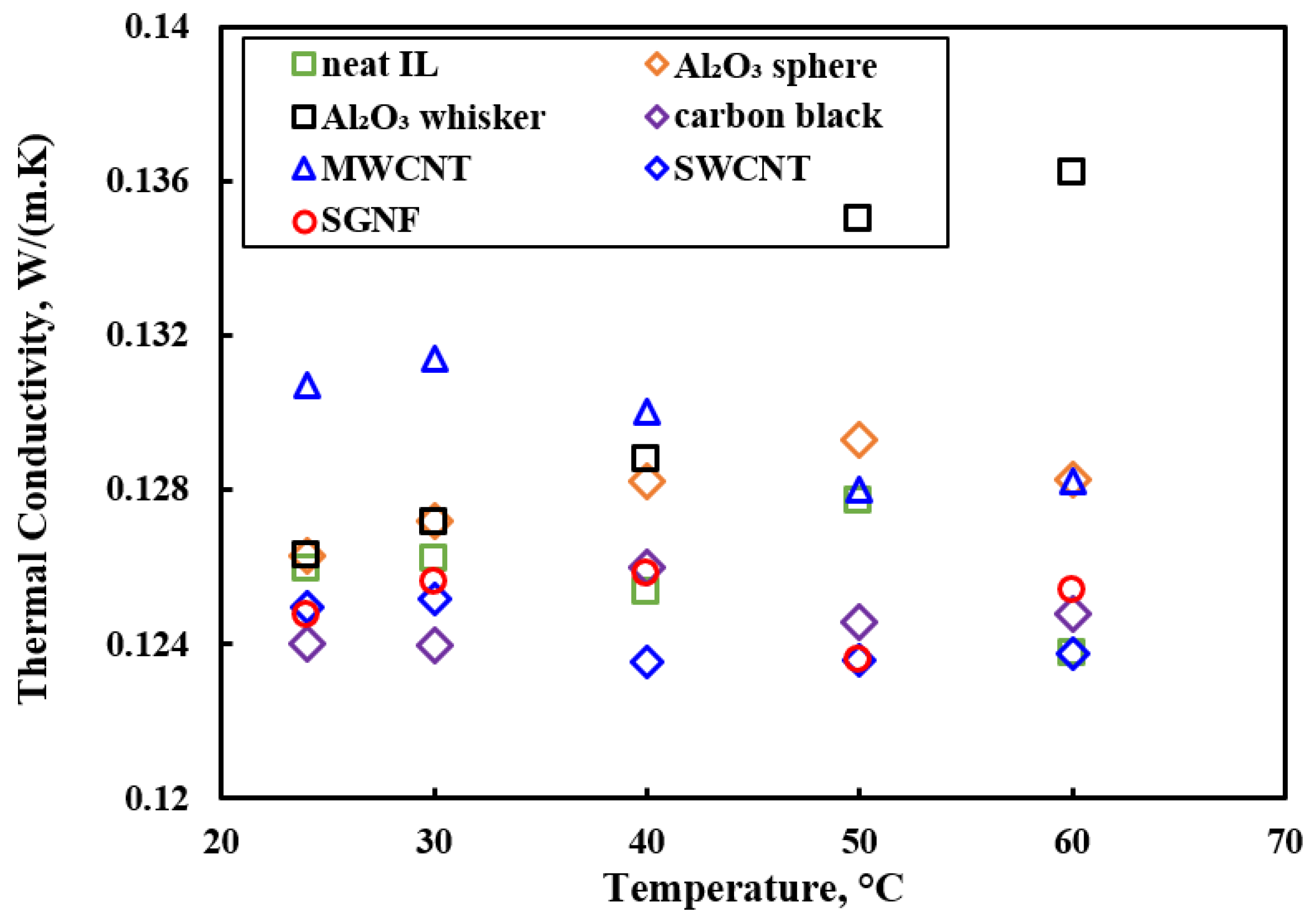
3.3. Thermal Conductivity
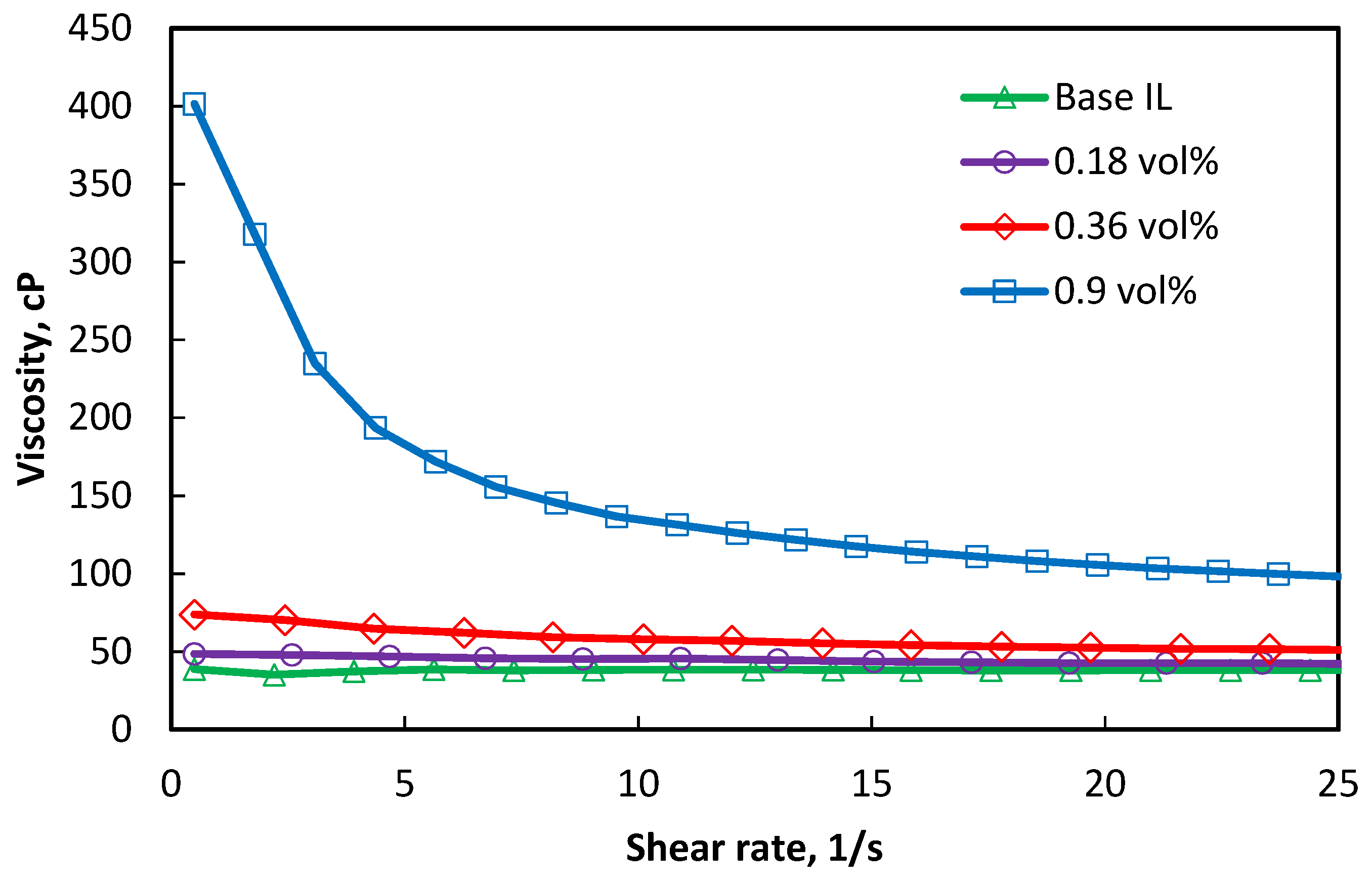
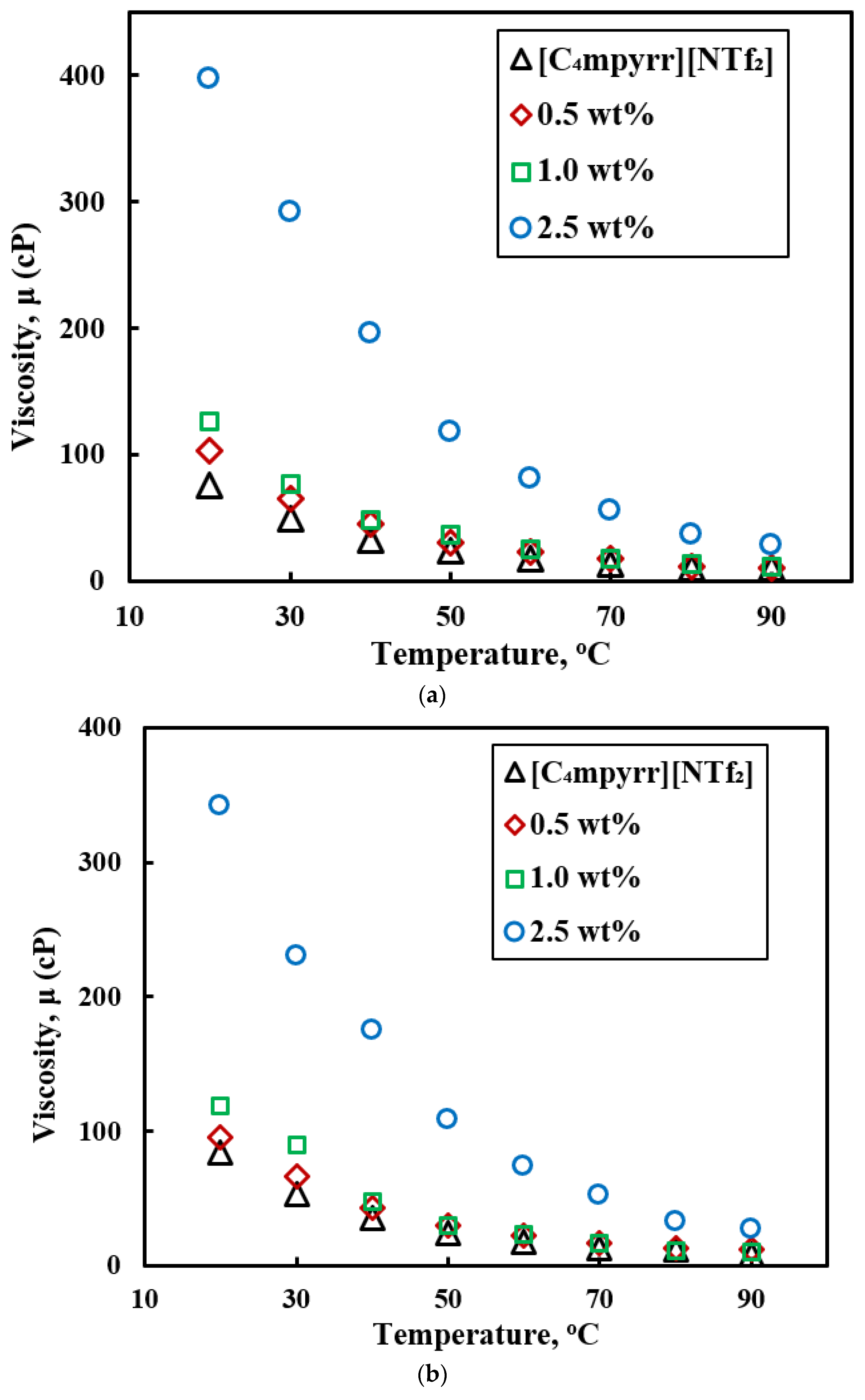
3.4. Viscosity.
4. Overall Comparison of Thermophysical Properties
5. Thermal Performance of Ionic Liquids-Based Nanofluids
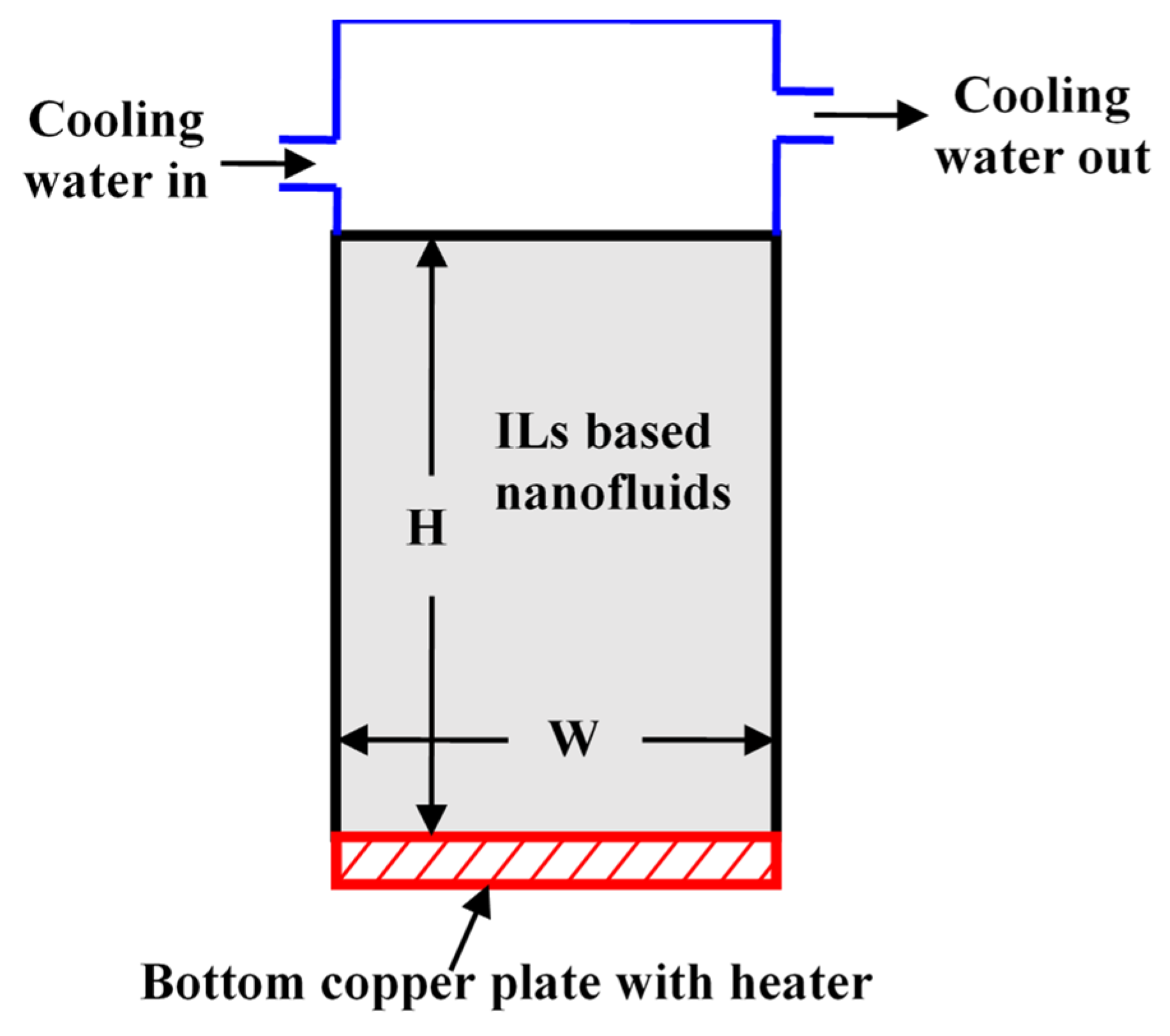
5.1. Natural Convection
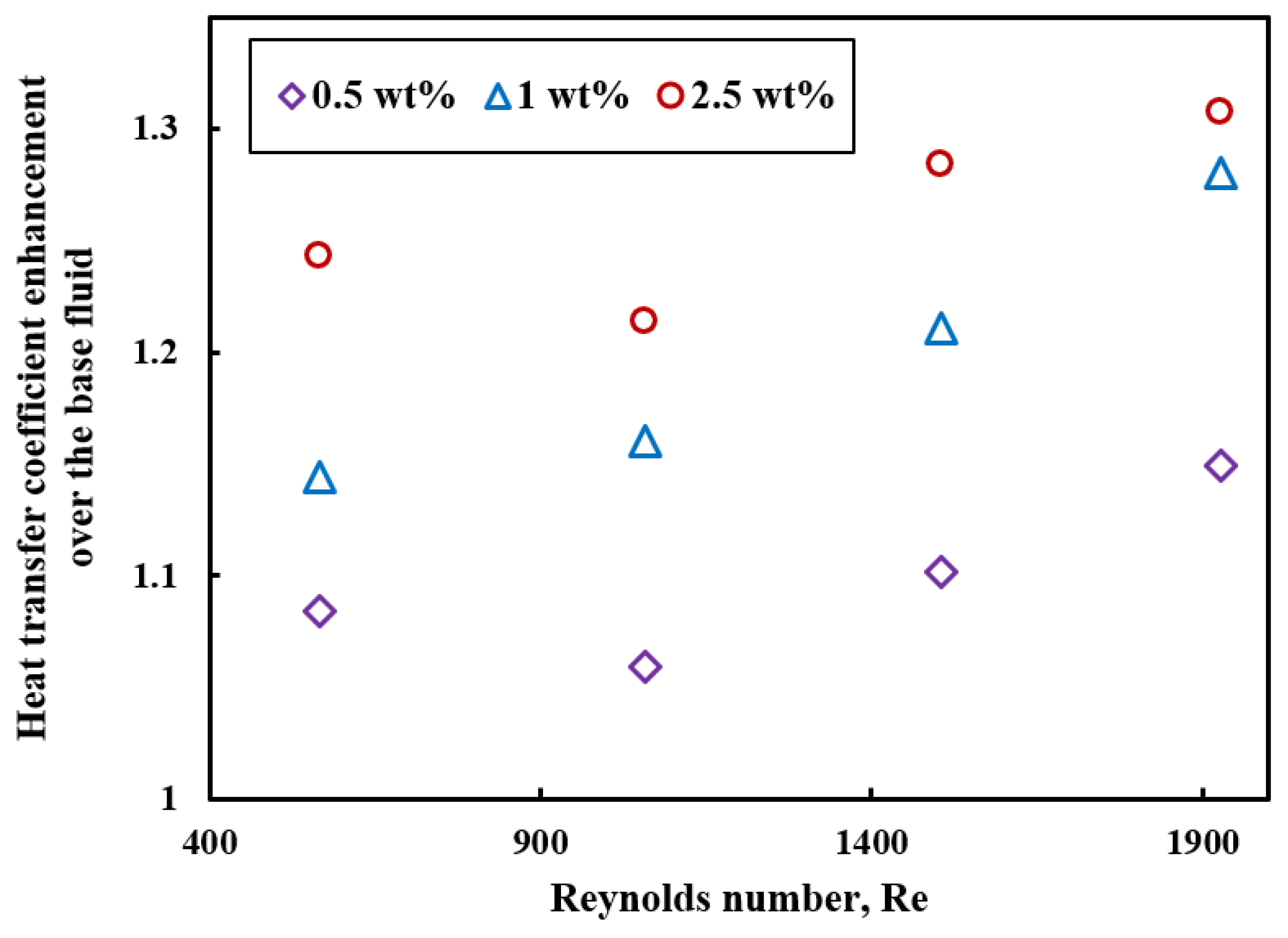
5.2. Forced Convection
6. Conclusions and Recommendations
- Only limited investigations of densities of ILs-based nanofluids exist. The density of ILs-based nanofluids increases compared to base ILs, as it adds highly denser nanoparticles in the base fluid. The density slightly decreases with an increase in temperature.
- Specific heat of ILs-based nanofluids shows scattered behavior with different nanoparticles. Graphene and single walled carbon nanotubes (SWCNTs)-based nanofluids show a lower specific heat compared to the base ILs. Multiwalled carbon nanotubes (MWCNTs)-based nanofluids show detraction and enhancement in heat capacity. All of the SiC and Al2O3 nanoparticles-based nanofluids studies reported enhancement in heat capacity compared to base ILs. Enhancement in specific heat was explained by the clustering of nanoparticles and interfacial layer of ILs into nanoparticles. However, there is no clear explanation for the scattered behavior of specific heat of ILs-based nanofluids.
- Extensive experimental investigation of the heat capacity of ILs-based nanofluids is required to better understand the detraction or enhancement in behavior of specific heat, since specific heat is one of the most important thermophysical properties for any heat storage medium.
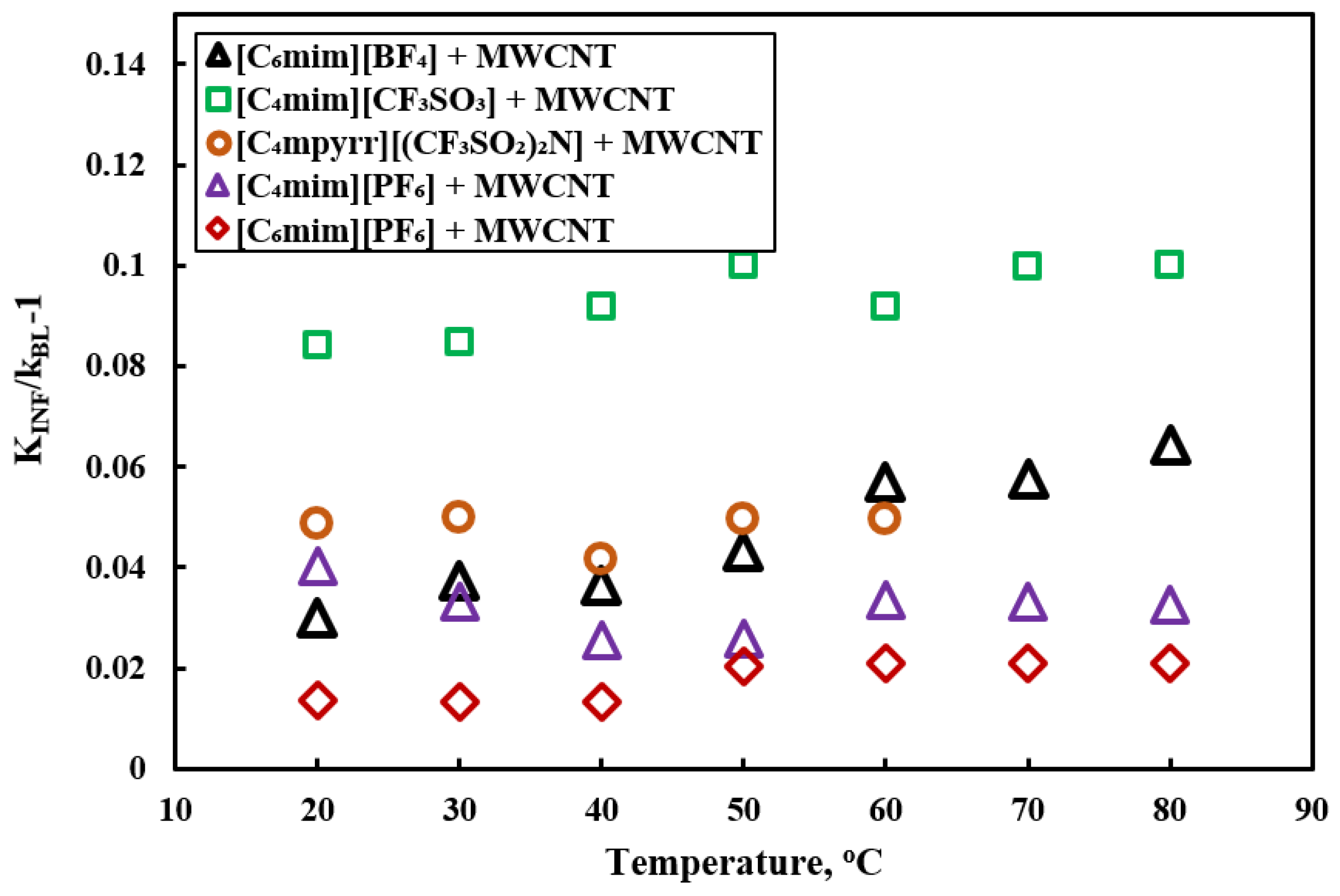
- All of the studies showed enhancement in the thermal conductivity of ILs-based nanofluids compared to base ILs, and thermal conductivity increased with nanoparticle concentrations. Enhancement in thermal conductivity was explained by the interfacial layer of base ILs into the nanoparticles, and interaction between ions and nanoparticles. However, extensive studies are required to explain the phenomenon of enhanced thermal conductivity of ILs-based nanofluids.
- All of the studies report shear thinning behavior of ILs-based nanofluids. Graphene and multiwalled carbon nanotubes (MWCNTs) show lower viscosity of ILs-based nanofluids compared to the base ILs. SiC and Al2O3 nanoparticles shows much higher viscosity of nanofluids compared to the base fluid and viscosity increases with nanoparticles concentrations. Although ILs-based nanofluids show enhanced viscosity compared to the based fluid, at high temperatures (≥300 °C) the viscosity of base ILs and ILs-based nanofluids are very low, which is beneficial as a heat transfer fluid. Enhancement in viscosity can be explained by clustering and agglomeration of nanoparticles in the ILs-based nanofluids, and those were evident with optical microscopic image and dynamic light scattering (DLS).
- Natural convection (bottom plate heated condition) of ILs-based nanofluids shows lower heat transfer performance compared to the base ILs, because of higher viscosity and possible sedimentation of nanoparticles. However, for sidewise heating, lower concentrations of ILs-based nanofluids show a higher heat transfer coefficient compared to water-based nanofluids.
- ILs-based nanofluids show an enhanced heat transfer coefficient in forced convection in experimental and numerical studies, and heat transfer coefficient increases with nanoparticles concentrations. One possible reason for the enhanced thermal performance of ILs-based nanofluids is high thermal conductivity, nanoparticles migration, and interactions between ion and nanoparticles. However, rigorous experimental and numerical studies are needed to explain the exact mechanism of heat transfer coefficient enhancement in ILs-based nanofluids.
7. Future Directions
- Experimental measurements, empirical correlations, and conventional numerical techniques are often insufficient to explain the effect of nanoparticles on base ionic liquids. Therefore, first-principle methods, such as density functional theory (DFT) or molecular dynamics (MD) simulation tools, can be used to accurately characterize the effect of nanoparticles size, shape, and concentration on ILs-based nanofluids properties.
- Machine learning based artificial intelligence can be utilized to characterize thermophysical properties and the thermal performance of new ILs-based nanofluids with minimal computational cost. However, machine learning tools need to be chosen wisely based on the available training data and computational resources. Molecular dynamics simulation results can be fed as training and validation data set in the machine learning/deep learning model.
- Thermophysical properties and thermal performance of ILs-based nanofluids need to be characterized at the appropriate operating conditions, i.e., high temperature of a solar thermal power plant.
- Overall thermal performance of ILs-based nanofluids need to be experimentally benchmarked for full-scale solar thermal power systems considering different geographical locations around the globe.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Thermal conductivity | [W/m·K] | |
| Heat capacity | [J/g·K] | |
| Fractal index | [~] | |
| Interfacial layer thickness | [nm] | |
| Average radius | [nm] | |
| Nusselt number | [~] | |
| Rayleigh number | [~] | |
| Prandtl number | [~] | |
| diameter | [m] | |
| x | axial distance | [m] |
| Greek Symbols | ||
| , | Constant | [~] |
| Density | [kg/m3] | |
| Dynamic viscosity | [centipoise] | |
| Nanoparticle volume fraction | [~] | |
| Effective volume fraction of aggregates | [~] | |
| Subscripts | ||
| Ionic liquids-based nanofluids | ||
| Base liquid | ||
| n | Nanoparticle | |
| Aggregate | ||
| Interfacial layer | ||
| MWCNTs | Multiwalled carbon nanotubes | |
| SWCNTs | Singlewalled carbon nanotubes | |
References
- Timilsina, G.R.; Kurdgelashvili, L.; Narbel, P.A. Solar energy: Markets, economics and policies. Renew. Sustain. Energy Rev. 2012, 16, 449–465. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Moens, L.; Blake, D.M.; Rudnicki, D.L.; Hale, M.J. Advanced Thermal Storage Fluids for Solar Parabolic trough Systems. J. Sol. Energy Eng. 2003, 125, 112. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Kubisa, P. Application of ionic liquids as solvents for polymerization processes. Prog. Polym. Sci. 2004, 29, 3–12. [Google Scholar] [CrossRef]
- Wishart, J.F. Energy applications of ionic liquids. Energy Environ. Sci. 2009, 2, 956–961. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, K.; Katyal, A.; Kalra, R.; Chandra, R. Copper nanoparticles in ionic liquid: An easy and efficient catalyst for selective carba-Michael addition reaction. Catal. Lett. 2009, 127, 119–125. [Google Scholar] [CrossRef]
- Reddy, R.G. Novel applications of ionic liquids in materials processing. J. Phys. Conf. Ser. 2009, 165, 012076. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium-steel contact. Part 3. Ti6Al4V lubricated with imidazolium ionic liquids with different alkyl chain lengths. Tribol. Lett. 2010, 40, 237–246. [Google Scholar] [CrossRef]
- Web of Sciences. Available online: https://apps.webofknowledge.com/WOS_GeneralSearch_input.do?product=WOS&search_mode=GeneralSearch&SID=2DlU9uF2bR6AJmsAz4a&preferencesSaved= (accessed on 11 June 2020).
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357–358, 97–102. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Masaki, N.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Dye-sensitized TiO2 solar cells using imidazolium-type ionic liquid crystal systems as effective electrolytes. J. Phys. Chem. B 2007, 111, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; Zimlich, T.K.; Mirjafari, A.; O’Brien, R.A.; Davis, J.H.; West, K.N. Thermophysical properties of imidazolium-based lipidic ionic liquids. J. Chem. Eng. Data 2013, 58, 1516–1522. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Veiga, H.I.; Esperança, J.M.; Rodríguez, A. Effect of temperature on the physical properties of two ionic liquids. J. Chem. Thermodyn. 2009, 41, 1419–1423. [Google Scholar] [CrossRef]
- Ge, R.; Hardacre, C.; Nancarrow, P.; Rooney, D.W. Thermal conductivities of ionic liquids over the temperature range from 293 K to 353 K. J. Chem. Eng. Data 2007, 53, 1819–1823. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Buoyancy driven heat transfer behavior of [C4mim][NTf2] ionic liquid: An experimental study. Appl. Therm. Eng. 2014, 66, 534–540. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Thermal performance of ionic liquids for solar thermal applications. Exp. Therm. Fluid Sci. 2014, 59, 88–95. [Google Scholar] [CrossRef]
- Shah, R.K. Thermal entry length solutions for the circular tube and parallel plates. In Proceedings of the 3rd National Heat and Mass Transfer Conference, Bombay, India, 11–13 December 1975; Indian Institute of Technology: Bombay, India, 1975; Volume 1. Paper No. HMT-11-75. [Google Scholar]
- Gnielinski, V. New equations for heat and mass transfer in the turbulent flow in pipes and channels. Int. J. Chem. Eng. 1976, 16, 359–368. [Google Scholar]
- Chen, H.; He, Y.; Zhu, J.; Alias, H.; Ding, Y.; Nancarrow, P.; Hardacre, C.; Rooney, D.; Tan, C. Rheological and heat transfer behaviour of the ionic liquid, [C4mim][NTf2]. Int. J. Heat Fluid Flow 2008, 29, 149–155. [Google Scholar] [CrossRef]
- He, G.D.; Fang, X.M.; Xu, T.; Zhang, Z.G.; Gao, X.N. Forced convective heat transfer and flow characteristics of ionic liquid as a new heat transfer fluid inside smooth and microfin tubes. Int. J. Heat Mass Transf. 2015, 91, 170–177. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Zhang, Y.; Li, B.; Sundén, B. Thermophysical properties and convection heat transfer behavior of ionic liquid [C4mim][NTf2] at medium temperature in helically corrugated tubes. Appl. Therm. Eng. 2018, 142, 457–465. [Google Scholar] [CrossRef]
- Wadekar, V.V. Ionic liquids as heat transfer fluids—An assessment using industrial exchanger geometries. Appl. Therm. Eng. 2017, 111, 1581–1587. [Google Scholar] [CrossRef]
- Wong, K.V.; De Leon, O. Applications of Nanofluids: Current and Future. Adv. Mech. Eng. 2010, 2, 1–12. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Mahian, O.; Wongwises, S. Thermal conductivity of Al2O3/water nanofluids: Measurement, correlation, sensitivity analysis, and comparisons with literature reports. J. Therm. Anal. Calorim. 2014, 117, 675–681. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C. Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int. Commun. Heat Mass Transf. 2013, 44, 7–14. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Rudyak, V.Y.; Minakov, A.V. Thermophysical properties of nanofluids. Eur. Phys. J. E 2018, 41, 1–12. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.D. Thermal properties of nanofluids. Adv. Colloid Interface Sci. 2012, 183–184, 30–45. [Google Scholar] [CrossRef]
- Asirvatham, L.G.; Raja, B.; Lal, D.M.; Wongwises, S. Convective heat transfer of nanofluids with correlations. Particuology 2011, 9, 626–631. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Fard, M.H.; Esfahany, M.N.; Talaie, M. Numerical study of convective heat transfer of nanofluids in a circular tube two-phase model versus single-phase model. Int. Commun. Heat Mass Transf. 2010, 37, 91–97. [Google Scholar] [CrossRef]
- Moghadassi, A.; Ghomi, E.; Parvizian, F. A numerical study of water based Al2O3 and Al2O3-Cu hybrid nanofluid effect on forced convective heat transfer. Int. J. Therm. Sci. 2015, 92, 50–57. [Google Scholar] [CrossRef]
- Putra, N.; Roetzel, W.; Das, S.K. Natural convection of nano-fluids. Heat Mass Transf. 2003, 39, 775–784. [Google Scholar] [CrossRef]
- Haddad, Z.; Abu-Nada, E.; Oztop, H.F.; Mataoui, A. Natural convection in nanofluids: Are the thermophoresis and Brownian motion effects significant in nanofluid heat transfer enhancement? Int. J. Therm. Sci. 2012, 57, 152–162. [Google Scholar] [CrossRef]
- Bridges, N.J.; Visser, A.E.; Fox, E.B. Potential of Nanoparticle-Enhanced Ionic Liquids (NEILs) as Advanced Heat-Transfer Fluids. Energy Fuels 2011, 25, 4862–4864. [Google Scholar] [CrossRef]
- Wittmar, A.; Ruiz-Abad, D.; Ulbricht, M. Dispersions of silica nanoparticles in ionic liquids investigated with advanced rheology. J. Nanoparticle Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Francìa, J.M.P.; Vieira, S.I.C.; Lourencìo, M.J.V.; Murshed, S.M.S.; de Castro, C.A.N. Thermal conductivity of [C4mim][(CF3SO 2)2N] and [C2mim][EtSO4] and their ionanofluids with carbon nanotubes: Experiment and theory. J. Chem. Eng. Data 2013, 58, 467–476. [Google Scholar] [CrossRef]
- Atashrouz, S.; Mozaffarian, M.; Pazuki, G. Modeling the Thermal Conductivity of Ionic Liquids and Ionanofluids Based on a Group Method of Data Handling and Modified Maxwell Model. Ind. Eng. Chem. Res. 2015, 54, 8600–8610. [Google Scholar] [CrossRef]
- Chereches, E.I.; Minea, A.A. A Study on Few Thermophysical Properties of Ionanofluids; IAPE: Oxford, UK, 2019; ISBN 978-1-912532-05-6. [Google Scholar]
- Chereches, I.E.; Chereches, M.; Minea, A.A. A study on specific heat of nanoparticle enhanced fluids. IOP Conf. Ser. Mater. Sci. Eng. 2019, 485, 012006. [Google Scholar] [CrossRef]
- Alizadeh, J.; Moraveji, M.K. An experimental evaluation on thermophysical properties of functionalized graphene nanoplatelets ionanofluids. Int. Commun. Heat Mass Transf. 2018, 98, 31–40. [Google Scholar] [CrossRef]
- Joseph, A.; Fal, J.; Żyła, G.; Mathew, S. Nanostructuring of 1-butyl-4-methylpyridinium chloride in ionic liquid–iron oxide nanofluids. J. Therm. Anal. Calorim. 2019, 135, 1373–1380. [Google Scholar] [CrossRef]
- Pamies, R.; Avilés, M.D.; Arias-Pardilla, J.; Carrión, F.J.; Sanes, J.; Bermúdez, M.D. Rheological study of new dispersions of carbon nanotubes in the ionic liquid 1-ethyl-3-methylimidazolium dicyanamide. J. Mol. Liq. 2019, 278, 368–375. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Zheng, F.-F.; Wu, X.-H.; Yin, Y.-L.; Chen, G. Variations of thermophysical properties and heat transfer performance of nanoparticle-enhanced ionic liquids. R. Soc. Open Sci. 2019, 6, 182040. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2011, 2012, 1–17. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Zhao, J.; Gao, H. Measurement of thermal conductivity, viscosity and density of ionic liquid [EMIM][DEP]-based nanofluids. Chin. J. Chem. Eng. 2016, 24, 331–338. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Zhang, X.; Li, T. Simulation and Experimental Study on Thermal Conductivity of [EMIM][DEP] + H2O + SWCNTs Nanofluids as a New Working Pairs. Int. J. Thermophys. 2018, 39, 41. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.; Fox, E.B.; Khan, J.A. Thermal performance of Al2O3 Nanoparticle Enhanced Ionic Liquids (NEILs) for Concentrated Solar Power (CSP) applications. Int. J. Heat Mass Transf. 2015, 85, 585–594. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Shevelyova, M.P.; Paulechka, Y.U.; Kabo, G.J.; Blokhin, A.V.; Kabo, A.G.; Gubarevich, T.M. Physicochemical Properties of Imidazolium-Based Ionic Nanofluids: Density, Heat Capacity, and Enthalpy of Formation. J. Phys. Chem. C 2013, 117, 4782–4790. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
- Wang, F.; Han, L.; Zhang, Z.; Fang, X.; Shi, J.; Ma, W. Surfactant-free ionic liquid-based nanofluids with remarkable thermal conductivity enhancement at very low loading of graphene. Nanoscale Res. Lett. 2012, 7, 314. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X. An investigation into the thermophysical and optical properties of SiC/ionic liquid nanofluid for direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2017, 163, 157–163. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Murshed, S.; Lourenço, M.; Santos, F.; Lopes, M.; França, J. Enhanced thermal conductivity and specific heat capacity of carbon nanotubes ionanofluids. Int. J. Therm. Sci. 2012, 62, 34–39. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.M.; Khan, J.A. Nanoparticle Enhanced Ionic Liquids (NEILS) as Working Fluid for the Next Generation Solar Collector. Procedia Eng. 2013, 56, 631–636. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.; Elsinawi, A. Understanding the heat capacity enhancement in ionic liquid-based nanofluids (ionanofluids). J. Mol. Liq. 2018, 253, 326–339. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Ionic liquid-based stable nanofluids containing gold nanoparticles. J. Colloid Interface Sci. 2011, 362, 5–14. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Gold-ionic liquid nanofluids with preferably tribological properties and thermal conductivity. Nanoscale Res. Lett. 2011, 6, 259. [Google Scholar] [CrossRef]
- Fox, E.B.; Visser, A.E.; Bridges, N.J.; Amoroso, J.W. Thermophysical Properties of Nanoparticle-Enhanced Ionic Liquids (NEILs) Heat-Transfer Fluids. Energy Fuels 2013, 27, 3385–3393. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Lourenc¸o, M.J.V.; Ribeiro, A.P.C.; Langa, E.; Vieira, S.I.C. Thermal Properties of Ionic Liquids and IoNanofluids of Imidazolium and Pyrrolidinium Liquids. J. Chem. Eng. Data 2010, 55, 653–661. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.; Fox, E.B.; Khan, J.A. Enhanced thermophysical properties of NEILs as heat transfer fluids for solar thermal applications. Appl. Therm. Eng. 2017, 110, 1–9. [Google Scholar] [CrossRef]
- França, J.M.P.M.; Lourenço, M.J.V.; Murshed, S.M.S.; Pádua, A.A.H.; De Castro, C.A.N. Thermal Conductivity of Ionic Liquids and IoNanofluids and Their Feasibility as Heat Transfer Fluids. Ind. Eng. Chem. Res. 2018, 57, 6516–6529. [Google Scholar] [CrossRef]
- Liu, J.; Xu, C.; Chen, L.; Fang, X.; Zhang, Z. Preparation and photo-thermal conversion performance of modified graphene/ionic liquid nanofluids with excellent dispersion stability. Sol. Energy Mater. Sol. Cells 2017, 170, 219–232. [Google Scholar] [CrossRef]
- Wang, B.; Hao, J.; Li, Q.; Li, H. New insights into thermal conduction mechanisms of multi-walled carbon nanotube/ionic liquid suspensions. Int. J. Therm. Sci. 2014, 83, 89–95. [Google Scholar] [CrossRef]
- Ferreira, A.; Simões, P.; Fonseca, M.; Oliveira, M.; Trino, A. Transport and thermal properties of quaternary phosphonium ionic liquids and IoNanofluids. J. Chem. Thermodyn. 2013, 64, 80–92. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Rheological and Tribological Properties of Ionic Liquid-Based Nanofluids Containing Functionalized Multi-Walled Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 8749–8754. [Google Scholar] [CrossRef]
- Neo, C.Y.; Ouyang, J. Functionalized carbon nanotube-induced viscosity reduction of an ionic liquid and performance improvement of dye-sensitized solar cells. Electrochim. Acta 2012, 85, 1–8. [Google Scholar] [CrossRef]
- Ho, C.; Liu, W.; Chang, Y.; Lin, C. Natural convection heat transfer of alumina-water nanofluid in vertical square enclosures: An experimental study. Int. J. Therm. Sci. 2010, 49, 1345–1353. [Google Scholar] [CrossRef]
- Maxwell, J.C. Treatise on Electricity and Magnetism, 3rd ed.; Clarendon Press: Oxford, UK, 1891. [Google Scholar]
- Jorjani, S.; Mozaffarian, M.; Pazuki, G. A novel Nanodiamond based IoNanofluid: Experimental and mathematical study of thermal properties. J. Mol. Liq. 2018, 271, 211–219. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; ElSinawi, A. Thermal Conductivity Enhancement Phenomena in Ionic Liquid-Based Nanofluids (Ionanofluids). Aust. J. Chem. 2019, 72, 21. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus About Wiley Online Library Privacy Policy Terms of Use. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; He, Y.; Tan, C. Rheological behaviour of ethylene glycol based titania nanofluids. Chem. Phys. Lett. 2007, 444, 333–337. [Google Scholar] [CrossRef]
- Leong, K.; Yang, C.; Murshed, S. A model for the thermal conductivity of nanofluids—The effect of interfacial layer. J. Nanoparticle Res. 2006, 8, 245–254. [Google Scholar] [CrossRef]
- Einstein, A. Eine neue Bestimmung der Moleküldimensionen. Ann. Phys. 1906, 324, 289–306. [Google Scholar] [CrossRef]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Nielsen, L.E. A Generalized Equation for the Elastic Moduli of Composite Materials. A Gen. Equ. Elastic Modul. Compos. Mater. 1970, 41, 4626–4627. [Google Scholar] [CrossRef]
- Krieger, M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and Heat Transfer Study of Dispersed Fluids with Submicron Metallic Oxide Particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Conceptions for heat transfer correlation of nanofluids. Int. J. Heat Mass Transf. 2000, 43, 3701–3707. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.; Fox, E.B.; Khan, J.A. Experimental investigation of natural convection heat transfer of Al2O3 Nanoparticle Enhanced Ionic Liquids (NEILs). Int. J. Heat Mass Transf. 2015, 83, 753–761. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Pradob, J.I.; Cherecheş, M.; Minea, A.A.; Lugo, L. Experimental study on thermophysical properties of alumina nanoparticle enhanced ionic liquids. J. Mol. Liq. 2019, 291, 111332. [Google Scholar] [CrossRef]
- Patil, V.S.; Cera-Manjarres, A.; Salavera, D.; Rode, C.V.; Patil, K.R.; De Castro, C.A.N.; Coronas, A. Ru-Imidazolium Halide IoNanofluids: Synthesis, Structural, Morphological and Thermophysical Properties. J. Nanofluids 2016, 5, 191–208. [Google Scholar] [CrossRef]
- Sadi, M. Determination of heat capacity of ionic liquid based nanofluids using group method of data handling technique. Heat Mass Transf. 2017, 54, 49–57. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Murshed, S.; Leong, K.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; He, Y.; Hu, Y.; Zhu, J.; Jiang, B. Experimental investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluids. Appl. Therm. Eng. 2015, 88, 363–368. [Google Scholar] [CrossRef]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.J.; Townsend, J.; Christianson, R.J.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-W.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Thermal conductivity and viscosity of Al2O3nanofluid based on car engine coolant. J. Phys. D Appl. Phys. 2010, 43. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.; Mohammed, H. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Zyła, G.; Fal, J.; Gizowska, M.; Witek, A.; Cholewa, M. Dynamic viscosity of aluminum oxide-ethylene glycol (Al2O3-EG) nanofluids. Acta Phys. Pol. A 2015, 128, 240–242. [Google Scholar] [CrossRef]
- Wang, X.; Heinemann, F.W.; Yang, M.; Melcher, B.U.; Fekete, M.; Mudring, A.-V.; Wasserscheid, P.; Meyer, K. A new class of double alkyl-substituted, liquid crystalline imidazolium ionic liquids—A unique combination of structural features, viscosity effects, and thermal properties. Chem. Commun. 2009, 7405–7407. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Khan, J.A. Numerical investigation of natural convection of nanoparticle enhanced ionic liquids (NEILs) in enclosure heated from below. In Proceedings of the International Conference of Mechanical Engineering ICME 2015, Dhaka, Bangladesh, 18–20 December 2015; Volume 1754. AIP Conference Proceedings. [Google Scholar]
- Minea, A.-A.; El-Maghlany, W.M. Natural convection heat transfer utilizing ionic nanofluids with temperature-dependent thermophysical properties. Chem. Eng. Sci. 2017, 174, 13–24. [Google Scholar] [CrossRef]
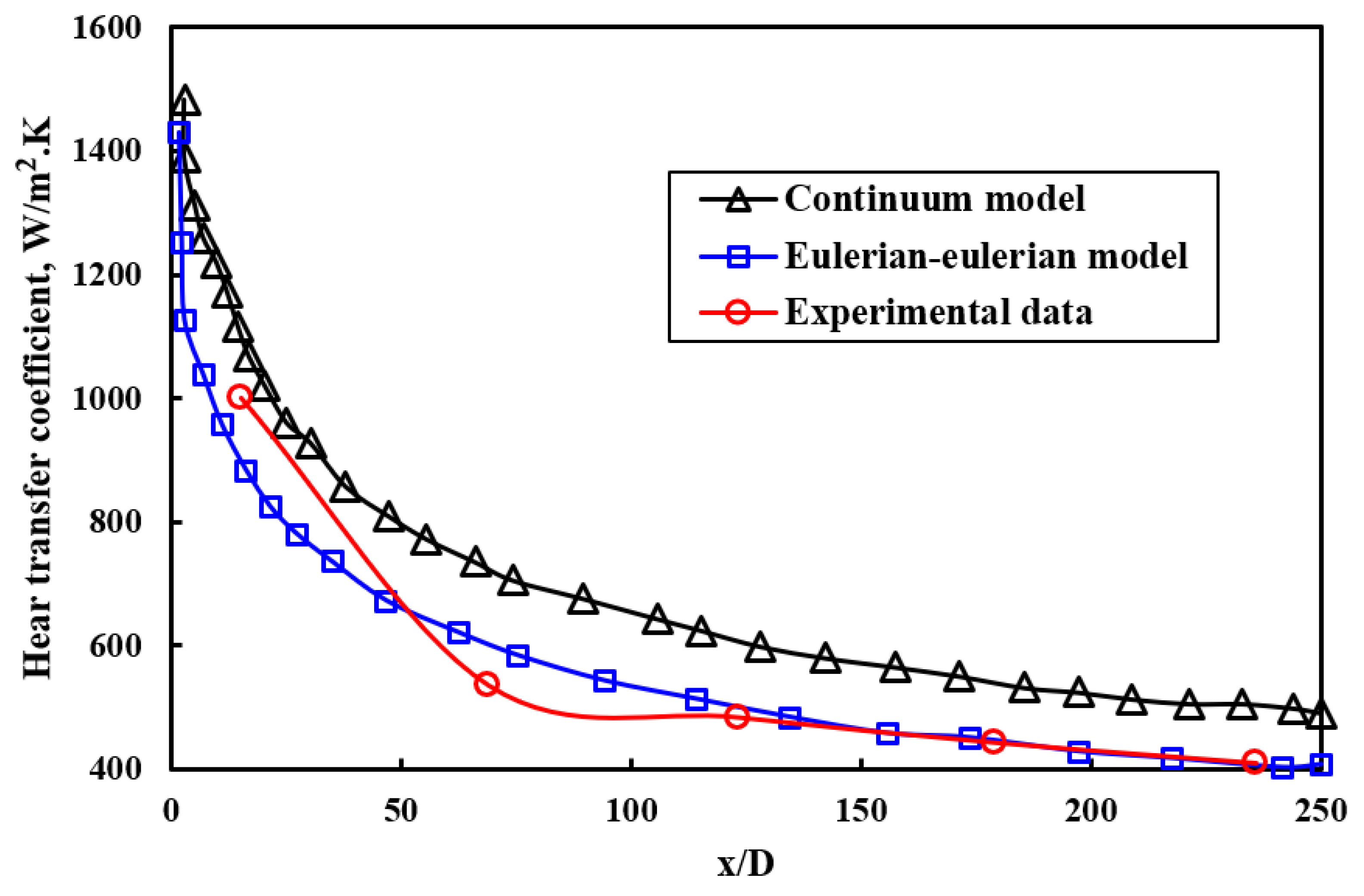
- Paul, T.C.; Mahamud, R.; Khan, J.A. Multiphase modeling approach for ionic liquids (ILs) based nanofluids: Improving the performance of heat transfer fluids (HTFs). Appl. Therm. Eng. 2019, 149, 165–172. [Google Scholar] [CrossRef]
- Chereches, E.I.; Sharma, K.V.; Minea, A.A. A numerical approach in describing ionanofluids behavior in laminar and turbulent flow. Contin. Mech. Thermodyn. 2018, 30, 657–666. [Google Scholar] [CrossRef]
- Minea, A.A.; Murshed, S.M.S. A review on development of ionic liquid based nanofluids and their heat transfer behavior. Renew. Sustain. Energy Rev. 2018, 91, 584–599. [Google Scholar] [CrossRef]








| Ionic Liquids | Abbreviations |
|---|---|
| 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide | [C4mmim][NTf2] |
| 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide | [C4mim][[NTf2] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | [C4mim][PF6] |
| 1-Butyl-3-methylimidazolium tetrafluoroborate | [C4mim][BF4]) |
| N-butyl-N-methylpyrrolidiniumbis{trifluoromethyl)sulfonyl} imide | [C4mpyrr][NTf2] |
| 1-butyl-3-methylimidazolium dicyanamide | [C4mim][Dca] |
| 1-butyl-3-methylimidazolium trifluoromethanesulfonate | [C4mim][CF3SO3] |
| 1-n-butyl-3-methyl-imidazolium thiocyanate | [C4mim][SCN] |
| N-butyl-N,N,N-trimetylammonium bis(trifluorme thylsulfonyl)imide | [N4111][NTf2] |
| 1-ethyl-3-methylimidazolium ethylsulfate | [C2mim][EtSO4] |
| 1-ethyl-3-methylimidazolium methanesulfonate | [C2mim][CH3SO3] |
| 1-ethyl-3-methylimidazolium dicyanamide | [C2mim][DCA] |
| 1-ethyl-3-methylimidazolium acetate | [C2mim]Ac |
| 1-ethyl-3-methylimidazolium diethylphosphate | [C2mim][DEP] |
| 1-ethyl-3-methyl-imidazolium thiocyanate | [C2mim][SCN] |
| 1-ethyl-3-methylimidazolium ethylsulfate | [C2mim][C2SO4] |
| 1-ethyl-3-methyl-imidazolium tricyanomethanide | [C2mim][C(CN)3] |
| 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonil)imide | [C6mim][NTf2] |
| 1-hexyl-3-methylimidazolium tetrafloroborate | [C6mim][BF4] |
| 1-hexyl-3-methylimidazolium hexafluorophosphate | [C6mim][PF6] |
| 1-propyl-3-methylimidazolium Iodide | [PMII] |
| Trihexyltetradecylphosphonium dicyanamide | [P66614][N-(CN)2] |
| Trihexyltetradecylphosphonium bromide | [P66614][Br] |
| Ionic Liquids | Nanoparticles | Concentration | Particle Size/Shape | Study Parameters | Mixing Method | Ref. |
|---|---|---|---|---|---|---|
| [C4mmim][NTf2] | Al2O3 | 0.5, 1, 2.5 wt% | 50 nm spherical | Density and heat capacity | Vortex mixture | [36] |
| [C6mim][NTf2] [C6mim][BF4] | SiO2 | 0.1, 0.3, 0.5, 1, and 2 wt% | 10–20 nm spherical | Viscosity and rheological behavior | Ultrasound treatment in a BANDELIN-Sonorex Digitec ultrasound bath | [37] |
| [C4mim][ NTf2] [C2mim][EtSO4] | Multiwalled carbon nanotubes (MWCNTs) | 0.5, 1, 3 wt% | 13−16 nm dia., 1−10 µm length | Thermal conductivity | Sonication | [38] |
| [C2mim][CH3SO3] | Al2O3 | 1, 2, 3 wt% | Not mentioned | Heat capacity | Not mentioned | [40] |
| [C4mim][PF6] | Graphene | 1, 2, 3 wt% | Not mentioned | Surface tension, electrical conductivity, viscosity | Ultrasonic sonicator | [42] |
| 1-butyl-4-methylpyridinium chloride | FeSO4 | 4, 6.8, 14.7 and 15.6 mass% | 5–10 nm | viscosity | One step method | [43] |
| [C2mim][DCA] | MWCNTs, Aligned MWCNTs, SWCNTs | 1 wt.% | MWCNTs-8 nm dia., 0.5–2 μm length, Aligned MWCNTs-10–20 nm dia., 5–15 μm length, SWCNTs-1–2 nm dia., 5–30 μm length | viscosity | Agatemortar | [44] |
| [C2mim][Ac] | Graphene nanoplatelets | 0.05, 0.3, 0.5, 1, 2, 3, 5 mass fraction | 5–10 µm dia., 4–20 nm thickness | Viscosity, thermal conductivity | Ultrasonication | [45] |
| [C2mim][DEP] | Multi-wall carbon nanotubes (MWCNTs) | 0.2, 0.5, 1 mass fraction | 20–40 nm dia., 1–2 μm length | thermal conductivity, viscosity and density | Magnetic stirrer | [47] |
| [C2mim][DEP] | Single-wall carbon nanotubes (SWCNTs) | 0.5, 1, 2 wt% | 1–2 nm dia., 5–30 μm length | Thermal conductivity | Ultrasonic vibration | [48] |
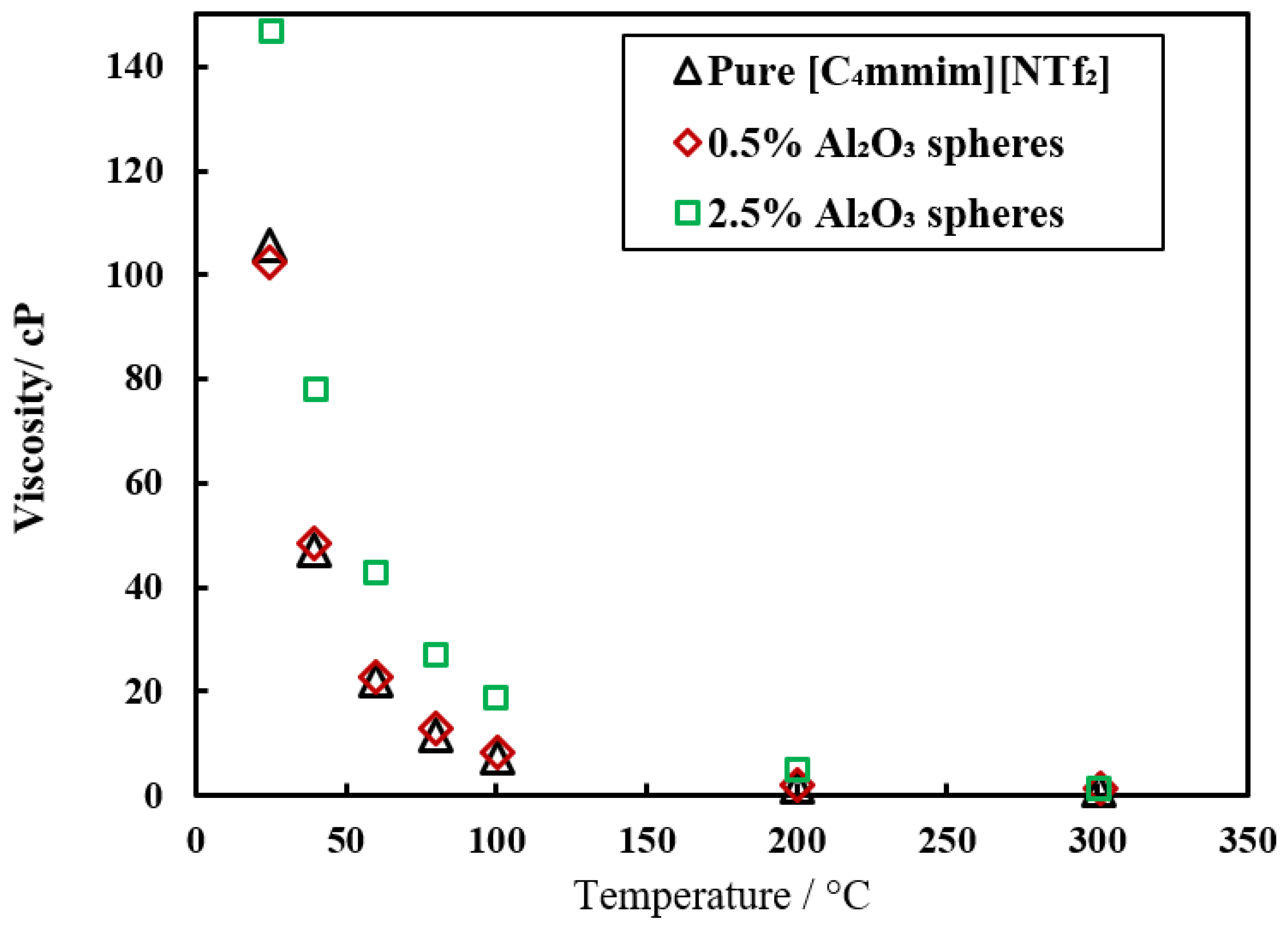
| [C4mim][NTf2] | Al2O3 | 0.5, 1, 2.5 wt% | 50 nm | Density, viscosity, thermal conductivity | Vortex mixture | [49] |
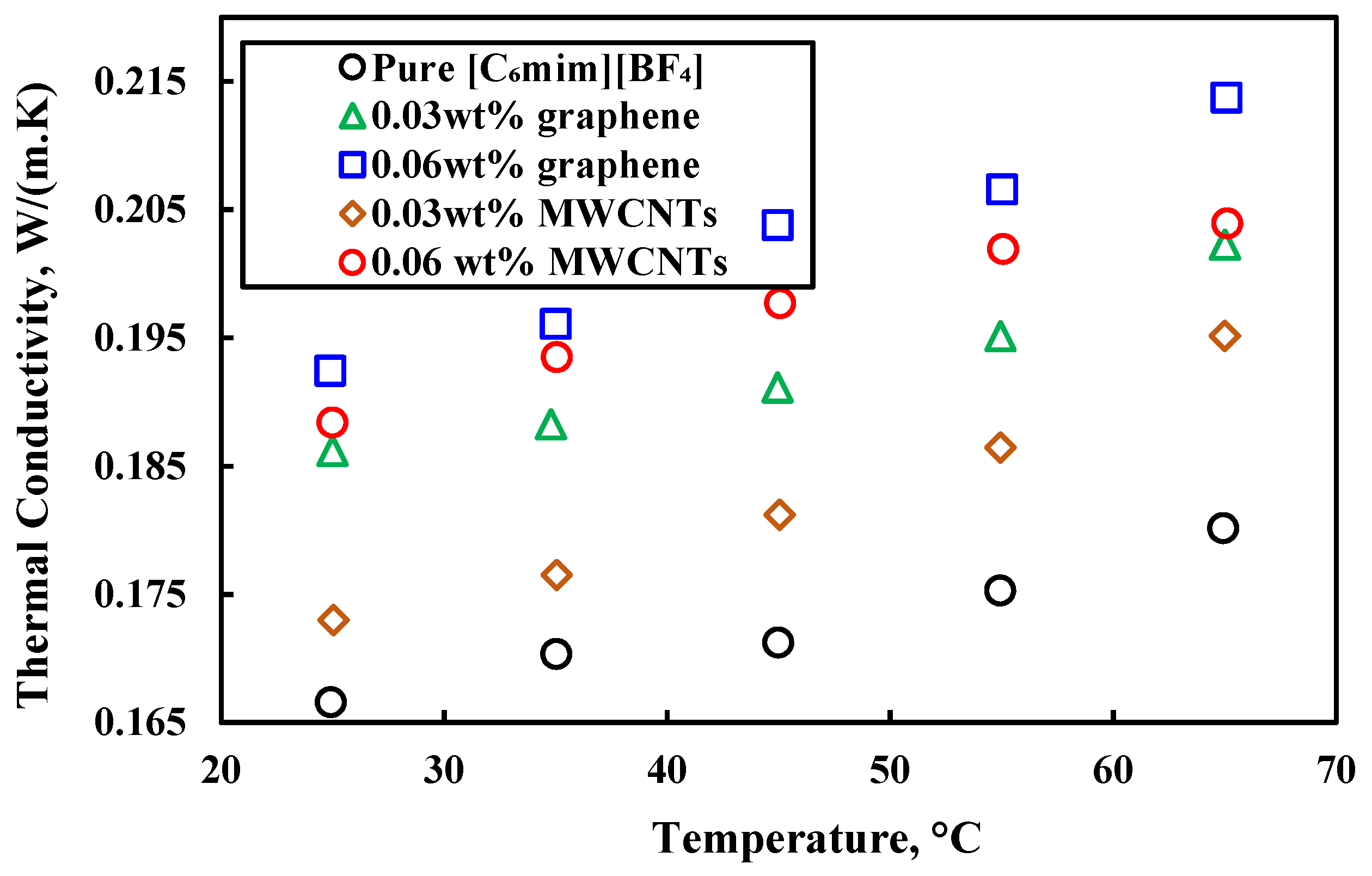
| [C6mim][BF4] | Graphene | 0.03, 0.06 wt% | Not mentioned | Thermal conductivity, viscosity, specific heat and density | Ultrasonic vibration | [50] |
| [C4mim][BF4] [C4mim][PF6] | Multiwalled carbon nanotubes (MWCNTs) | 12.4 11.4 wt% | 10–80 nm dia., 1–2 μm length | Specific heat | Agate mortar and grinding | [51] |
| [C6mim][BF4] | Graphite nanoparticles (GNPs), single-wall carbon nanotubes (SWCNTs) and graphene (GE) | 0.005, 0.01 wt% | 30 nm, 2 nm, and lengths range from 5 to 30 mm, 0.8 nm | Specific heat, thermal conductivity, viscosity | Ultrasonic apparatus, magnetic stirrer | [52] |
| [C6mim][BF4] | Graphene, MWCNTs | 0.03, 0.06, 0.09 wt% | Not mentioned | Thermal conductivity, Specific heat, Viscosity | Ultrasonicator | [53] |
| [C6mim][BF4] | SiC | 0.01, 0.03, 0.06 wt% | 30 nm | Thermal conductivity, Specific heat, Viscosity, optical properties | Ultrasonication homogenizer Sonifier | [54] |
| [Cnmim][NTf2] (where n = 4, 6, and 8), [C4mim][CF3SO3], [C2mim][EtSO4], [C4mim][BF4], and [C4mim][PF6] | MWCNTs | 1, 1.5 wt.% | length of nanotubes were 13–16 nm and 1–10 m | Thermal conductivity, specific heat | Sonication | [55] |
| [C4mim][NTf2] [C4mpyrr][NTf2] | Al2O3 | 1 wt% | 50 nm | Thermal conductivity, heat capacity, heat transfer coefficient | Vortex mixture | [56] |
| [C4mim][Dca] [C4mim][NTf2] [C4mpyrr][NTf2] [C4mim][PF6] [C2mim][C2SO4] | MWCNTs | 0.5, 1, 3 wt% | 13–16 nm diameter, 1–10 mm length | Density, Heat capacity | [57] | |
| [C4mim][PF6] | Gold nanoparticles | 1 wt% | 5.2, 18.4, 18.4, 29.9 and 56.4 nm | Thermal conductivity, viscosity | Sonication | [58] |
| [C4mim][PF6] | Gold nanoparticles | 1 wt% | 5.2 nm | Thermal conductivity, friction coefficient | Sonication | [59] |
| [C4mmim][Tf2N] | Al2O3 MWCNTs SWCNTs carbon black ZnO Fe2O3 SiO2 CuO Au | 0.5, 2.5 wt% | 50 nm 2−4 nm × 2800 nm 20−25 nm × 1−5 μm 0.7–2.5 nm × 0.5–10 μm 4 nm 50 nm 5−15 nm 100 nm | Viscosity, thermal conductivity | Vortex mixture | [60] |
| [C6mim][BF4] [C4mim][PF6] [C6mim][PF6] [C4mim][CF3SO3] [C4mpyrr][NTf2] | MWCNTs | 1, 1.5 wt% | 13–16 nm dia., 1–10 mm length | Thermal conductivity, heat capacity | Sonication | [61] |
| [C4mim][NTf2] [C4mpyrr][NTf2], [N4111][NTf2] [C4mmim][NTf2] | Al2O3 | 0.5, 1, 2.5 wt% | 50 nm | Viscosity, thermal conductivity, heat capacity | Vortex mixture | [62] |
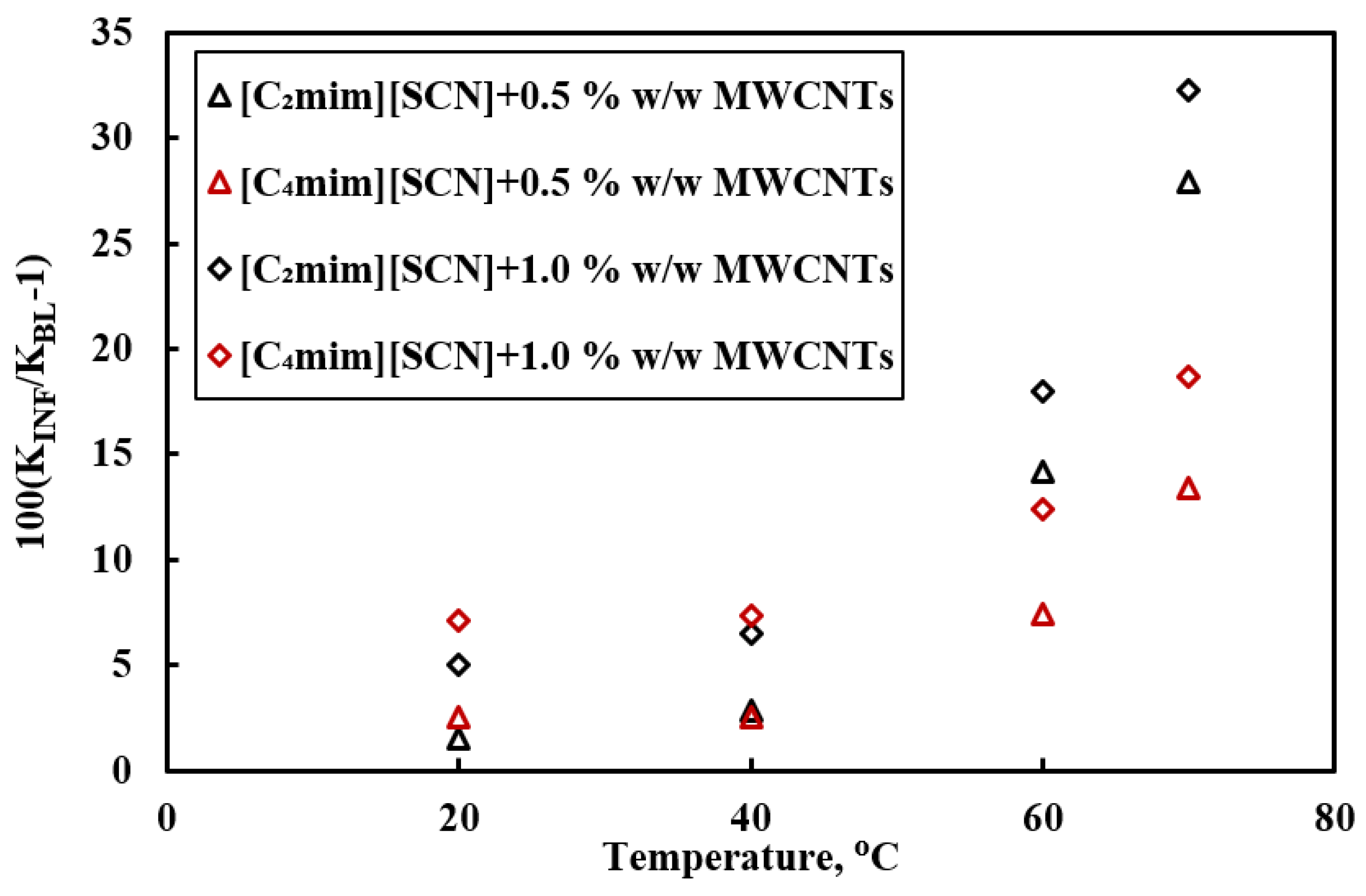
| [P66614][N-(CN)2] [P66614][Br] [C2mim][SCN] [C4mim][SCN] [C2mim][C(CN)3] [C4mim][C(CN)3] | MWCNTs | 0.5, 1 wt% | 13–16 nm dia., 1–10 mm length | Thermal conductivity | Sonication | [63] |
| [C4mim][BF4] | Modified graphene | 0.01%, 0.03% and 0.05% wt% | Not mentioned | Thermal conductivity, Radiative properties | Mildly stirring | [64] |
| [C4mim][PF6] | MWCNTs | 0.05, 0.1 wt% | Diameter, 20–40 nm; length, 5–15 µm | Thermal conductivity | [65] | |
| [(C6)3PC14)] [Phosph] [(C4)3PC1)] [C1SO4] [(C6)3PC14] [NTf2] | MWCNTs | 0.1, 0.2 wt% | 13–16 nm dia., 1–10 mm length | Thermal conductivity, viscosity, heat capacity, thermal stability | Sonication | [66] |
| [C4mim][PF6] | Functionalized multiwalled carbon nanotubes (F-MWCNTs) | 0.01, 0.02, 0.04, 0.06 wt% | Diameter, 20–40 nm; length, 5–15 μm | Viscosity | Ultrasonication | [67] |
| [PMII] | MWCNTs | 0.1 wt% | Outer diameter of 8–15 nm, inner diameter of 3–5 nm, length of approximately 50 µm | Viscosity | Not mentioned | [68] |
| Models | Equations | Ref. |
|---|---|---|
| Maxwell model [70] | [54,61,62,64,71] | |
| Hamilton and Crosser model [72] | [47,52,63,65,73] | |
| Bruggeman model [74] | [62] | |
| Aggregation model [75] | where | [62] |
| Interfacial layer [76] | where ; here considered. | [47,55,61,62,63,66,73] |
| Equations | Ref. |
|---|---|
Where | [71] |
| [49] | |
| [65] |
| Models | Equations | Ref. |
|---|---|---|
| Einstein model [77] | [45,62] | |
| Brinkman model [78] | [45,47,62] | |
| Batchelor model [79] | [45,54,62] | |
| Nielson [80] | where is the maximum particle packing fraction which is typically considered 0.605. | [62] |
| Krieger-Dougherty (K-D) model [81] | where, and are the average radii of the aggregate and primary nanoparticles, respectively. | [62] |
| Equations | Ref. |
|---|---|
| [45] | |
| [49] |
| Models | Equations | Ref. |
|---|---|---|
| Pak and Cho [82] | [41] | |
| Xuan and Roetzel [83] | [41,57,62,66] |
| Property | IL-Based Nanofluids | Temperature Range (°C) | Ref. | |
|---|---|---|---|---|
 | Density | [C6mim][BF4] + 0.06 wt% graphene | 30–210 | [50] |
| [C2mim][DEP] + 1 wt% MWCNT | 25–50 | [47] | ||
| [C6mim][BF4] + 0.06 wt% SiC | 25–65 | [54] | ||
| Viscosity | [C4mmim][NTf2] + 2.5 wt% Al2O3 | 25–300 | [60] | |
| [C6mim][BF4] + 0.06 wt% graphene | 25–215 | [50] | ||
| [C4mim][BF4] + 0.01 wt% graphene | 25–150 | [52] | ||
 | Thermal conductivity | [C2mim][DEP] + 2 wt% SWCNT | 30–80 | [48] |
| [C4mmim][NTf2] + 2.5 wt% whiskers Al2O3 | 25–60 | [60] | ||
| [C4mim][NTf2] + 2.5 wt% Al2O3 | 10–70 | [62] | ||
| Specific heat | [C4mpyrr][NTf2] + 2.5 wt% Al2O3 | 25–340 | [84] | |
| [C4mmim][NTf2] + 2.5 wt% Al2O3 | 25–340 | [62] | ||
| [C6mim][BF4] + 0.06 wt% SiC | 0–80 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, T.C.; Tikadar, A.; Mahamud, R.; Salman, A.S.; Morshed, A.K.M.M.; Khan, J.A. A Critical Review on the Development of Ionic Liquids-Based Nanofluids as Heat Transfer Fluids for Solar Thermal Energy. Processes 2021, 9, 858. https://doi.org/10.3390/pr9050858
Paul TC, Tikadar A, Mahamud R, Salman AS, Morshed AKMM, Khan JA. A Critical Review on the Development of Ionic Liquids-Based Nanofluids as Heat Transfer Fluids for Solar Thermal Energy. Processes. 2021; 9(5):858. https://doi.org/10.3390/pr9050858
Chicago/Turabian StylePaul, Titan C., Amitav Tikadar, Rajib Mahamud, Azzam S. Salman, A. K. M. Monjur Morshed, and Jamil A. Khan. 2021. "A Critical Review on the Development of Ionic Liquids-Based Nanofluids as Heat Transfer Fluids for Solar Thermal Energy" Processes 9, no. 5: 858. https://doi.org/10.3390/pr9050858
APA StylePaul, T. C., Tikadar, A., Mahamud, R., Salman, A. S., Morshed, A. K. M. M., & Khan, J. A. (2021). A Critical Review on the Development of Ionic Liquids-Based Nanofluids as Heat Transfer Fluids for Solar Thermal Energy. Processes, 9(5), 858. https://doi.org/10.3390/pr9050858






